Abstract
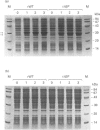
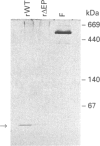
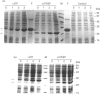
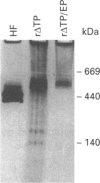
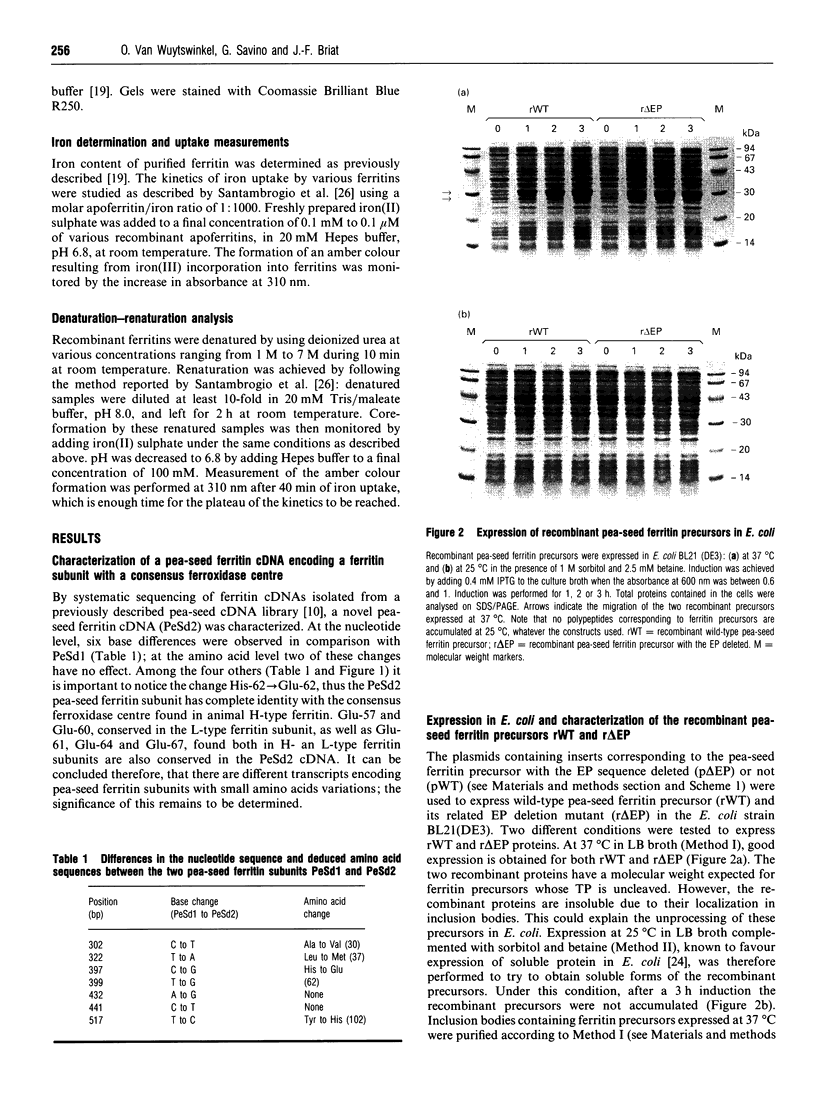
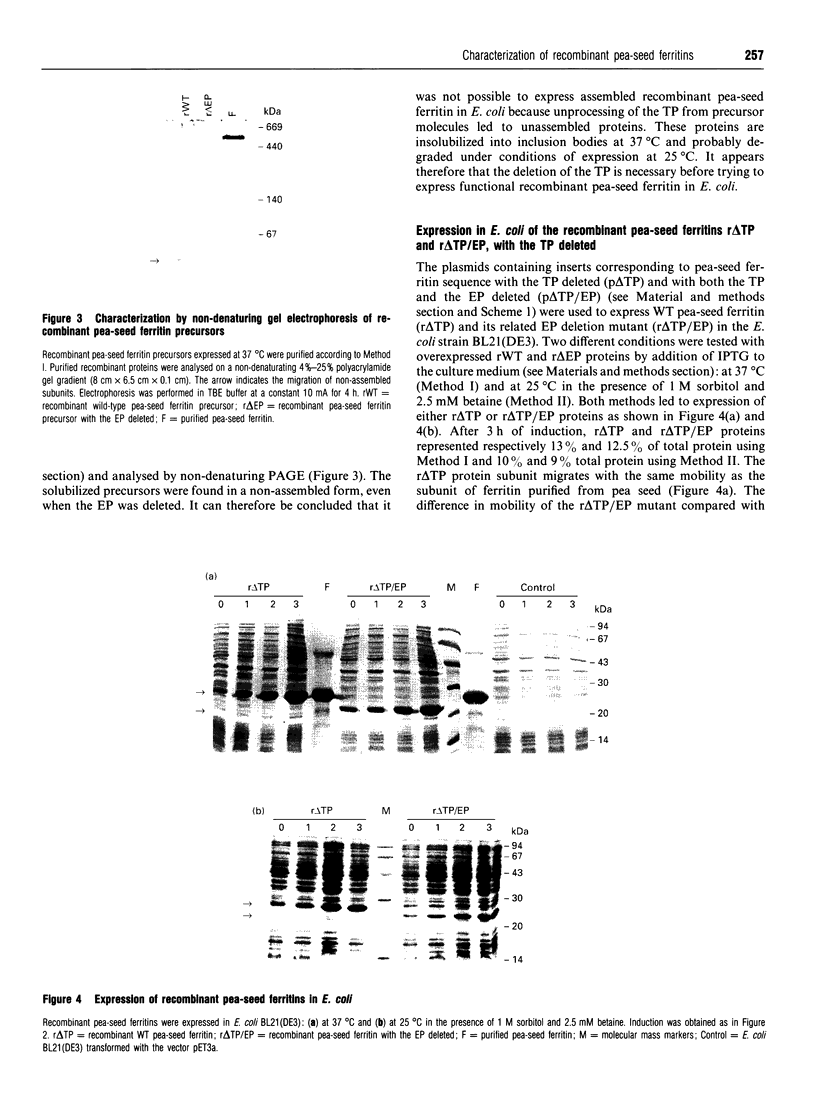
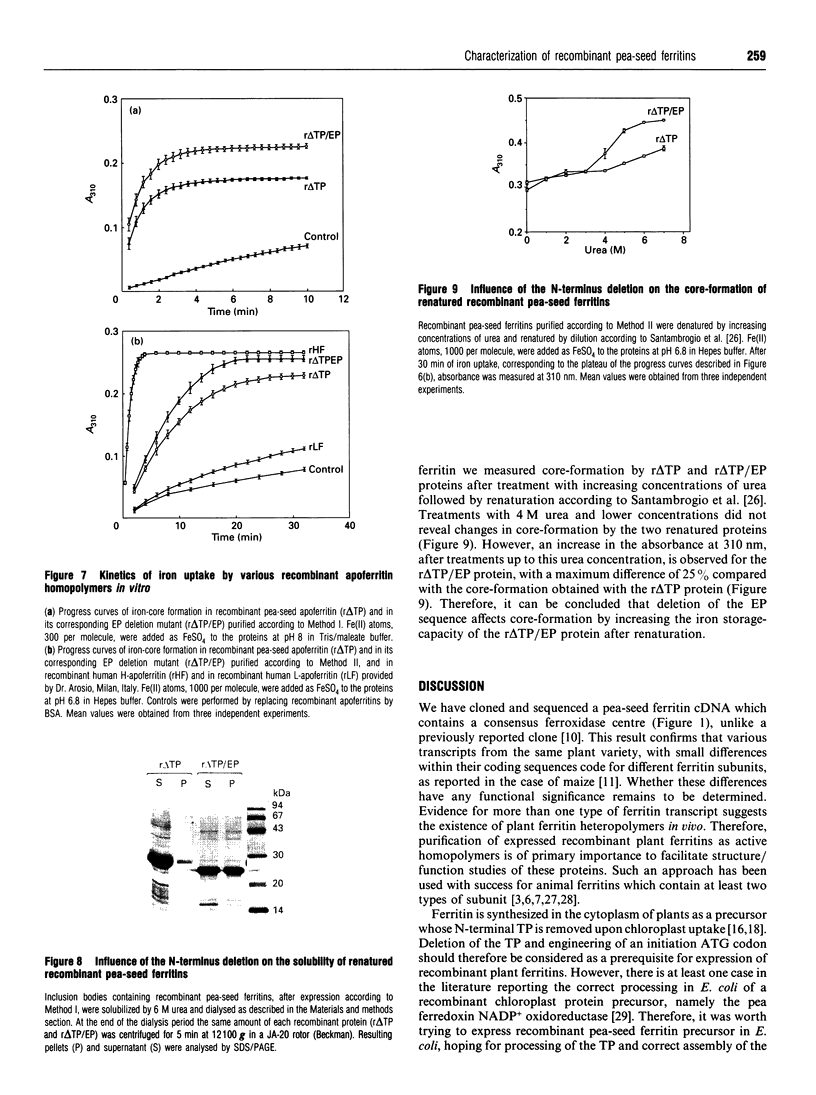
Plant ferritin subunits are synthesized as precursor molecules; the transit peptide (TP) in their NH2 extremity, responsible for plastid targeting, is cleaved during translocation to this compartment. In addition, the N-terminus of the mature subunit contains a plant-specific sequence named extension peptide (EP) [Ragland, Briat, Gagnon, Laulhère, Massenet, and Theil, E.C. (1990) J. Biol. Chem. 265, 18339-18344], the function of which is unknown. A novel pea-seed ferritin cDNA, with a consensus ferroxidase centre conserved within H-type animal ferritins has been characterized. This pea-seed ferritin cDNA has been engineered using oligonucleotide-directed mutagenesis to produce DNA fragments (1) corresponding to the wild-type (WT) ferritin precursor, (2) with the TP deleted, (3) with both the TP and the plant specific EP sequences deleted and (4) containing the TP but with the EP deleted. These four DNA fragments have been cloned in an Escherichia coli expression vector to produce the corresponding recombinant pea-seed ferritins. Expression at 37 degrees C led to the accumulation of recombinant pea-seed ferritins in inclusion bodies, whatever the construct introduced in E. coli. Expression at 25 degrees C in the presence of sorbitol and betaine allowed soluble proteins to accumulate when constructs with the TP deleted were used; under this condition, E. coli cells transformed with constructs containing the TP were unable to accumulate recombinant protein. Recombinant ferritins purified from inclusion bodies were found to be assembled only when the TP was deleted; however assembled ferritin under this condition had a ferroxidase activity undetectable at acid pH. On the other hand, soluble recombinant ferritins with the TP deleted and expressed at 25 degrees C were purified as 24-mers containing