Abstract
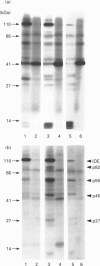
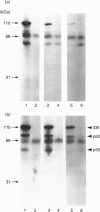
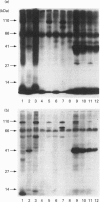
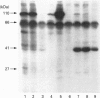
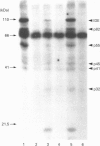
We previously demonstrated that internalized insulin enters the cytoplasm before accumulating in nuclei of H35 rat hepatoma cells. This finding raises the possibility that insulin may interact with cytosolic proteins in addition to insulin-degrading enzyme (IDE). In the present study, cytosol from H35 hepatoma cells, rat liver or muscle was incubated with A14- or B26-125I-insulin at 4 degrees C for 5-120 min in the absence or presence of 25 micrograms/ml unlabelled insulin. 125I-insulin was cross-linked to cytosolic proteins by disuccinimidyl suberate and analysed by reducing or non-reducing SDS/PAGE and autoradiography. Our results demonstrate the presence of both tissue-specific and common cytosolic proteins which specifically bind insulin. In muscle cytosol, only two proteins of 27 and 110 kDa were specifically labelled with B26-125I-insulin. Seven major bands, of 27, 45, 55, 60, 76, 82 and 110 kDa, were labelled in rat liver cytosol. Detection of cytosolic insulin-binding proteins in H35-cell cytosol was dependent on cell-culture conditions. Labelling in cytosol from serum-deprived cells was decreased or absent compared with cytosol prepared from serum-fed or serum-deprived cells treated with 100 ng/ml insulin for 1 h before preparation of the cytosol, in which six bands, of 32, 41, 45, 55, 82 and 110 kDa, were specifically labelled with B26-125I-insulin. This result suggests that the concentration or binding activity of some cytosolic insulin-binding proteins is rapidly regulated. Labelling of both rat liver and H35 cytosolic insulin-binding proteins was time-dependent, and decreased or disappeared at 120 min in parallel with the degradation of labelled insulin. Fewer bands were specifically labelled with A14-125I-insulin than with B26-125I-insulin. The number of labelled bands observed under reducing and non-reducing conditions was not different in any of the cytosols. The 110 kDa band in all cytosols was identified as IDE by Western-blot analysis; the other proteins did not react with anti-IDE antibody and remain unidentified. 1,10-Phenanthroline (2 mM) increased IDE labelling, but decreased the labelling of 82 and 27 kDa bands. The marked difference in the number of cytosolic insulin-binding proteins in muscle and either H35 cells or liver suggests both that the labelling is specific and that these proteins serve a function and may be involved in some heretofore unknown mechanism of the signalling pathway by which insulin regulates cell growth or differentiation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H., Shii K., Yokono K., Yonezawa K., Sato S., Watanabe K., Baba S. Cellular localization of insulin-degrading enzyme in rat liver using monoclonal antibodies specific for this enzyme. Biochem Biophys Res Commun. 1988 Sep 15;155(2):914–922. doi: 10.1016/s0006-291x(88)80583-7. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Kahn C. R., White M. F. The dissociation and degradation of internalized insulin occur in the endosomes of rat hepatoma cells. J Biol Chem. 1990 Sep 5;265(25):14828–14835. [PubMed] [Google Scholar]
- Berezney R. The nuclear matrix: a heuristic model for investigating genomic organization and function in the cell nucleus. J Cell Biochem. 1991 Oct;47(2):109–123. doi: 10.1002/jcb.240470204. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Rosen O. M., Rubin C. S. Identification and characterization of a latent pool of insulin receptors in 3T3-L1 adipocytes. J Biol Chem. 1982 May 25;257(10):5350–5358. [PubMed] [Google Scholar]
- Ding L., Becker A. B., Suzuki A., Roth R. A. Comparison of the enzymatic and biochemical properties of human insulin-degrading enzyme and Escherichia coli protease III. J Biol Chem. 1992 Feb 5;267(4):2414–2420. [PubMed] [Google Scholar]
- Duckworth W. C. Insulin degradation: mechanisms, products, and significance. Endocr Rev. 1988 Aug;9(3):319–345. doi: 10.1210/edrv-9-3-319. [DOI] [PubMed] [Google Scholar]
- Frank B. H., Peavy D. E., Hooker C. S., Duckworth W. C. Receptor binding properties of monoiodotyrosyl insulin isomers purified by high performance liquid chromatography. Diabetes. 1983 Aug;32(8):705–711. doi: 10.2337/diab.32.8.705. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J. Binding of insulin to isolated nuclei. Proc Natl Acad Sci U S A. 1976 May;73(5):1427–1431. doi: 10.1073/pnas.73.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammons G. T., Smith R. M., Jarett L. Inhibition by bacitracin of rat adipocyte plasma membrane degradation of 125I-insulin is associated with an increase in plasma membrane bound insulin and a potentiation of glucose oxidation by adipocytes. J Biol Chem. 1982 Oct 10;257(19):11563–11570. [PubMed] [Google Scholar]
- Harada S., Loten E. G., Smith R. M., Jarett L. Nonreceptor mediated nuclear accumulation of insulin in H35 rat hepatoma cells. J Cell Physiol. 1992 Dec;153(3):607–613. doi: 10.1002/jcp.1041530323. [DOI] [PubMed] [Google Scholar]
