Abstract
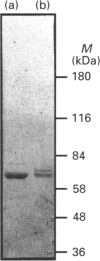
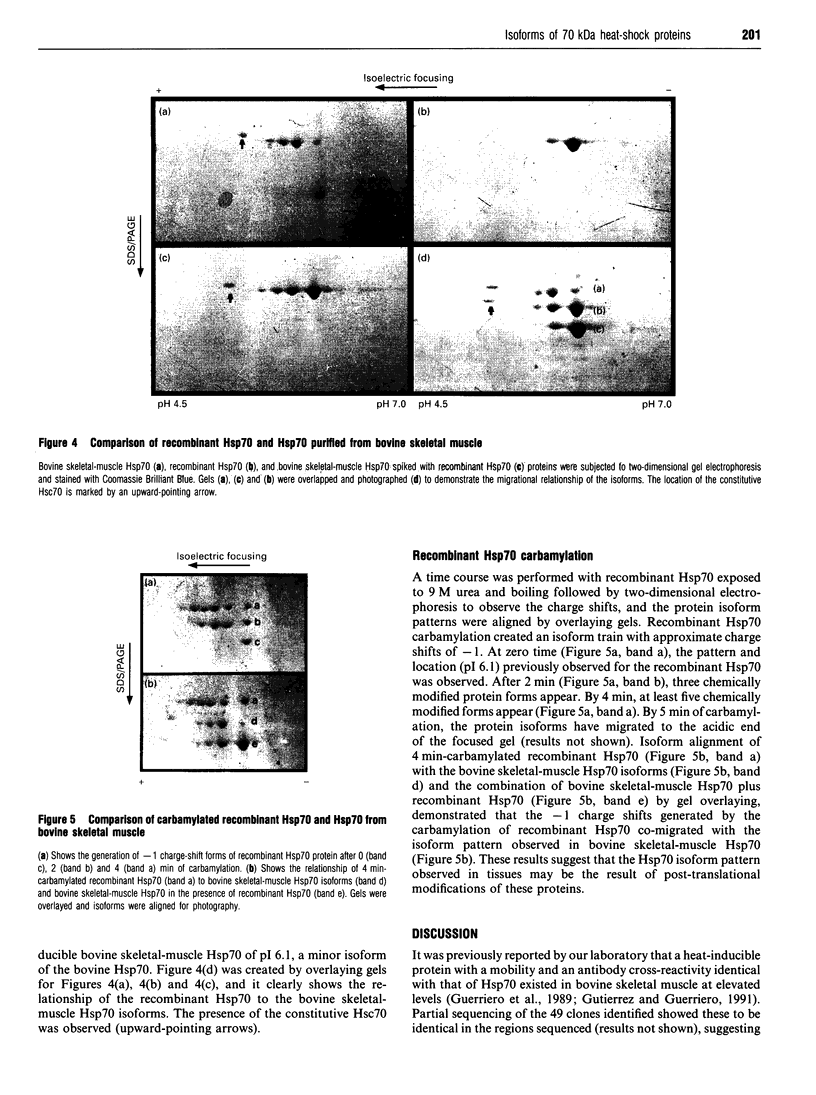
A cDNA clone for the stress-inducible 70 kDa heat-shock protein (Hsp70) has been isolated from a bovine skeletal-muscle cDNA library. This mRNA encodes a protein with a calculated molecular mass of 70250 Da. The cDNA has one continuous open reading frame capable of encoding a 641-amino-acid protein. Expression of this cDNA in a bacterial expression system produced a protein with a mobility identical with that of the inducible Hsp70 protein from bovine skeletal muscle as determined by SDS/PAGE. Two-dimensional gel electrophoresis demonstrated this protein to have focusing properties identical with that of a minor isoform from bovine skeletal muscle. Upon carbamylation of this bacterially expressed protein, a train of charged proteins with charge differences of -1 were produced. These carbamylated proteins were shown to have similar focusing mobilities to the Hsp70 isoforms isolated from bovine skeletal muscle. These results demonstrate the identification of a skeletal-muscle inducible Hsp70 gene and suggest that the presence of multiple Hsp70 isoforms may be the product of post-translational modifications to the Hsp70 proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. L., O'Brien D. A., Eddy E. M. A novel hsp70-like protein (P70) is present in mouse spermatogenic cells. Mol Cell Biol. 1988 Feb;8(2):828–832. doi: 10.1128/mcb.8.2.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond-Elguindi S., Fourie A. M., Sambrook J. F., Gething M. J. Peptide-dependent stimulation of the ATPase activity of the molecular chaperone BiP is the result of conversion of oligomers to active monomers. J Biol Chem. 1993 Jun 15;268(17):12730–12735. [PubMed] [Google Scholar]
- Craig E. A., McCarthy B. J., Wadsworth S. C. Sequence organization of two recombinant plasmids containing genes for the major heat shock-induced protein of D. melanogaster. Cell. 1979 Mar;16(3):575–588. doi: 10.1016/0092-8674(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Currie R. W., White F. P. Characterization of the synthesis and accumulation of a 71-kilodalton protein induced in rat tissues after hyperthermia. Can J Biochem Cell Biol. 1983 Jun;61(6):438–446. doi: 10.1139/o83-059. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frohman M. A. Rapid amplification of complementary DNA ends for generation of full-length complementary DNAs: thermal RACE. Methods Enzymol. 1993;218:340–356. doi: 10.1016/0076-6879(93)18026-9. [DOI] [PubMed] [Google Scholar]
- Gallagher D. S., Jr, Grosz M. D., Womack J. E., Skow L. C. Chromosomal localization of HSP70 genes in cattle. Mamm Genome. 1993;4(7):388–390. doi: 10.1007/BF00360590. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Grosz M. D., Womack J. E., Skow L. C. Syntenic conservation of HSP70 genes in cattle and humans. Genomics. 1992 Dec;14(4):863–868. doi: 10.1016/s0888-7543(05)80106-5. [DOI] [PubMed] [Google Scholar]