an average of 40-50 iron atoms per molecule. Despite the conservation in the plant ferritin subunit of a consensus ferroxidase centre, the iron uptake activity in vitro at pH 6.8 was found to be lower than that of the recombinant human H-ferritin, though it was much more active than the recombinant human L-ferritin. The recombinant ferritin with both the TP and the EP deleted (r delta TP/EP) assembled correctly as a 24-mer; it has slightly higher ferroxidase activity and decreased solubility compared with the wild-type protein with the TP deleted (r delta TP). In addition, on denaturation by urea followed by renaturation by dialysis the r delta TP/EP protein showed a 25% increase in core-formation in vitro compared with the r delta TP protein.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Arosio P., Bottke W., Briat J. F., von Darl M., Harrison P. M., Laulhère J. P., Levi S., Lobreaux S., Yewdall S. J. Structure, function, and evolution of ferritins. J Inorg Biochem. 1992 Aug 15;47(3-4):161–174. doi: 10.1016/0162-0134(92)84062-r. [DOI] [PubMed] [Google Scholar]
- Bauminger E. R., Harrison P. M., Hechel D., Nowik I., Treffry A. Mössbauer spectroscopic investigation of structure-function relations in ferritins. Biochim Biophys Acta. 1991 Dec 11;1118(1):48–58. doi: 10.1016/0167-4838(91)90440-b. [DOI] [PubMed] [Google Scholar]
- Blackwell J. R., Horgan R. A novel strategy for production of a highly expressed recombinant protein in an active form. FEBS Lett. 1991 Dec 16;295(1-3):10–12. doi: 10.1016/0014-5793(91)81372-f. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ceccarelli E. A., Viale A. M., Krapp A. R., Carrillo N. Expression, assembly, and processing of an active plant ferredoxin-NADP+ oxidoreductase and its precursor protein in Escherichia coli. J Biol Chem. 1991 Aug 5;266(22):14283–14287. [PubMed] [Google Scholar]
- Laulhere J. P., Laboure A. M., Briat J. F. Mechanism of the transition from plant ferritin to phytosiderin. J Biol Chem. 1989 Feb 25;264(6):3629–3635. [PubMed] [Google Scholar]
