Abstract
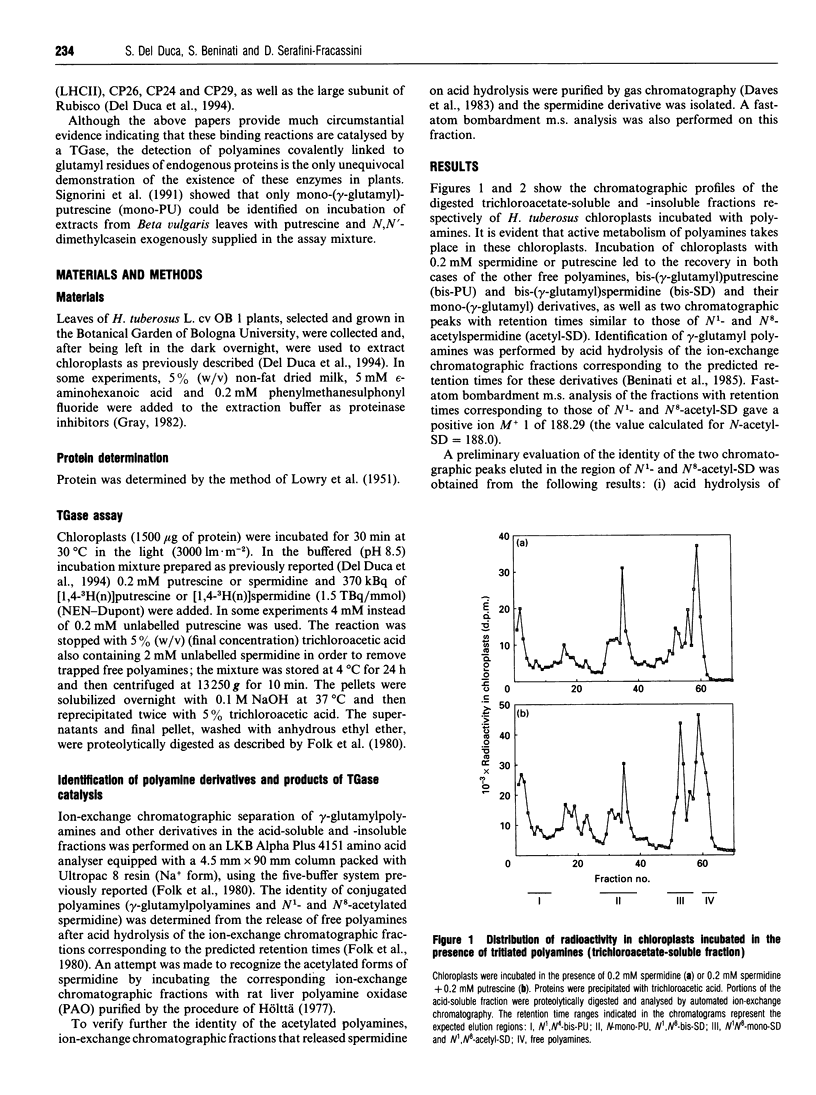
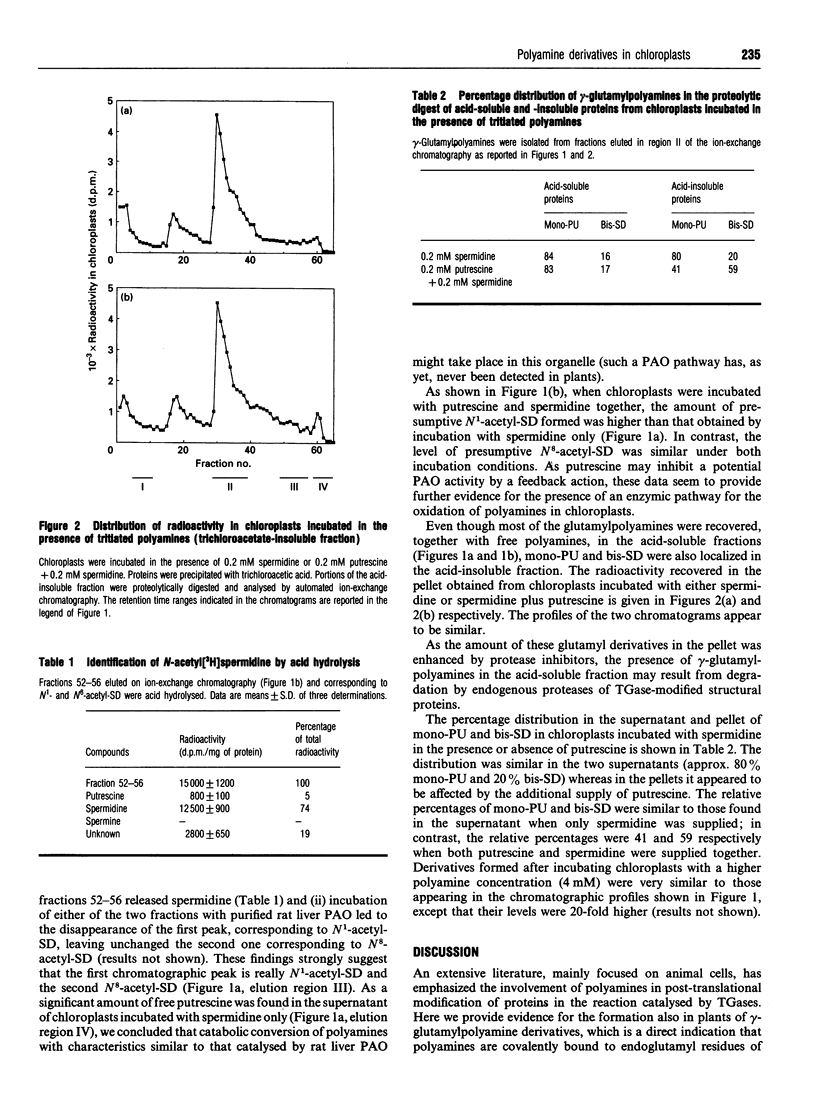
Incubation of chloroplasts of Helianthus tuberosus with labelled putrescine and/or spermidine and proteolytic digestion of their trichloroacetate-soluble and -insoluble proteins revealed the presence of N-(gamma-glutamyl)-putrescine, N1,N4-bis-(gamma-glutamyl)-putrescine and N1,N8-bis-(gamma-glutamyl)spermidine. This finding may be regarded as unequivocal proof of the presence of transglutaminase activity in chloroplasts. In addition, the recovery of spermidine or putrescine and acetylspermidine from chloroplasts incubated with [3H]putrescine or [3H]spermidine respectively indicates the existence of biosynthetic and oxidative pathways. These results suggest that polyamines may have an important function in chloroplasts both in their free form and by covalently binding to proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beninati S., Abbruzzese A., Cardinali M. Differences in the post-translational modification of proteins by polyamines between weakly and highly metastatic B16 melanoma cells. Int J Cancer. 1993 Mar 12;53(5):792–797. doi: 10.1002/ijc.2910530515. [DOI] [PubMed] [Google Scholar]
- Beninati S., Piacentini M., Argento-Cerù M. P., Russo-Caia S., Autuori F. Presence of di- and polyamines covalently bound to protein in rat liver. Biochim Biophys Acta. 1985 Jul 26;841(1):120–126. doi: 10.1016/0304-4165(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. Acetylderivatives as intermediates in polyamine catabolism. Int J Biochem. 1981;13(3):287–292. doi: 10.1016/0020-711x(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Daves G. D., Jr, Smith R. G., Valkenburg C. A. Gas chromatographic-mass spectrometric analysis of polyamines and polyamine conjugates. Methods Enzymol. 1983;94:48–54. doi: 10.1016/s0076-6879(83)94009-0. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Galston A. W., Sawhney R. K. Polyamines in plant physiology. Plant Physiol. 