- Guerriero V., Jr, Raynes D. A., Gutierrez J. A. HSP70-related proteins in bovine skeletal muscle. J Cell Physiol. 1989 Sep;140(3):471–477. doi: 10.1002/jcp.1041400310. [DOI] [PubMed] [Google Scholar]
- Gutierrez J. A., Guerriero V., Jr Quantitation of Hsp70 in tissues using a competitive enzyme-linked immunosorbent assay. J Immunol Methods. 1991 Sep 20;143(1):81–88. doi: 10.1016/0022-1759(91)90275-k. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hunt C. R., Gasser D. L., Chaplin D. D., Pierce J. C., Kozak C. A. Chromosomal localization of five murine HSP70 gene family members: Hsp70-1, Hsp70-2, Hsp70-3, Hsc70t, and Grp78. Genomics. 1993 Apr;16(1):193–198. doi: 10.1006/geno.1993.1158. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Slater M. R., Craig E. A. Saccharomyces cerevisiae contains a complex multigene family related to the major heat shock-inducible gene of Drosophila. Mol Cell Biol. 1982 Nov;2(11):1388–1398. doi: 10.1128/mcb.2.11.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leno G. H., Ledford B. E. Reversible ADP-ribosylation of the 78 kDa glucose-regulated protein. FEBS Lett. 1990 Dec 10;276(1-2):29–33. doi: 10.1016/0014-5793(90)80499-9. [DOI] [PubMed] [Google Scholar]
- Leustek T., Amir-Shapira D., Toledo H., Brot N., Weissbach H. Autophosphorylation of 70 kDa heat shock proteins. Cell Mol Biol. 1992 Feb;38(1):1–10. [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Moran L. A. Proteins related to the mouse L-cell major heat shock protein are synthesized in the absence of heat shock gene expression. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2317–2321. doi: 10.1073/pnas.81.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. K., Rijli F., Appella E. Characterization of the mouse 84-kD heat shock protein gene family. DNA Cell Biol. 1990 Jul-Aug;9(6):387–400. doi: 10.1089/dna.1990.9.387. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelman L. J., Van de Weghe A. R., Coppieters W. R., Van Zeveren A. J., Bouquet Y. H. Complete nucleotide sequence of a porcine HSP70 gene. Immunogenetics. 1992;35(4):286–289. doi: 10.1007/BF00166836. [DOI] [PubMed] [Google Scholar]
- Reikofski J., Tao B. Y. Polymerase chain reaction (PCR) techniques for site-directed mutagenesis. Biotechnol Adv. 1992;10(4):535–547. doi: 10.1016/0734-9750(92)91451-j. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Wang C., Lin J. M., Lazarides E. Methylations of 70,000-Da heat shock proteins in 3T3 cells: alterations by arsenite treatment, by different stages of growth and by virus transformation. Arch Biochem Biophys. 1992 Aug 15;297(1):169–175. doi: 10.1016/0003-9861(92)90656-h. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992 Oct;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]