- Lawson D. M., Artymiuk P. J., Yewdall S. J., Smith J. M., Livingstone J. C., Treffry A., Luzzago A., Levi S., Arosio P., Cesareni G. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature. 1991 Feb 7;349(6309):541–544. doi: 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. M., Treffry A., Artymiuk P. J., Harrison P. M., Yewdall S. J., Luzzago A., Cesareni G., Levi S., Arosio P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989 Aug 28;254(1-2):207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- Lescure A. M., Proudhon D., Pesey H., Ragland M., Theil E. C., Briat J. F. Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8222–8226. doi: 10.1073/pnas.88.18.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S., Salfeld J., Franceschinelli F., Cozzi A., Dorner M. H., Arosio P. Expression and structural and functional properties of human ferritin L-chain from Escherichia coli. Biochemistry. 1989 Jun 13;28(12):5179–5184. doi: 10.1021/bi00438a040. [DOI] [PubMed] [Google Scholar]
- Levi S., Yewdall S. J., Harrison P. M., Santambrogio P., Cozzi A., Rovida E., Albertini A., Arosio P. Evidence of H- and L-chains have co-operative roles in the iron-uptake mechanism of human ferritin. Biochem J. 1992 Dec 1;288(Pt 2):591–596. doi: 10.1042/bj2880591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobreaux S., Briat J. F. Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem J. 1991 Mar 1;274(Pt 2):601–606. doi: 10.1042/bj2740601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobreaux S., Massenet O., Briat J. F. Iron induces ferritin synthesis in maize plantlets. Plant Mol Biol. 1992 Jul;19(4):563–575. doi: 10.1007/BF00026783. [DOI] [PubMed] [Google Scholar]
- Lobreaux S., Yewdall S. J., Briat J. F., Harrison P. M. Amino-acid sequence and predicted three-dimensional structure of pea seed (Pisum sativum) ferritin. Biochem J. 1992 Dec 15;288(Pt 3):931–939. doi: 10.1042/bj2880931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudhon D., Briat J. F., Lescure A. M. Iron induction of ferritin synthesis in soybean cell suspensions. Plant Physiol. 1989 Jun;90(2):586–590. doi: 10.1104/pp.90.