Abstract
Working life is associated with lifestyle, screening uptake, and occupational health risks that may explain differences in cancer onset. To better understand the association between working life and cancer risk, we need to account for the entire employment history. We investigated whether lifetime employment trajectories are associated with cancer risk. We used data from 6809 women and 5716 men, average age 70 years, from the Survey of Health, Ageing, and Retirement in Europe. Employment history from age 16 to 65 was collected retrospectively using a life calendar and trajectories were constructed using sequence analysis. Associations between employment trajectories and self-reported cancer were assessed using logistic regression. We identified eight employment trajectories for women and two for men. Among women, the risk of cancer was higher in the trajectories “Mainly full-time to home/family”, “Full-time or home/family to part-time”, “Mainly full-time”, and “Other” compared with the “Mainly home/family” trajectory. Among men, the risk of cancer was lower in the “Mainly self-employment” trajectory compared with “Mainly full-time”. We could show how employment trajectories were associated with cancer risk, underlining the potential of sequence analysis for life course epidemiology. More research is needed to understand these associations and determine if causal relationships exist.
Keywords: Cancer, Employment, Epidemiology, Life course, Sequence analysis, Social determinants of health
Subject terms: Cancer, Risk factors
Introduction
Cancer is associated with early life course influences, such as socioeconomic circumstances1–5 and quality of parenting6 in childhood, or main occupation in early adulthood7 and middle age8,9. There are many association studies correlating cancer with socioeconomic circumstances at a given moment in the life course, such as childhood10, or with two or three life course moments3,7,11,12, but little is known about how entire life course trajectories (with information on each year of life) can predict the onset of cancer.
Life course epidemiology, and the study of social determinants of health, have shown that where people work and live throughout the life course13 shapes their health and how they adapt to stress and age14–17. Life course epidemiology studies how exposures over the life course—timing, duration and sequencing—can increase the risk of disease or the course of disease development. The study of exposures over the life course links to several life course models, such as the sensitive period, the accumulation, the pathway and the social mobility models17. Cancer determinants research is interested in understanding how these conditions throughout the life course may influence the early development of cancer18,19. For example, the life course approach could enrich the understanding of the association between adiposity and cancer, by understanding how the timing, duration and sequencing of adipose tissue increases or decreases the risk of cancer20,21. One set of conditions in particular, our working lives, is central to our life course as we spend a large proportion of our time at work22. Exposure to work throughout life therefore has both positive and negative effects on health in later in life23–26, and may be associated with the development of cancer27–32. Previous studies have shown that past labour market status (hereafter, employment status) is associated with health in old age33–53 and cancer8,54. However, these studies have focused on one-off indicators of current or past employment status, which do not take into account the complex nature of individual work histories. One challenge of life course epidemiology is to study the complexity of the life course, i.e., potentially include data covering the entire life course of the individual, data from various domains such as health, working life, family life and leisure life.
Another approach to assessing the influence of the life course on health in old age is to use the information on the whole history of the participant’s life course, for example from adolescence to the middle age (or the beginning of old age), collected through life history interviews55. The information is then organized into sequences, where each sequence represents a unique individual’s life trajectory over time. These sequences are grouped using various techniques. This data-driven approach allows exploration of when events or transitions occur within life course trajectories, providing insights into timing mechanisms and revealing typical or atypical sequences of events that contribute to understanding sequencing mechanisms. Using this approach, studies have described employment trajectories over the life course56–59, with women’s trajectories being more diverse than those of men56. Other studies have shown that employment trajectories are associated with quality of life60,61, self-rated health33,62,63, mental health60,62,64–70, and functional health68,71, but no study has investigated a possible association with cancer over the life course.
We hypothesise, therefore, that cancer onset is associated with employment trajectories over the life course. Using data from a large population-based cohort study, we first described the men’s and women’s employment history from 16 to 65 years of age by classifying them into types of trajectories using sequence analysis; second, we analysed the associations between men’s and women’s employment trajectories and cancer onset over the life course. For exploratory purposes, we also examined the associations between these trajectories and breast cancer.
Results
Participant characteristics
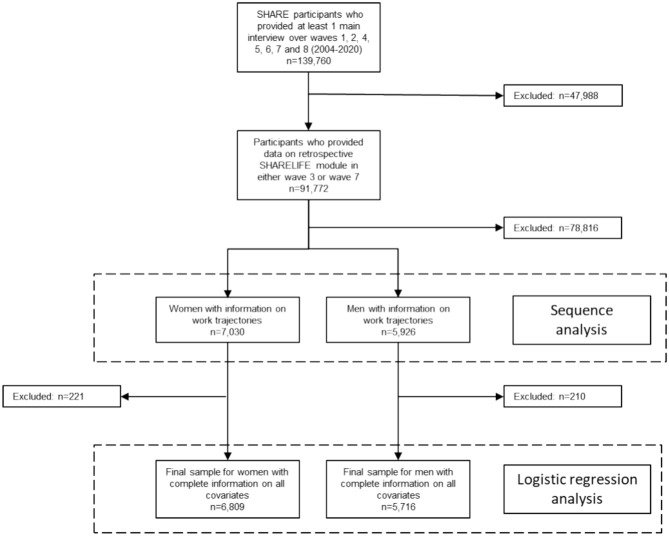
The flow chart (Fig. 1) describes the exclusion of participants to obtain the analytical sample for this study. First, we excluded participants who did not participate in the SHARELIFE module (n = 47,988 excluded). Second, we excluded participants who did not provide any information on their work or employment history between the ages of 16 to 65 (n = 78,816 excluded). After excluding participants with missing information on covariates, the final analytic sample consisted of 6,809 women and 5,716 men living in 14 European countries (Austria, Belgium, Czech Republic, Denmark, France, Germany, Greece, Ireland, Italy, Netherlands, Poland, Spain, Sweden, Switzerland). Women and men participated in an average of 5 waves (median 5, minimum 2, maximum 8).
Fig. 1.
Flow chart of respondents’ inclusion.
The mean age at baseline was 70.7 years (SD 7.0) for women without diagnosed cancer and 70.1 years (6.7) for women with diagnosed cancer. Mean age was similar in men. The mean BMI was 26.5 in women and 26.8 in men, and the mean BMI was similar in participants with and without cancer in both genders. The majority of women and men were non-smokers and were physically active (i.e., engaged in any activity more than once a week). The proportion of women with fewer than two chronic conditions was slightly higher in the group without cancer (52.8%) than in the group with cancer (46.2%); this difference was slightly smaller in men: 62.4% in the group without cancer and 57.4% in the group with cancer.
A total of 781 women and 827 men were diagnosed with cancer at any time during their lifetime (Table 1). The distribution of age at cancer diagnosis is reported in Figs. S1, S2 (Supplementary material). 31.0% of women and 17.9% of men were diagnosed before the age of 65.
Table 1.
