Abstract
This report describes the complete translated gene sequence, predicted secondary structure and lipid bilayer association of a novel kinetoplastid membrane protein (KMP-11) from Leishmania donovani promastigotes. KMP-11 was previously referred to as the lipophosphoglycan-associated protein (LPGAP). The isolation, species distribution and chemical characterization, including a partial protein sequence analysis and post-translational modifications, of this major membrane component have been described [Jardim, Funk, Caprioli and Olafson (1995) Biochem. J. 305, 307-313]. C.d. measurements of KMP-11 indicated a very high helical content estimated to be approximately 86% in trifluoroethanol. This was in agreement with computer-based secondary-structure analyses which predicted KMP-11 to be almost exclusively alpha-helical, with the protein adopting a helix-loop-helix motif. Arrangement of the residues located in the putative helical regions on an Edmundson helical wheel showed that this molecule could have a strongly amphipathic conformation and provided an explanation for how such a highly charged protein might be inserted into the plasma membrane. Evidence in support of KMP-11 association with lipid bilayers was provided by showing that KMP-11 could mediate carboxyfluorescein release from liposomes. These findings suggested that KMP-11 may function in part to increase bilayer pressure, stabilizing molecules such as lipophosphoglycan within the parasite pellicular membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J., Schneider P., Etges R., Bordier C. Peptide substrate specificity of the membrane-bound metalloprotease of Leishmania. Biochemistry. 1990 Oct 30;29(43):10113–10119. doi: 10.1021/bi00495a015. [DOI] [PubMed] [Google Scholar]
- Cevc G. How membrane chain melting properties are regulated by the polar surface of the lipid bilayer. Biochemistry. 1987 Oct 6;26(20):6305–6310. doi: 10.1021/bi00394a002. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is a protease. J Biol Chem. 1986 Jul 15;261(20):9098–9101. [PubMed] [Google Scholar]