- Harada S., Smith R. M., Jarett L. 1,10-Phenanthroline increases nuclear accumulation of insulin in response to inhibiting insulin degradation but has a biphasic effect on insulin's ability to increase mRNA levels. DNA Cell Biol. 1994 May;13(5):487–493. doi: 10.1089/dna.1994.13.487. [DOI] [PubMed] [Google Scholar]
- Harada S., Smith R. M., Smith J. A., Jarett L. Inhibition of insulin-degrading enzyme increases translocation of insulin to the nucleus in H35 rat hepatoma cells: evidence of a cytosolic pathway. Endocrinology. 1993 Jun;132(6):2293–2298. doi: 10.1210/endo.132.6.8504733. [DOI] [PubMed] [Google Scholar]
- Hari J., Shii K., Roth R. A. In vivo association of [125I]-insulin with a cytosolic insulin-degrading enzyme: detection by covalent cross-linking and immunoprecipitation with a monoclonal antibody. Endocrinology. 1987 Feb;120(2):829–831. doi: 10.1210/endo-120-2-829. [DOI] [PubMed] [Google Scholar]
- Heyner S., Rao L. V., Jarett L., Smith R. M. Preimplantation mouse embryos internalize maternal insulin via receptor-mediated endocytosis: pattern of uptake and functional correlations. Dev Biol. 1989 Jul;134(1):48–58. doi: 10.1016/0012-1606(89)90077-8. [DOI] [PubMed] [Google Scholar]
- Horvat A., Li E., Katsoyannis P. G. Cellular binding sites for insulin in rat liver. Biochim Biophys Acta. 1975 Apr 8;382(4):609–620. doi: 10.1016/0005-2736(75)90226-6. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kahn C. R. Insulin induces rapid accumulation of insulin receptors and increases tyrosine kinase activity in the nucleus of cultured adipocytes. J Cell Physiol. 1993 Nov;157(2):217–228. doi: 10.1002/jcp.1041570203. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller D. S. Stimulation of RNA and protein synthesis by intracellular insulin. Science. 1988 Apr 22;240(4851):506–509. doi: 10.1126/science.2451860. [DOI] [PubMed] [Google Scholar]
- Ogawa W., Shii K., Yonezawa K., Baba S., Yokono K. Affinity purification of insulin-degrading enzyme and its endogenous inhibitor from rat liver. J Biol Chem. 1992 Jan 15;267(2):1310–1316. [PubMed] [Google Scholar]
- Perlman R. K., Gehm B. D., Kuo W. L., Rosner M. R. Functional analysis of conserved residues in the active site of insulin-degrading enzyme. J Biol Chem. 1993 Oct 15;268(29):21538–21544. [PubMed] [Google Scholar]
- Posner B. I., Raquidan D., Josefsberg Z., Bergeron J. J. Different regulation of insulin receptors in intracellular (Golgi) and plasma membranes from livers of obese and lean mice. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3302–3306. doi: 10.1073/pnas.75.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purrello F., Burnham D. B., Goldfine I. D. Insulin regulation of protein phosphorylation in isolated rat liver nuclear envelopes: potential relationship to mRNA metabolism. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1189–1193. doi: 10.1073/pnas.80.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purrello F., Vigneri R., Clawson G. A., Goldfine I. D. Insulin stimulation of nucleoside triphosphatase activity in isolated nuclear envelopes. Science. 1982 May 28;216(4549):1005–1007. doi: 10.1126/science.6281885. [DOI] [PubMed] [Google Scholar]
- Schumm D. E., Webb T. E. Effect of physiological concentrations of insulin and antidiabetic drugs on RNA release from isolated liver nuclei. J Cell Biochem. 1983;23(1-4):223–229. doi: 10.1002/jcb.240230119. [DOI] [PubMed] [Google Scholar]
- Shii K., Baba S., Yokono K., Roth R. A. Covalent linkage of 125I-insulin to a cytosolic insulin-degrading enzyme. J Biol Chem. 1985 Jun 10;260(11):6503–6506. [PubMed] [Google Scholar]
- Shii K., Roth R. A. Inhibition of insulin degradation by hepatoma cells after microinjection of monoclonal antibodies to a specific cytosolic protease. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4147–4151. doi: 10.1073/pnas.83.12.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. Partial characterization of mechanism of insulin accumulation in H35 hepatoma cell nuclei. Diabetes. 1990 Jun;39(6):683–689. doi: 10.2337/diab.39.6.683. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. Ultrastructural evidence for the accumulation of insulin in nuclei of intact 3T3-L1 adipocytes by an insulin-receptor mediated process. Proc Natl Acad Sci U S A. 1987 Jan;84(2):459–463. doi: 10.1073/pnas.84.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler A. P., Smith R. M., Jarett L. Insulin stimulates accumulation and efflux of macromolecules in isolated nuclei from H35 hepatoma cells. Diabetes. 1992 Feb;41(2):194–201. doi: 10.2337/diab.41.2.194. [DOI] [PubMed] [Google Scholar]
- Soler A. P., Thompson K. A., Smith R. M., Jarett L. Immunological demonstration of the accumulation of insulin, but not insulin receptors, in nuclei of insulin-treated cells. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6640–6644. doi: 10.1073/pnas.86.17.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. A., Soler A. P., Smith R. M., Jarett L. Intranuclear localization of insulin in rat hepatoma cells: insulin/matrix association. Eur J Cell Biol. 1989 Dec;50(2):442–446. [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]