Women and men characteristics at baseline, Survey of Health, Ageing, and Retirement in Europe (SHARE), 2004–2020.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| All 6,809 women | No cancer (n = 6,028) | Diagnosed with cancer (n = 781) | All 5,716 men | No cancer (n = 4,889) | Diagnosed with cancer (n = 827) | |
| Age at baseline, years (SD) | 70.6 (7.0) | 70.7 (7.0) | 70.1 (6.7) | 70.0 (6.5) | 70.0 (6.6) | 69.9 (6.3) |
| Birth cohort | ||||||
| No crisis/war | 1387 (20.4) | 1,236 (20.5) | 151 (19.3) | 999 (17.5) | 857 (17.5) | 142 (17.2) |
| War period | 2367 (34.8) | 2,086 (34.6) | 281 (36) | 2030 (35.5) | 1,743 (35.7) | 287 (34.7) |
| Great depression | 3055 (44.9) | 2,706 (44.9) | 349 (44.7) | 2687 (47.0) | 2,289 (46.8) | 398 (48.1) |
| Attrition | ||||||
| No dropout | 3043 (44.7) | 2,651 (44) | 392 (50.2) | 2340 (40.9) | 1,951 (39.9) | 389 (47) |
| Dropout | 2098 (30.8) | 1,929 (32) | 169 (21.6) | 1713 (30.0) | 1,524 (31.2) | 189 (22.9) |
| Deceased | 1668 (24.5) | 1,448 (24) | 220 (28.2) | 1663 (29.1) | 1,414 (28.9) | 249 (30.1) |
| BMI, kg/m2 (SD) | 26.5 (4.5) | 26.5 (4.5) | 26.6 (4.7) | 26.8 (3.6) | 26.8 (3.6) | 26.8 (3.6) |
| Smoking status at baseline | ||||||
| Non-smoker | 6150 (90.3) | 5,459 (90.6) | 691 (88.5) | 4740 (82.9) | 4,047 (82.8) | 693 (83.8) |
| Smoker | 659 (9.7%) | 569 (9.4) | 90 (11.5) | 976 (17.1) | 842 (17.2) | 134 (16.2) |
| No. of chronic conditions | ||||||
| < 2 | 3543 (52.0) | 3,182 (52.8) | 361 (46.2) | 3524 (61.7) | 3,049 (62.4) | 475 (57.4) |
| ≥ 2 | 3266 (48.0) | 2,846 (47.2) | 420 (53.8) | 2192 (38.3) | 1,840 (37.6) | 352 (42.6) |
| Physical activity | ||||||
| Engage in any activity more than once a week | 4644 (68.2) | 4,096 (67.9) | 548 (70.2) | 4298 (75.2) | 3,672 (75.1) | 626 (75.7) |
| Did not engage in any activity more than once a week | 2165 (31.8) | 1,932 (32.1) | 233 (29.8) | 1418 (24.8) | 1,217 (24.9) | 201 (24.3) |
| Women’s employment trajectory | ||||||
| Mainly full-time | 1950 (27.7) | 1,649 (27.4) | 266 (34.1) | – | – | – |
| Mainly home/family | 1662 (23.6) | 1,507 (25) | 143 (18.3) | – | – | – |
| Mainly full-time to home/family | 1285 (18.3) | 1,149 (19.1) | 152 (19.5) | – | – | – |
| Mainly self-employment | 583 (8.3) | 520 (8.6) | 37 (4.7) | – | – | – |
| Full-time or home/family to part time | 624 (8.9) | 435 (7.2) | 89 (11.4) | – | – | – |
| Home/family to full-time | 433 (6.2) | 334 (5.5) | 40 (5.1) | – | – | – |
| Mainly unemployment | 95 (1.4) | 90 (1.5) | 9 (1.2) | – | – | – |
| Other | 398 (5.7) | 344 (5.7) | 45 (5.8) | – | – | – |
| Men’s employment trajectory | ||||||
| Mainly full-time | – | – | – | 4691 (82.1) | 3,984 (81.5) | 707 (85.5) |
| Mainly self-employment | – | – | – | 1025 (17.9) | 905 (18.5) | 120 (14.5) |
| Self-reported site of cancer* | ||||||
| Lung | – | – | 27 (3.5) | – | – | 66 (8) |
| Colon | – | – | 105 (13.4) | – | – | 126 (15.2) |
| Skin | – | – | 88 (11.3) | – | – | 97 (11.7) |
| Breast | – | – | 353 (45.2) | – | – | 10 (1.2) |
| Cervix | – | – | 56 (7.2) | – | – | – |
Data are n (%) unless indicated.
BMI body mass index, SD standard deviation.
*Numbers do not add up to 100% due to other cancers present in the population, but not mentioned in this table.
Employment trajectories of women: sequence analysis
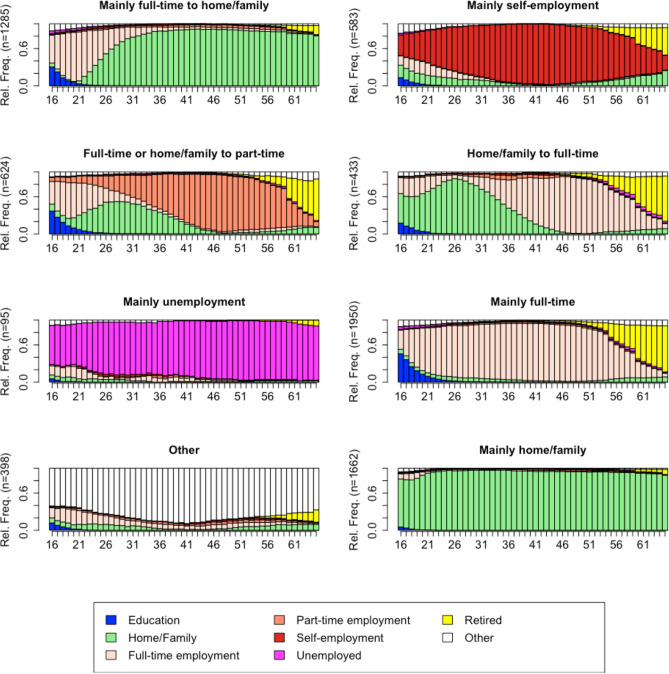
For women, a solution with 8 employment trajectories (clusters) was chosen (Fig. 2). Here, the ASW of 0.34 indicated that the resulting clustering solution was of reasonable quality. The largest employment trajectory regrouped women who worked mainly full-time throughout their working lives (n = 1,950, 27.7%, this group was named “Mainly full-time”). This group of women also spent some time in education during adolescence. The second largest employment trajectory grouped women who were mainly involved in home and family, with very little time either in education or in the labour market (n = 1,662, 23.6%, “Mainly home/family”). Another employment trajectory (n = 1,285, 18.3%) included women who moved from full-time work to home / family (“Mainly full-time to home/family”). This group of women also spent some time in education during adolescence. These first three groups represented more than 70% of the sample, while the remaining five groups each represented less than 10% of the sample. Among the last five groups, we found one employment trajectory (n = 583, 8.3%) that included women who were mainly self-employed during their working life (“Mainly self-employment”). Another employment trajectory (n = 624, 8.9%) included women who started their working lives in either full-time or part-time employment, with a significant proportion moving to home/family in their twenties, and then moving to mainly part-time employment (“Full-time or home/family to part time”). A small number of women (n = 433, 6.2%) had an employment trajectory in which they spent the first half of their working lives mainly at home and then worked full-time in the second half of their working lives (“Home/Family to full-time”). An even smaller group of women (n = 95, 1.4%) were mainly unemployed during their working lives (“Mainly unemployed”). 398 women (5.7%) had an employment trajectory mostly in the “other” category, indicating more unusual trajectories characterised by illness or disability, voluntary work, and travelling (“Other”).
Fig. 2.
Chronogram of the eight employment trajectories for women aged 16–65. Data from the Survey of Health, Ageing, and Retirement in Europe (SHARE). Average silhouette width of 0.34.