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland M., Briat J. F., Gagnon J., Laulhere J. P., Massenet O., Theil E. C. Evidence for conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J Biol Chem. 1990 Oct 25;265(30):18339–18344. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio P., Levi S., Cozzi A., Rovida E., Albertini A., Arosio P. Production and characterization of recombinant heteropolymers of human ferritin H and L chains. J Biol Chem. 1993 Jun 15;268(17):12744–12748. [PubMed] [Google Scholar]
- Spence M. J., Henzl M. T., Lammers P. J. The structure of a Phaseolus vulgaris cDNA encoding the iron storage protein ferritin. Plant Mol Biol. 1991 Sep;17(3):499–504. doi: 10.1007/BF00040644. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sun S., Arosio P., Levi S., Chasteen N. D. Ferroxidase kinetics of human liver apoferritin, recombinant H-chain apoferritin, and site-directed mutants. Biochemistry. 1993 Sep 14;32(36):9362–9369. doi: 10.1021/bi00087a015. [DOI] [PubMed] [Google Scholar]
- Takeda S., Ohta M., Ebina S., Nagayama K. Cloning, expression and characterization of horse L-ferritin in Escherichia coli. Biochim Biophys Acta. 1993 Aug 19;1174(2):218–220. doi: 10.1016/0167-4781(93)90121-s. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Wade V. J., Treffry A., Laulhère J. P., Bauminger E. R., Cleton M. I., Mann S., Briat J. F., Harrison P. M. Structure and composition of ferritin cores from pea seed (Pisum sativum). Biochim Biophys Acta. 1993 Jan 15;1161(1):91–96. doi: 10.1016/0167-4838(93)90201-2. [DOI] [PubMed] [Google Scholar]
- Waldo G. S., Ling J., Sanders-Loehr J., Theil E. C. Formation of an Fe(III)-tyrosinate complex during biomineralization of H-subunit ferritin. Science. 1993 Feb 5;259(5096):796–798. doi: 10.1126/science.8430332. [DOI] [PubMed] [Google Scholar]
- van der Mark F., van den Briel W., Huisman H. G. Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Studies on the synthesis and the uptake in vitro of the precursors of ferritin and ferredoxin by intact chloroplasts. Biochem J. 1983 Sep 15;214(3):943–950. doi: 10.1042/bj2140943. [DOI] [PMC free article] [PubMed] [Google Scholar]