- Fitch W. M. Phylogenies constrained by the crossover process as illustrated by human hemoglobins and a thirteen-cycle, eleven-amino-acid repeat in human apolipoprotein A-I. Genetics. 1977 Jul;86(3):623–644. doi: 10.1093/genetics/86.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gierasch L. M. Signal sequences. Biochemistry. 1989 Feb 7;28(3):923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe C. L., Shor R., Trau H., Passwell J. H. Parasite antigens recognized by patients with cutaneous leishmaniasis. Clin Exp Immunol. 1990 Apr;80(1):77–82. doi: 10.1111/j.1365-2249.1990.tb06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
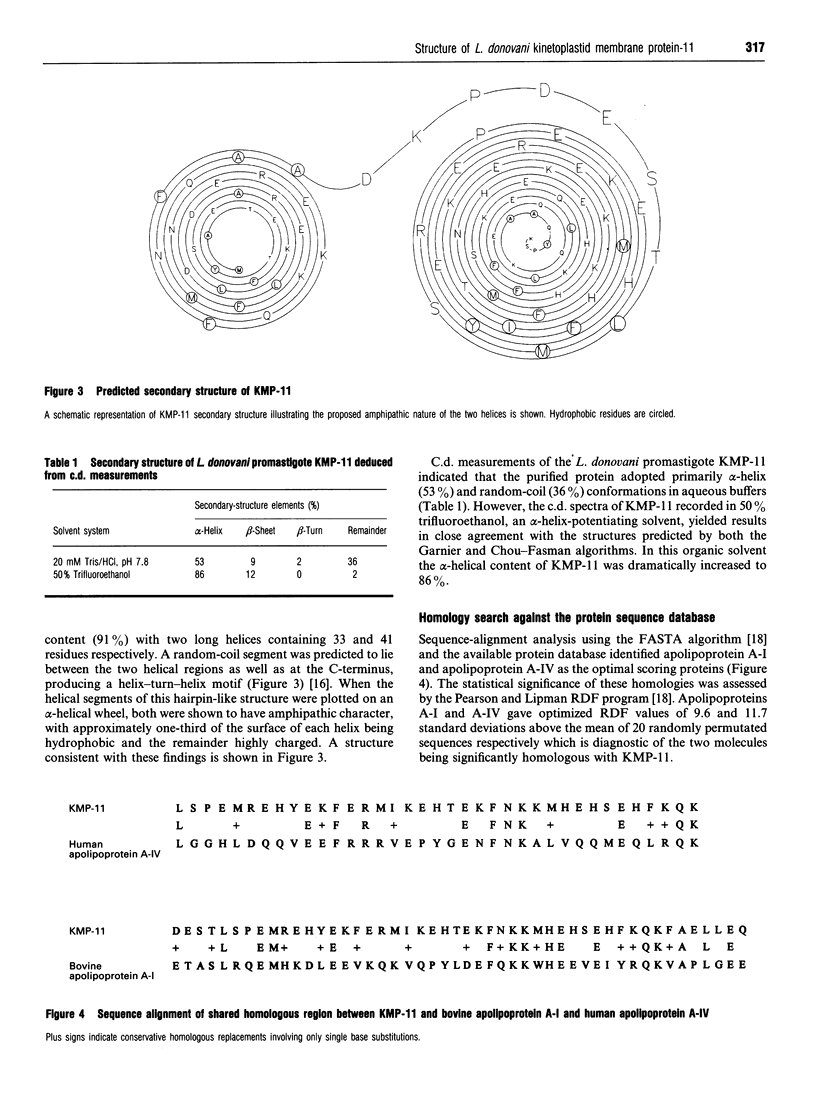
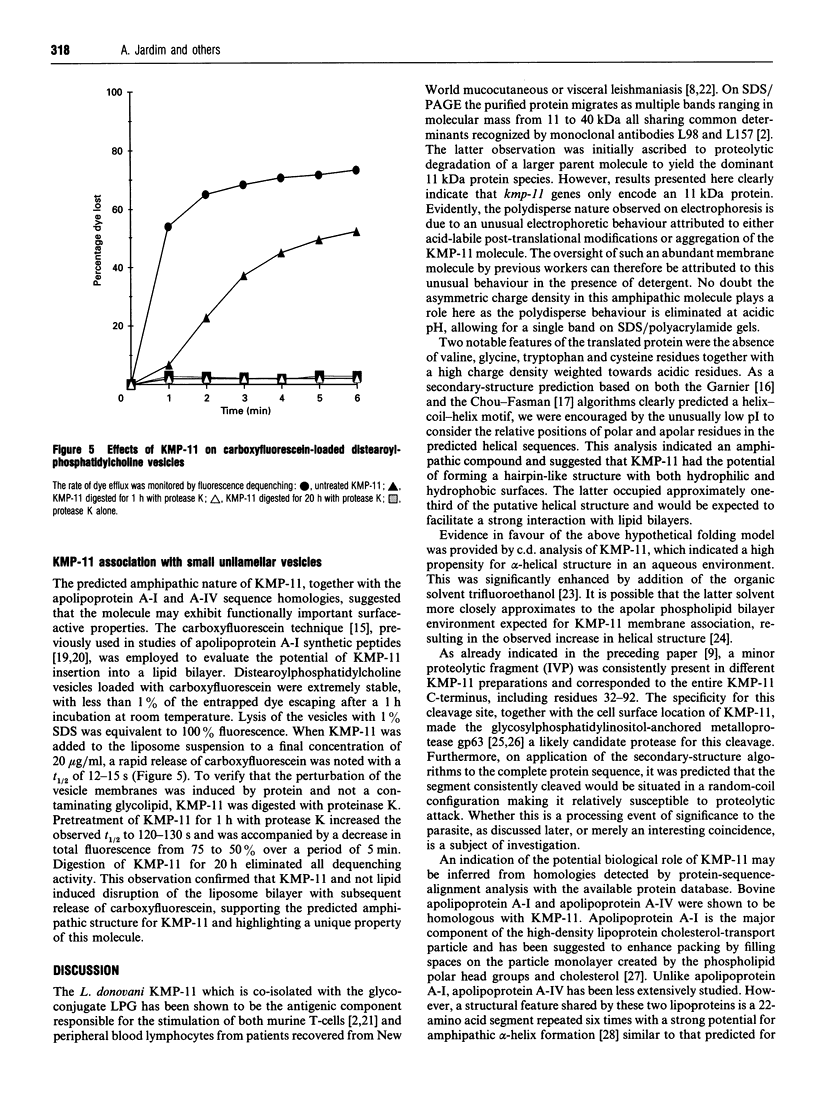
- Jardim A., Funk V., Caprioli R. M., Olafson R. W. Isolation and structural characterization of the Leishmania donovani kinetoplastid membrane protein-11, a major immunoreactive membrane glycoprotein. Biochem J. 1995 Jan 1;305(Pt 1):307–313. doi: 10.1042/bj3050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardim A., Tolson D. L., Turco S. J., Pearson T. W., Olafson R. W. The Leishmania donovani lipophosphoglycan T lymphocyte-reactive component is a tightly associated protein complex. J Immunol. 1991 Nov 15;147(10):3538–3544. [PubMed] [Google Scholar]
- Kanellis P., Romans A. Y., Johnson B. J., Kercret H., Chiovetti R., Jr, Allen T. M., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Effect of charged amino acid residue topography on lipid affinity. J Biol Chem. 1980 Dec 10;255(23):11464–11472. [PubMed] [Google Scholar]
- Kemp M., Theander T. G., Handman E., Hey A. S., Kurtzhals J. A., Hviid L., Sørensen A. L., Were J. O., Koech D. K., Kharazmi A. Activation of human T lymphocytes by Leishmania lipophosphoglycan. Scand J Immunol. 1991 Feb;33(2):219–224. doi: 10.1111/j.1365-3083.1991.tb03752.x. [DOI] [PubMed] [Google Scholar]
- King D. L., Chang Y. D., Turco S. J. Cell surface lipophosphoglycan of Leishmania donovani. Mol Biochem Parasitol. 1987 May;24(1):47–53. doi: 10.1016/0166-6851(87)90114-9. [DOI] [PubMed] [Google Scholar]
- Langford C. K., Ullman B., Landfear S. M. Leishmania: codon utilization of nuclear genes. Exp Parasitol. 1992 May;74(3):360–361. doi: 10.1016/0014-4894(92)90161-3. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Bacic A., Mitchell G. F., Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Bacic A. The glycoinositolphospholipid profiles of two Leishmania major strains that differ in lipophosphoglycan expression. Mol Biochem Parasitol. 1990 Jan 1;38(1):57–67. doi: 10.1016/0166-6851(90)90205-z. [DOI] [PubMed] [Google Scholar]