Employment trajectories of men: sequence analysis
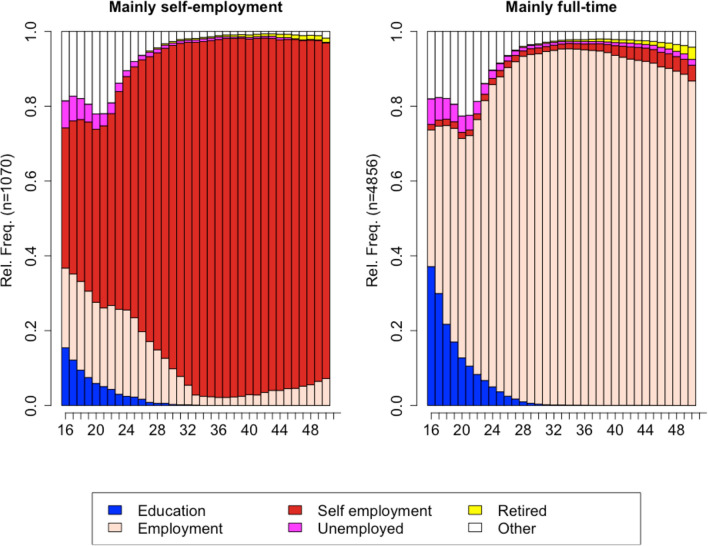
For men, a solution with two clusters was retained based on an ASW of 0.70, indicating a strong clustering structure (Fig. 3). The first employment trajectory included men whose working life was mainly characterised by self-employment (n = 1,070). The second employment trajectory included men (n = 4,856) who spent most of their working lives in full-time employment.
Fig. 3.
Chronogram of the two employment trajectories for men aged 16–65. Data from the Survey of Health, Ageing, and Retirement in Europe (SHARE). Average silhouette width of 0.70.
Associations of women’s employment trajectories with cancer
Table 2 shows the results of the logistic regression analyses. After adjustment with covariates and attrition, compared with women in the “Mainly home/family” employment trajectory (reference category), women in the “Mainly full-time to home/family” trajectory, the “Full-time or home/family to part-time” trajectory, the “Mainly full-time” trajectory, and the “Other” trajectory had a higher risk of overall cancer over the life course (odds ratios (ORs) of 1.51, 2.28, 1.73, and 1.47, respectively). The sensitivity analysis with prospective overall cancer (i.e., cancer diagnosed at 65 years of age or later) yielded comparable results (Table S1). The sensitivity analysis adjusting for partnership status did not alter the results for overall cancer over the life course (Table S2) or for prospective overall cancer after 65 years (Table S1). Upon adjustment for healthy diet, the results remained similar, with the exception of the “Other” trajectories which became nonsignificant for overall cancer over the life course (Table S2) but remained significant for prospective overall cancer after 65 years (Table S1).
Table 2.
Associations of women’s employment trajectories from age 16 to 65 with cancer (all sites, n = 781) over the life course. Survey of Health, Ageing, and Retirement in Europe (SHARE), 2004–2020.
| Types of employment trajectories | OR (95%CI) |
|---|---|
| Mainly home/family | Ref. |
| Mainly full-time | 1.73 (1.40–2.16) |
| Mainly full-time to home/family | 1.51 (1.19–1.93) |
| Mainly self-employment | 0.76 (0.51–1.09) |
| Full-time or home/family to part time | 2.28 (1.70–3.05) |
| Home/family to full-time | 1.23 (0.84–1.77) |
| Mainly unemployment | 1.05 (0.48–2.03) |
| Other | 1.47 (1.02–2.09) |
The model is adjusted with covariates (age, birth cohort, BMI at baseline, smoking at baseline, number of chronic conditions, and physical activity) and attrition (reason for dropout).
Odds ratio (OR) and 95% confidence intervals (CIs); Ref. reference category.
Results for breast cancer are reported in the supplementary appendix (Table S3). The association between types of employment trajectories and breast cancer risk was similar to that for overall cancer, but with slightly higher ORs (1.68, 2.38, 1.92, 1.79, respectively). The sensitivity analysis adjusting for partnership status did not alter the results for breast cancer over the life course (Table S3) or for prospective breast cancer after 65 years (Table S4). Upon adjustment for healthy diet, the results remained similar, with the exception of the “Other” trajectories which became nonsignificant for breast cancer over the life course (Table S3) and for prospective breast cancer after 65 years (Table S4).
Associations of men’s employment trajectories with cancer
After adjustment with covariates and attrition, compared with men in the “Mainly full-time” employment trajectory (reference category), those in the “Mainly self-employment” trajectory had a lower risk of overall cancer (OR 0.74 95%CI 0.60–0.90). The sensitivity analysis with prospective overall cancer (i.e., cancer diagnosed at 65 years of age or later) yielded comparable results (Table S5). The sensitivity analysis adjusting for partnership status did not alter the results for overall cancer over the life course (Table S6) or for prospective overall cancer after 65 years (Table S5). When adjusting for healthy diet, we observed that the association of being “Mainly self-employment” became nonsignificant for the risk of overall cancer over the life course (0.82 95%CI 0.65–1.02, Table S6), but remained significant for prospective overall cancer risk (0.74 95%CI 0.58–0.95, Table S5).
Discussion
In this study, we first described the employment trajectories from age 16 to 65 of a cohort of Europeans born between 1914 and 1945, and second, we assessed whether these employment trajectories could predict the onset of cancer at any time in the life course. First, using sequence analysis, we grouped the employment histories from the ages of 16 to 65 into eight and two patterned trajectories for women and men, respectively. For women, we named the eight trajectories “Mainly full-time”, “Mainly home/family”, “Mainly full-time to home/family”, “Mainly self-employment”, “Full-time or home/family to part time”, “Home/family to full-time”, “Mainly unemployment” and “Other”. In terms of the proportions of the sample, none of the eight trajectories was in the majority. In this “prediction exercise”72, and thanks to sequence analysis, we found that, compared to women who spent their lives mainly caring for the home/family, four employment trajectories were associated with a higher risk of cancer over the life course: women who worked predominantly full-time, women who worked full-time then transitioned to taking care of home and the family, women who either worked full-time or took care of the home/the family and then transitioned to working part time, and women with unusual employment trajectories (“Other”), i.e. trajectories characterised by illness or disability, voluntary work, and travelling. When we looked at breast cancer, we also found associations with the same four employment trajectories. For men, we named the two solutions “Mainly full-time” and “Mainly self-employment”, with the first one representing the largest, majority group. In sum, this study has shown that the onset of cancer over the life course can be predicted by classifying women and men into typical groups of lifetime employment trajectory, and that these associations were independent of factors known to be associated with cancer (smoking, physical activity, number of chronic conditions, and body mass index).
The finding of eight employment trajectories for women suggested that women’s employment trajectories were more heterogeneous, or less “socially standardised” by the culture and local contexts, than men’s, who had only two trajectories, with one predominant type being full-time work throughout their working lives. The heterogeneity of women’s employment trajectories and the homogeneity of men’s employment trajectories are consistent with previous studies in Germany58, England73 and Switzerland56, and, at least for women, with previous studies using the same dataset61,74. However, using the same dataset, another study found two patterned employment trajectories for women, but using multi-channel sequence analysis75. The finding of heterogeneity in women’s employment trajectories was to be expected for these cohorts as women’s life course trajectories, compared with men’s, are socially normed towards multiple social roles, such as parenthood, family responsibilities, including contribution to breadwinning, and—to a lesser extent—professional career76–79.
Individuals’ employment trajectories are influenced by the policies of the country in which they live80,81. Indeed, policies can affect employment opportunities, job security, wages and career paths. For example, labour market policies implemented by governments (such as employment protection legislation, labour market flexibility measures, reintegration measures), social security and welfare policies (such as unemployment benefits, income support programmes), family policies (childbearing) and health policies (health insurance, disability insurance) can have direct or indirect effects on employment trajectories. Such effects were not investigated in this study but it could be assumed that country policies might moderate some of the associations observed in this study. Such research may prove challenging as it would require historical research on labour market, social security, family and health policies in each of the countries included for the period covering the participants’ employment trajectories.