1990 Oct;94(2):406–410. doi: 10.1104/pp.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1980;74:101–139. [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Icekson I., Apelbaum A. Evidence for transglutaminase activity in plant tissue. Plant Physiol. 1987 Aug;84(4):972–974. doi: 10.1104/pp.84.4.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. D., Guzman E., Kuehn G. D. Purification and partial characterization of transglutaminase from Physarum polycephalum. J Bacteriol. 1992 Apr;174(8):2599–2605. doi: 10.1128/jb.174.8.2599-2605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamppa G. K., Abad M. S. Processing of a wheat light-harvesting chlorophyll a/b protein precursor by a soluble enzyme from higher plant chloroplasts. J Cell Biol. 1987 Dec;105(6 Pt 1):2641–2648. doi: 10.1083/jcb.105.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Margosiak S. A., Dharma A., Bruce-Carver M. R., Gonzales A. P., Louie D., Kuehn G. D. Identification of the Large Subunit of Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase as a Substrate for Transglutaminase in Medicago sativa L. (Alfalfa). Plant Physiol. 1990 Jan;92(1):88–96. doi: 10.1104/pp.92.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E. The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J Biol Chem. 1983 Aug 25;258(16):9941–9948. [PubMed] [Google Scholar]
- Piacentini M., Fesus L., Farrace M. G., Ghibelli L., Piredda L., Melino G. The expression of "tissue" transglutaminase in two human cancer cell lines is related with the programmed cell death (apoptosis). Eur J Cell Biol. 1991 Apr;54(2):246–254. [PubMed] [Google Scholar]
- Serafini-Fracassini D., Del Duca S., D'Orazi D. First evidence for polyamine conjugation mediated by an enzymic activity in plants. Plant Physiol. 1988 Jul;87(3):757–761. doi: 10.1104/pp.87.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]