- Mendonça S. C., Russell D. G., Coutinho S. G. Analysis of the human T cell responsiveness to purified antigens of Leishmania: lipophosphoglycan (LPG) and glycoprotein 63 (gp 63). Clin Exp Immunol. 1991 Mar;83(3):472–478. doi: 10.1111/j.1365-2249.1991.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Russo D. M., Turco S. J., Burns J. M., Jr, Reed S. G. Stimulation of human T lymphocytes by Leishmania lipophosphoglycan-associated proteins. J Immunol. 1992 Jan 1;148(1):202–207. [PubMed] [Google Scholar]
- Segrest J. P., Chung B. H., Brouillette C. G., Kanellis P., McGahan R. Studies of synthetic peptide analogs of the amphipathic helix. Competitive displacement of exchangeable apolipoproteins from native lipoproteins. J Biol Chem. 1983 Feb 25;258(4):2290–2295. [PubMed] [Google Scholar]
- Shen B. W., Scanu A. M., Kézdy F. J. Structure of human serum lipoproteins inferred from compositional analysis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):837–841. doi: 10.1073/pnas.74.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen F. D., Van Eyk J. E., Hodges R. S., Sykes B. D. Effect of trifluoroethanol on protein secondary structure: an NMR and CD study using a synthetic actin peptide. Biochemistry. 1992 Sep 22;31(37):8790–8798. doi: 10.1021/bi00152a015. [DOI] [PubMed] [Google Scholar]
- Turco S. J. The leishmanial lipophosphoglycan: a multifunctional molecule. Exp Parasitol. 1990 Feb;70(2):241–245. doi: 10.1016/0014-4894(90)90105-l. [DOI] [PubMed] [Google Scholar]
- Webb J. R., Button L. L., McMaster W. R. Heterogeneity of the genes encoding the major surface glycoprotein of Leishmania donovani. Mol Biochem Parasitol. 1991 Oct;48(2):173–184. doi: 10.1016/0166-6851(91)90113-k. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- Yang D. M., Rogers M. V., Liew F. Y. Identification and characterization of host-protective T-cell epitopes of a major surface glycoprotein (gp63) from Leishmania major. Immunology. 1991 Jan;72(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Wu C. S., Martinez H. M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Fukushima D., Kupferberg J. P., Kézdy F. J., Kaiser E. T. The mechanism of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I and an amphiphilic peptide. J Biol Chem. 1980 Aug 10;255(15):7333–7339. [PubMed] [Google Scholar]