The diversity of employment trajectories was associated with higher cancer risk over the life course. In our study, we identified several groups of lifetime employment trajectories in which work in general (either full-time or part-time) was associated with cancer in women firstly, and, secondly, some sequencings in the lifetime employment trajectories (Mainly full-time then home/family; Full-time then home/family then part time). First, we found that a lifetime trajectory of working full-time as an employee was associated with a higher risk of cancer compared with a lifetime trajectory of caring for home and family. In addition, working full-time as an employee in the first half of the working life and then transitioning into the family sphere was also associated with a higher risk of cancer. There are three possible interpretations of these findings. First, given that the association with cancer reflects breast cancer risk, it is possible that women who entered the labour market at some point in their life course were more likely to be informed and screened for breast cancer, and therefore had more cancer diagnoses. A European cross-sectional study suggested that being inactive was associated with a lower likelihood of being up to date with cancer screening82. Second, if full-time employment does increase women’s cancer risk, it is not per se, but because employment may be an indirect marker of the poor quality of women’s jobs and of occupational stress. Indeed, in the second half of the twentieth century women were known to have a higher proportion of low-skilled jobs83. Women from these cohorts increased their participation in the labour market but were more likely to be employed in manual occupations and in low-value jobs84. It is also possible that employed women are more exposed to occupational stress than women who have been self-employed throughout their working lives. Work can expose individuals to social and psychological stressors85, and stress has a biopsychosocial pathway to cancer development32,86–88. Gender insensitive working environments may affect mental health89, which may also predict cancer incidence through depression and psychological distress87. Exposure to more or less long-term poor working conditions90 may dysregulate physiological systems (e.g., immunity91,92), or the general physiological wear and tear19 and DNA methylation93,94, and predict the onset of cancer95,96. It is likely that the employment status—the indicator on which our sequence analysis was based—is an imperfect marker of the resources (income, job-related skills, social network at work) and status (social status of occupations) that women derive from their occupations and from the types of responsibilities they have at work, and ultimately of the levels of stress to which they are exposed97,98. Thus, without lifetime data on occupation and on psychosocial stress at work (e.g., effort-reward balance), the association between employment exposure and increased cancer risk should be treated with great caution. Second, some sequences in the lifetime employment trajectories (Mainly full-time then home/family; Full-time then home/family then part time) were associated with cancer. Interpretation of theses sequences should be done with great caution, given the limitation that a cancer diagnosis may occur in the course of an employment trajectory. Nevertheless, we could hypothesise that women who worked full-time in early years of adulthood may have been exposed to poor quality of women’s jobs and of occupational stress, suggesting a support for the sensitive period model.
Among men, we observed an association between a lifetime trajectory of self-employment and cancer risk. Compared with men who worked full-time as employees throughout their working lives, those who were self-employed had a lower risk of cancer. There is little research on the relationship between self-employment and better health for men. Self-employed people may generally have better health compared to employees for the following reasons: firstly, self-employed men may have more control over their work environment and workload, and therefore be better able to manage the stress levels; secondly, self-employed men may have more flexibility in their working hours, which may allow them to engage in healthy behaviours such as exercise and healthy eating, or cancer screening. On the other hand, we cannot ignore that the self-employed are exposed to the risks and responsibilities that come with running a business, which can lead to stress99,100, thus limiting the proposed explanation.
This study has limitations. First, we cannot exclude that reporting bias on the outcome may be significant, for two reasons: (a) cancer diagnosis was self-reported rather than from cancer registries. Self-reported cancer has an overall false-negative rate of 39.2%, with wide variation by cancer site101; (b) respondents in this cohort were older, which may be associated with more frequent false-positive reporting102. Second, survivor bias is inherent in the design of ageing studies such as SHARE, whose baseline included participants aged 50 years and over. Consequently, our findings may be more representative of cancer risks in older populations who survived until the study period. However, the survivor bias may be limited by the low probability of dying from cancer before the age of 50103. Third, as shown in the flowchart of respondent’s inclusion, the analytical sample (6,809 + 5,716 = 12,525) was a reduction compared to the original dataset (n = 139,760), signifying that selection bias may affect the results. Fourth, due to the lack of data in SHARE, our study does not take into account other life course factors such as intrauterine life, perinatal characteristics, child growth, and hormonal factors, which are associated with the incidence of several cancers5,104. Fifth, like all longitudinal studies of ageing, SHARE is characterised by attrition at follow-up105, and we have partially controlled for this in the analysis. Sixth, by controlling for cancer risk factors such as smoking106, sedentary behaviour107 and BMI108,109 measured at baseline, as well as partnership status and healthy diet, we confirmed that employment trajectories were independent of these risk factors, however we do not have information about the individual life course history of these factors, which may interact with employment over the working life. Seventh, the eight trajectories are not a definitive solution for categorising women’s trajectories because the clustering methods impose numerous choices, such as the clustering algorithm and the distance metric, which could influence the results. The eight trajectories are the result of a data mining construction that seeks the best solution for grouping the great heterogeneity of respondents’ trajectories (each individual sequence), taking into account the importance of the change (its timing and its order in the sequence) as well as the duration of a state (no change) in the employment trajectory. Eighth, the decisions made by SHARE about the coding of the employment status in 8 categories are critical in sequence analysis, as it will deeply influence the structure of the sequences. Ninth, the use of the life calendar allowed the reconstruction of the entire life course, retrospectively, on a yearly basis, between the ages of 16 and 65. Such a tool is useful for collecting comprehensive information on the life course of respondents. However, life calendars are subject to mnemonic and cognitive biases, but these are limited by the instrument’s ability to enhance respondent’s memory and correct for inconsistencies compared to a list of standardised questions110. Tenth, this study was based on participants who spent most of their lives between the ages of 16 and 65 in the twentieth century, a context marked by gender inequalities in access to education and the labour market, which is assumed to influence the ageing of women in good health111. Due to changes in the social stratification and in social norms112, in particular progress in socioeconomic equality between women and men83,113, we expect that our findings may not be applicable to cohorts who will spend their life course in the twenty-first century. Eleventh, this study examined employment trajectories cross-nationally by merging respondents from 14 countries. Our approach should not mask large cross-national differences in employment trajectories80,81. Twelfth, the timing of exposure (employment trajectories from 16 to 65 years) overlapped with the onset of the outcome (cancer diagnoses from 0 to 99 years), with about three in ten female cancer diagnoses occurring before the age of 65 years. However, the sensitivity analysis, which included only those cases of cancer diagnosed at 65 years of age or later, yielded comparable results to those of the main analysis.
Not with standing these challenges, we are confident in the robustness of our findings. The association between employment trajectories and cancer risk remained significant even after adjusting for various cancer-related factors, including body mass index, chronic conditions, smoking, physical activity, diet and partnership. Furthermore, our sensitivity analysis, which limited the analysis to prospective cancer cases occurring after the age of 65 years, confirmed the persistence of these associations.
Lifetime employment trajectories of European women are diverse and some of these trajectories were associated with a higher risk of self-reported cancer over the life course if women participated in the labour market compared with women who stayed at home. Lifetime employment trajectories of European men were less diverse, and being self-employed throughout working life was associated with a lower risk of cancer.
We believe that examining the full history of the participant’s life course using life calendar data and identifying patterned trajectories in these data using sequence analysis, has a promising potential for studies in life course epidemiology, although it has been rather rare to date in the field of epidemiology and health studies61,73,114. Although the lifetime employment trajectories approach looks promising, our association study remained a predictive exercise72. More research is needed to better understand these associations and to establish whether they are causal. In particular, factors such as health-related behaviours and occupational health risks, and their timing over the life course, should be considered in further studies.
Methods
Study design and population
The Survey of Health, Ageing, and Retirement in Europe (SHARE) is an ongoing longitudinal and cross-national survey designed to investigate population ageing processes and includes individuals aged 50 years and older115. SHARE was approved by the Ethics Council of the Max Planck Society and the relevant national research ethics committees in the participating countries, confirming that the survey was conducted in accordance with the Declaration of Helsinki as well as all relevant legal and ethical guidelines and regulations. All participants provided written informed consent.
Currently, SHARE includes eight waves of data that were collected every two years between 2004 and 2020 from 28 countries (12 countries in 2004). In the third and seventh wave, retrospective life history data were collected in the SHARELIFE module on past work or employment history from age 16 to 65 using a life calendar116.
Employment trajectories
The analysis of employment trajectories utilized the Harmonized Share Life History Dataset, Version B, February 2020, provided by the Gateway to Global Aging Data. Only individuals who had information on employment trajectories until the age of 65 were retained. The data was then split based on gender (variable “ragender”) and the clustering of trajectories was performed on each gender-specific dataset.
The analyses of employment trajectories are based on the variable “workstate” which is available from the ages 15–80. This variable describes the labour market status of the participants and is constructed using available information on paid work (as an employee or self-employed), unpaid work (domestic work or family work), or whether a person was not working (education, episodes of illness, etc.). Thus, for each year, the employment status of the participant is characterised according to one of these eight situations: 1. Employed full-time, 2. Employed part-time, 3. Self-employed, 4. Unemployed, 5. Home/family, 6. Retired, 7. Full-time education, and 8. Other. The “Other” category includes: Illness or disability, voluntary work, military service, and travelling.
Cancer
The question “Has a doctor ever told you that you had/Do you currently have any of the conditions on this card?” was used to operationalize cancer1. Participants who selected the ‘Cancer: ever diagnosed/currently having’ option were included in the analyses as having cancer. This included participants whose doctor had told the participants that they had cancer and who were currently being treated for or bothered by cancer. The follow-up question on specific cancer sites was used to run the analyses by site.
Covariates
The analyses were adjusted for attrition (no dropout, dropout, deceased) and covariates. The latter includes birth cohort, body mass index (BMI), smoking status, chronic conditions and physical activity levels. Birth cohort was categorized as born not during a crisis or war period (i.e., born before 1914, between 1919 and 1928, or after 1945), born during a war period (i.e., born between 1914 and 1918 or between 1939 and 1945), or born during the Great Depression (i.e., born between 1929 and 1938). BMI was derived using self-reported weight and height at baseline. Smoking status at baseline was used to categorize participants into non-smokers or smokers. The number of chronic conditions was calculated based on the following conditions at baseline and then transformed into a binary variable indicating (0) less or (1) two or more chronic conditions: stroke, heart attack, hypertension, high blood cholesterol, diabetes, chronic lung disease, stomach or duodenal ulcer, peptic ulcer, Parkinson disease, Alzheimer’s disease, affective or emotional disorders, including anxiety, nervous or psychiatric problems, rheumatoid arthritis, osteoarthritis, or other rheumatism, chronic kidney disease, and asthma. Physical activity was assessed in all waves, except for wave 3, and was based on two questions about the level of daily life physical activity: “How often do you engage in vigorous physical activity, such as sports, heavy housework, or a job that involves physical labour?” and “How often do you engage in activities that require a low or moderate level of energy, such as gardening, cleaning the car, or walking?”. Answers were based on a 4-point scale (1, more than once a week; 2, once a week; 3, one to three times a month; 4, hardly ever, or never). In this study, we used the baseline level of physical activity to classify participants as low (i.e. not doing any activity more than once a week) or high.
Statistical analyses
The statistical analysis was carried out in two stages, in accordance with the two objectives (Fig. 1).
The first objective was to describe and identify clusters in the employment trajectories of men and women. To do this, we used sequence analysis. Sequence analysis is a statistical method for describing the patterns and dynamics of trajectories coded as sequences, i.e. an ordered set of states (status). This approach is commonly used in the social sciences to analyse intensive longitudinal data when the temporal ordering of states needs to be understood. Technically, it is based on methods developed to study DNA, RNA or protein sequences in biology. Sequence analysis in the social sciences focuses on sequences of individual-level trajectories. Each sequence consists of a series of discrete states of the individuals occurring at different points in time. For example, sequences might represent the educational trajectory of young adults, or the occupational trajectory of middle-aged and older workers, or the trajectory of health behaviours over a life course period. It involves collecting information in each year of the trajectory of interest.
Sequence analysis typically involves several steps. First, the sequences are encoded in a standardised format, such as a matrix or string of symbols, where each element represents a particular state. The next step is to align the sequences. In our case, we aligned sequences from age 16 to age 65, so that each participant was assigned a sequence with a length of 49 states. Sequence analysis then involves comparing sequences to identify similarities and differences. We relied on the Hamming distance algorithm117 in TraMineR118. This distance is known to be sensitive to timing and therefore well suited to the analysis of employment trajectories, as employment status is expected to have different effects depending on when it occurs in the life course: for example, being unemployed at age 25 does not have the same implications as being unemployed at age 55. In determining the number of clusters119, priority was given to the “Average Silhouette Width” (ASW) measure120. Finally, similar sequences were grouped using cluster analysis based on the partitioning around medoids (PAM) algorithm. A cluster represented an employment trajectory with a typical, representative pattern.
For the second objective, we assessed the associations of employment trajectory clusters with the risk of cancer over the life course. To this end, we used logistic regression. Dates of diagnosis covered the whole life course (Figures S1 and S2 in Supplementary materials). All models were adjusted for the following covariates: age, birth cohort, body mass index at baseline, smoking at baseline, number of chronic conditions at baseline, and physical activity at baseline. In this study, baseline was defined as the first wave in which an individual participated in SHARE and completed the prospective questionnaire. Odds ratios (OR) and 95% confidence intervals were estimated. Models were also adjusted with a categorical variable representing participant attrition in the cohort (no dropout, dropout, death). Analyses were run for self-reported overall cancer, and for breast cancer in women. We did not run analyses for other cancer sites due to low prevalence rates (e.g., cervix, colorectal, lung, skin, ovarian, stomach). Statistical analyses were performed with R version 4.3.0121.
In line with the second objective, we conducted two sensitivity analyses with further covariate adjustments. First, we included partnership status (living alone or with a partner), which can influence cancer screening decisions and subsequently impact cancer incidence. This factor has been well-documented in the literature as influencing health behaviours including cancer screening122–124. In our regression models, we included the covariate whether individuals were living with or without a partner at baseline. Second, we included eating vegetables and fruits (healthy diet), which is a proxy capturing the tendency of respondents to have a healthy diet at baseline. Consumption of vegetables and fruit reduce the risk of cancer125 and some diet, such as the Mediterranean diet, have been shown weak and suggestive evidence with specific cancers126. A third sensitivity analysis was conducted in which the exposure (types of employment trajectories) and the outcome (diagnosis of cancer) were chronologically ordered, with the exposure occurring from 16 to 65 years of age and the diagnosis of cancer occurring prospectively from 65 years of age onwards. This was conducted in order to reduce the potential bias related to the possibility of the outcome occurring during the exposure period, and to ascertain whether the results would differ from those of the main analysis.
Supplementary Information
Acknowledgements
This study uses data from SHARE waves 1, 2, 3, 4, 5, 6, 7 and 8 (DOIs: 10.6103/SHARE.w1.710, 10.6103/SHARE.w2.710, 10.6103/SHARE.w3.710, 10.6103/SHARE.w4.710, 10.6103/SHARE.w5.710, 10.6103/SHARE.w6.710, 10.6103/SHARE.w7.710, 10.6103/SHARE.w8.710), see Börsch-Supan et al. (113) for methodological details. This study uses data or information from the Harmonized SHARE dataset and Codebook, Version B as of February 2020 developed by the Gateway to Global Aging Data in collaboration with the University of Duesseldorf.
Author contributions
Conceptualization: all authors; Data curation: SS, RG, BWAvdL; Formal analysis: SS, RG, BWAvdL; Funding acquisition: SC; Project administration: SC; Roles/Writing—original draft:SC, BWAvdl; and Writing—review & editing: all authors.
Funding
The SHARE data collection has been funded by the European Commission, DG RTD through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006-062193, COMPARE: CIT5-CT-2005-028857, SHARELIFE: CIT4-CT-2006-028812), FP7 (SHARE-PREP: GA N°211909, SHARE-LEAP: GA N°227822, SHARE M4: GA N°261982, DASISH: GA N°283646) and Horizon 2020 (SHARE-DEV3: GA N°676536, SHARE-COHESION: GA N°870628, SERISS: GA N°654221, SSHOC: GA N°823782) and by DG Employment, Social Affairs & Inclusion through VS 2015/0195, VS 2016/0135, VS 2018/0285, VS 2019/0332, and VS 2020/0313. Additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C, RAG052527A) and from various national funding sources is gratefully acknowledged (see www.share-project.org). The development of the Harmonized SHARE Life History was funded by the National Institute on Aging (R01 AG030153, RC2 AG036619, 1R03AG043052). For more information, please refer to www.g2aging.org. This work was supported by the Swiss National Centre of Competence in Research ’LIVES—overcoming vulnerability: Life course perspectives’, which is financed by the Swiss National Science Foundation (SNSF; 51NF40-160590).
Data availability
This SHARE dataset is available at http://www.share-project.org/data-access.html.
Competing interests
The authors declare no competing interests.
Ethical approval
This study was part of the SHARE study, approved by the relevant research ethics committees in the participating countries.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stéphane Cullati, Email: stephane.cullati@unifr.ch.
Bernadette W.A. van der Linden, Email: bernadette.vanderlinden@unifr.ch
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70909-2.
References
- 1.van der Linden, B. W. A. et al. Effect of childhood socioeconomic conditions on cancer onset in later life: An ambidirectional cohort study. Int. J. Public Health63, 799–810. 10.1007/s00038-018-1111-9 (2018). 10.1007/s00038-018-1111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widding-Havneraas, T. & Pedersen, S. H. The role of welfare regimes in the relationship between childhood economic stress and adult health: A multilevel study of 20 European countries. SSM Popul. Health12, 100674. 10.1016/j.ssmph.2020.100674 (2020). 10.1016/j.ssmph.2020.100674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santini, Z. I. et al. Cumulative risk of compromised physical, mental and social health in adulthood due to family conflict and financial strain during childhood: A retrospective analysis based on survey data representative of 19 European countries. BMJ Global Health6, e004144. 10.1136/bmjgh-2020-004144 (2021). 10.1136/bmjgh-2020-004144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stroup, A. M. et al. Baby boomers and birth certificates: Early-life socioeconomic status and cancer risk in adulthood. Cancer Epidemiol. Biomark. Prev.26, 75–84. 10.1158/1055-9965.EPI-16-0371 (2017). 10.1158/1055-9965.EPI-16-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lope, V. et al. Perinatal and childhood factors and risk of breast cancer subtypes in adulthood. Cancer Epidemiol.40, 22–30. 10.1016/j.canep.2015.11.004 (2016). 10.1016/j.canep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Demakakos, P., Chrousos, G. P. & Biddulph, J. P. Childhood experiences of parenting and cancer risk at older ages: Findings from the English Longitudinal Study of Ageing (ELSA). Int. J. Public Health63, 823–832. 10.1007/s00038-018-1117-3 (2018). 10.1007/s00038-018-1117-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolau, B. et al. Shared social mechanisms underlying the risk of nine cancers: A life course study. Int. J. Cancer144, 59–67. 10.1002/ijc.31719 (2019). 10.1002/ijc.31719 [DOI] [PubMed] [Google Scholar]
- 8.McNamara, C. L., Toch-Marquardt, M., Albani, V., Eikemo, T. A. & Bambra, C. The contribution of employment and working conditions to occupational inequalities in non-communicable diseases in Europe. Eur. J. Public Health31, 181–185. 10.1093/eurpub/ckaa175 (2021). 10.1093/eurpub/ckaa175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadkhale, K. et al. Occupational variation in incidence of bladder cancer: A comparison of population-representative cohorts from Nordic countries and Canada. BMJ Open7, e016538. 10.1136/bmjopen-2017-016538 (2017). 10.1136/bmjopen-2017-016538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akinyemiju, T. F. et al. The association of early life socioeconomic position on breast cancer incidence and mortality: A systematic review. Int. J. Public Health63, 787–797. 10.1007/s00038-017-1060-8 (2018). 10.1007/s00038-017-1060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly-Irving, M. et al. Childhood adversity as a risk for cancer: Findings from the 1958 British birth cohort study. BMC Public Health13, 767. 10.1186/1471-2458-13-767 (2013). 10.1186/1471-2458-13-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly-Irving, M., Mabile, L., Grosclaude, P., Lang, T. & Delpierre, C. The embodiment of adverse childhood experiences and cancer development: Potential biological mechanisms and pathways across the life course. Int. J. Public Health58, 3–11. 10.1007/s00038-012-0370-0 (2013). 10.1007/s00038-012-0370-0 [DOI] [PubMed] [Google Scholar]
- 13.Kuh, D. J. L. & Ben-Shlomo, Y. A Life Course Approach to Chronic Disease Epidemiology (Oxford University Press, 2004). [Google Scholar]
- 14.Ben-Shlomo, Y., Cooper, R. & Kuh, D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int. J. Epidemiol.45, 973–988. 10.1093/ije/dyw096 (2016). 10.1093/ije/dyw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton-Jeangros, C., Cullati, S., Sacker, A. & Blane, D. Vol. Collection “Life Course Research and Social Policies n°4” (Springer, New York, 2015).
- 16.Cullati, S., Kliegel, M. & Widmer, E. Development of reserves over the life course and onset of vulnerability in later life. Nat. Hum. Behaviour2, 551–558. 10.1038/s41562-018-0395-3 (2018). 10.1038/s41562-018-0395-3 [DOI] [PubMed] [Google Scholar]
- 17.Wagner, C. et al. Life course epidemiology and public health. Lancet Public Health9, e261–e269. 10.1016/S2468-2667(24)00018-5 (2024). 10.1016/S2468-2667(24)00018-5 [DOI] [PubMed] [Google Scholar]
- 18.Wright, R. J. & Hanson, H. A. A tipping point in cancer epidemiology: Embracing a life course exposomic framework. Trends Cancer8, 280–282. 10.1016/j.trecan.2022.01.016 (2022). 10.1016/j.trecan.2022.01.016 [DOI] [PubMed] [Google Scholar]
- 19.Kelly-Irving, M. & Vineis, P. Reducing Social Inequalities in Cancer: Evidence and Priorities for Research (International Agency for Research on Cancer Scientific Publications, 2019). [PubMed] [Google Scholar]
- 20.Andersen, Z. J. et al. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: A register-based cohort study. Breast Cancer Res.16, R4. 10.1186/bcr3596 (2014). 10.1186/bcr3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmichael, A. R. Obesity and prognosis of breast cancer. Obes. Rev.7, 333–340. 10.1111/j.1467-789X.2006.00261.x (2006). 10.1111/j.1467-789X.2006.00261.x [DOI] [PubMed] [Google Scholar]
- 22.OECD. Time Use (Edition 2021). (OECD Social and Welfare Statistics (database), 10.1787/dfdd9f12-en (accessed on 04 May 2023), 2020).
- 23.Lu, W. et al. Relationship between employment histories and frailty trajectories in later life: Evidence from the English Longitudinal Study of Ageing. J. Epidemiol. Community Health71, 439–445. 10.1136/jech-2016-207887 (2017). 10.1136/jech-2016-207887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsen, C., Andel, R., Darin-Mattsson, A. & Kåreholt, I. Psychosocial working conditions across working life may predict late-life physical function: A follow-up cohort study. BMC Public Health19, 1125. 10.1186/s12889-019-7473-y (2019). 10.1186/s12889-019-7473-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baxter, S., Blank, L., Cantrell, A. & Goyder, E. Is working in later life good for your health? A systematic review of health outcomes resulting from extended working lives. BMC Public Health21, 1356. 10.1186/s12889-021-11423-2 (2021). 10.1186/s12889-021-11423-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waddell, G. & Burton, A. K. Is Work Good for your Health and Well-Being? (The Stationery Office, 2006). [Google Scholar]
- 27.Bayer, O. et al. Occupation and cancer of the larynx: A systematic review and meta-analysis. Eur. Arch. Oto Rhino Laryngol.273, 9–20. 10.1007/s00405-014-3321-y (2016). 10.1007/s00405-014-3321-y [DOI] [PubMed] [Google Scholar]
- 28.Reulen, R. C., Kellen, E., Buntinx, F., Brinkman, M. & Zeegers, M. P. A meta-analysis on the association between bladder cancer and occupation. Scand. J. Urol. Nephrol.42, 64–78. 10.1080/03008880802325192 (2008). 10.1080/03008880802325192 [DOI] [PubMed] [Google Scholar]
- 29.Krstev, S. & Knutsson, A. Occupational risk factors for prostate cancer: A meta-analysis. J. Cancer Prev.24, 91–111. 10.15430/JCP.2019.24.2.91 (2019). 10.15430/JCP.2019.24.2.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivera-Izquierdo, M. et al. Shift work and prostate cancer: An updated systematic review and meta-analysis. Int. J. Environ. Res. Public Health17, 1345 (2020). 10.3390/ijerph17041345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oddone, E., Modonesi, C. & Gatta, G. Occupational exposures and colorectal cancers: A quantitative overview of epidemiological evidence. World J. Gastroenterol.20, 12431–12444. 10.3748/wjg.v20.i35.12431 (2014). 10.3748/wjg.v20.i35.12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang, T. et al. Work stress and the risk of cancer: A meta-analysis of observational studies. Int. J. Cancer144, 2390–2400. 10.1002/ijc.31955 (2019). 10.1002/ijc.31955 [DOI] [PubMed] [Google Scholar]
- 33.Stone, J., Evandrou, M., Falkingham, J. & Vlachantoni, A. Women’s economic activity trajectories over the life course: Implications for the self-rated health of women aged 64+ in England. J. Epidemiol. Community Health69, 873–879. 10.1136/jech-2014-204777 (2015). 10.1136/jech-2014-204777 [DOI] [PubMed] [Google Scholar]
- 34.Platts, L. G., Webb, E., Zins, M., Goldberg, M. & Netuveli, G. Mid-life occupational grade and quality of life following retirement: A 16 year follow-up of the French GAZEL study. Aging Mental Health19, 634–646. 10.1080/13607863.2014.955458 (2015). 10.1080/13607863.2014.955458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karimi, M., Geoffroy-Perez, B., Fouquet, A., Latouche, A. & Rey, G. Socioprofessional trajectories and mortality in France, 1976–2002: A longitudinal follow-up of administrative data. J. Epidemiol. Community Health69, 339–346. 10.1136/jech-2014-204615 (2015). 10.1136/jech-2014-204615 [DOI] [PubMed] [Google Scholar]
- 36.Astrom, A. N., Ekback, G., Lie, S. A. & Ordell, S. Life-course social influences on tooth loss and oral attitudes among older people: Evidence from a prospective cohort study. Eur. J. Oral Sci.123, 30–38. 10.1111/eos.12160 (2015). 10.1111/eos.12160 [DOI] [PubMed] [Google Scholar]
- 37.Benavides, F. G., Duran, X., Gimeno, D., Vanroelen, C. & Martinez, J. M. Labour market trajectories and early retirement due to permanent disability: A study based on 14 972 new cases in Spain. Eur. J. Public Health25, 673–677. 10.1093/eurpub/cku204 (2015). 10.1093/eurpub/cku204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berkman, L. F. et al. Mothering alone: Cross-national comparisons of later-life disability and health among women who were single mothers. J. Epidemiol. Community Health69, 865–872. 10.1136/jech-2014-205149 (2015). 10.1136/jech-2014-205149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blomgren, J., Martikainen, P., Grundy, E. & Koskinen, S. Marital history 1971–91 and mortality 1991–2004 in England & Wales and Finland. J. Epidemiol. Community Health66, 30. 10.1136/jech.2010.110635 (2012). 10.1136/jech.2010.110635 [DOI] [PubMed] [Google Scholar]
- 40.Stringhini, S., Zaninotto, P., Kumari, M., Kivimäki, M. & Batty, G. D. Lifecourse socioeconomic status and type 2 diabetes: The role of chronic inflammation in the English Longitudinal Study of Ageing. Sci. Rep.6, 24780. 10.1038/srep24780 (2016). 10.1038/srep24780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briggs, F. B. et al. Adverse socioeconomic position during the life course is associated with multiple sclerosis. J. Epidemiol. Community Health68, 622–629. 10.1136/jech-2013-203184 (2014). 10.1136/jech-2013-203184 [DOI] [PubMed] [Google Scholar]
- 42.Lacey, R. J., Belcher, J. & Croft, P. R. Does life course socio-economic position influence chronic disabling pain in older adults? A general population study. Eur. J. Public Health23, 534–540. 10.1093/eurpub/cks056 (2013). 10.1093/eurpub/cks056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollitt, R. A. et al. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J. Epidemiol. Community Health62, 484–491. 10.1136/jech.2006.054106 (2008). 10.1136/jech.2006.054106 [DOI] [PubMed] [Google Scholar]
- 44.Crandall, C. J. et al. Socioeconomic status over the life-course and adult bone mineral density: The Midlife in the U.S Study. Bone51, 107–113. 10.1016/j.bone.2012.04.009 (2012). 10.1016/j.bone.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruenewald, T. L. et al. History of socioeconomic disadvantage and allostatic load in later life. Soc. Sci. Med.74, 75–83. 10.1016/j.socscimed.2011.09.037 (2012). 10.1016/j.socscimed.2011.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merkin, S. S., Karlamangla, A., Roux, A. V., Shrager, S. & Seeman, T. E. Life course socioeconomic status and longitudinal accumulation of allostatic load in adulthood: Multi-ethnic study of atherosclerosis. Am. J. Public Health104, e48-55. 10.2105/AJPH.2013.301841 (2014). 10.2105/AJPH.2013.301841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robertson, T., Popham, F. & Benzeval, M. Socioeconomic position across the lifecourse & allostatic load: Data from the West of Scotland Twenty-07 cohort study. BMC Public Health14, 184. 10.1186/1471-2458-14-184 (2014). 10.1186/1471-2458-14-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elo, I. T., Martikainen, P. & Myrskyla, M. Socioeconomic status across the life course and all-cause and cause-specific mortality in Finland. Soc. Sci. Med.119, 198–206. 10.1016/j.socscimed.2013.11.037 (2014). 10.1016/j.socscimed.2013.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hostinar, C. E., Ross, K. M., Chen, E. & Miller, G. E. Modeling the association between lifecourse socioeconomic disadvantage and systemic inflammation in healthy adults: The role of self-control. Health Psychol.34, 580–590. 10.1037/hea0000130 (2015). 10.1037/hea0000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harper, S. et al. Life course socioeconomic conditions and adult psychosocial functioning. Int. J. Epidemiol.31, 395–403. 10.1093/ije/31.2.395 (2002). 10.1093/ije/31.2.395 [DOI] [PubMed] [Google Scholar]
- 51.McGovern, P. & Nazroo, J. Y. Patterns and causes of health inequalities in later life: A Bourdieusian approach. Sociol. Health Illn.37, 143–160. 10.1111/1467-9566.12187 (2015). 10.1111/1467-9566.12187 [DOI] [PubMed] [Google Scholar]
- 52.Moore, D. E. & Hayward, M. D. Occupational careers and mortality of elderly men. Demography27, 31–53 (1990). 10.2307/2061551 [DOI] [PubMed] [Google Scholar]
- 53.Aartsen, M. J. et al. Advantaged socioeconomic conditions in childhood are associated with higher cognitive functioning but stronger cognitive decline in older age. Proc. Natl. Acad. Sci.116, 5478–5486. 10.1073/pnas.1807679116 (2019). 10.1073/pnas.1807679116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andersen, Z. J., Lassen, C. F. & Clemmensen, I. H. Social inequality and incidence of and survival from cancers of the mouth, pharynx and larynx in a population-based study in Denmark, 1994–2003. Eur. J. Cancer44, 1950–1961 (2008). 10.1016/j.ejca.2008.06.019 [DOI] [PubMed] [Google Scholar]
- 55.Freedman, D., Thornton, A., Camburn, D., Alwin, D. & Young-DeMarco, L. The life history calendar: A technique for collecting retrospective data. Sociol. Methodol.18, 37–68. 10.2307/271044 (1988). 10.2307/271044 [DOI] [PubMed] [Google Scholar]
- 56.Widmer, E. D. & Ritschard, G. The de-standardization of the life course: Are men and women equal?. Adv. Life Course Res.14, 28–39. 10.1016/j.alcr.2009.04.001 (2009). 10.1016/j.alcr.2009.04.001 [DOI] [Google Scholar]
- 57.Van Winkle, Z. & Fasang, A. The complexity of employment and family life courses across 20th century Europe: More evidence for larger cross-national differences but little change across 1916–1966 birth cohorts. Demogr. Res.44, 775–810 (2021). 10.4054/DemRes.2021.44.32 [DOI] [Google Scholar]
- 58.Simonson, J., Gordo, L. R. & Titova, N. Changing employment patterns of women in Germany: How do baby boomers differ from older cohorts? A comparison using sequence analysis. Adv. Life Course Res.16, 65–82. 10.1016/j.alcr.2011.03.002 (2011). 10.1016/j.alcr.2011.03.002 [DOI] [Google Scholar]
- 59.Madero-Cabib, I., Undurraga, R. & Valenzuela, C. How have women’s employment patterns during young adulthood changed in Chile? A cohort study. Longitud. Life Course Stud.10, 375–397. 10.1332/175795919X15628474680736 (2019). 10.1332/175795919X15628474680736 [DOI] [Google Scholar]
- 60.Di Gessa, G., Corna, L., Price, D. & Glaser, K. Lifetime employment histories and their relationship with 10 year health trajectories in later life: Evidence from England. Eur. J. Public Health30, 793–799. 10.1093/eurpub/ckaa008 (2020). 10.1093/eurpub/ckaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahrendorf, M. Previous employment histories and quality of life in older ages: Sequence analyses using SHARELIFE. Ageing Soc.35, 1928–1959. 10.1017/S0144686X14000713 (2015). 10.1017/S0144686X14000713 [DOI] [Google Scholar]
- 62.Zella, S. & Harper, S. Employment trajectories and health: Cohort differences in English and French women. J. Public Health43, 587–594. 10.1093/pubmed/fdaa064 (2021). 10.1093/pubmed/fdaa064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madero-Cabib, I., Corna, L. & Baumann, I. Aging in different welfare contexts: A comparative perspective on later-life employment and health. J. Gerontol. Ser. B75, 1515–1526. 10.1093/geronb/gbz037 (2020). 10.1093/geronb/gbz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Gessa, G., Corna, L., Price, D. & Glaser, K. Lifetime employment histories and their relationship with 10 year health trajectories in later life: evidence from England. Eur. J. Public Health10.1093/eurpub/ckaa008 (2020). 10.1093/eurpub/ckaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahrendorf, M., Hoven, H., Deindl, C., Lunau, T. & Zaninotto, P. Adverse employment histories, later health functioning and national labor market policies: European findings based on life-history data from SHARE and ELSA. J. Gerontol. Ser. B76, S27–S40. 10.1093/geronb/gbaa049 (2021). 10.1093/geronb/gbaa049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoven, H., Dragano, N., Lunau, T., Deindl, C. & Wahrendorf, M. The role of pension contributions in explaining inequalities in depressive symptoms. Results from SHARE. Scand. J. Public Health10.1177/1403494820909011 (2020). 10.1177/1403494820909011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giudici, F. & Morselli, D. 20 years in the world of work: A study of (nonstandard) occupational trajectories and health. Soc. Sci. Med.224, 138–148. 10.1016/j.socscimed.2019.02.002 (2019). 10.1016/j.socscimed.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 68.Arpino, B., Gumà, J. & Julià, A. Early-life conditions and health at older ages: The mediating role of educational attainment, family and employment trajectories. PLoS ONE13, e0195320. 10.1371/journal.pone.0195320 (2018). 10.1371/journal.pone.0195320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMunn, A. et al. Work-family life courses and psychological distress: Evidence from three British birth cohort studies. Adv. Life Course Res.10.1016/j.alcr.2021.100429 (2021). 10.1016/j.alcr.2021.100429 [DOI] [PubMed] [Google Scholar]
- 70.Daly, M. & Delaney, L. The scarring effect of unemployment throughout adulthood on psychological distress at age 50: Estimates controlling for early adulthood distress and childhood psychological factors. Soc. Sci. Med.80, 19–23. 10.1016/j.socscimed.2012.12.008 (2013). 10.1016/j.socscimed.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 71.Lu, W. et al. Relationship between employment histories and frailty trajectories in later life: Evidence from the English Longitudinal Study of Ageing. J. Epidemiol. Community Health71, 439. 10.1136/jech-2016-207887 (2017). 10.1136/jech-2016-207887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hernán, M. A., Hsu, J. & Healy, B. A second chance to get causal inference right: A classification of data science tasks. Chance32, 42–49. 10.1080/09332480.2019.1579578 (2019). 10.1080/09332480.2019.1579578 [DOI] [Google Scholar]
- 73.Juliet, S., Maria, E., Jane, F. & Athina, V. Women’s economic activity trajectories over the life course: Implications for the self-rated health of women aged 64+ in England. J. Epidemiol. Community Health69, 873. 10.1136/jech-2014-204777 (2015). 10.1136/jech-2014-204777 [DOI] [PubMed] [Google Scholar]
- 74.Schmitz, W., Naegele, L., Frerichs, F. & Ellwardt, L. Gendered late working life trajectories, family history and welfare regimes: Evidence from SHARELIFE. Eur. J. Ageing20, 5. 10.1007/s10433-023-00752-3 (2023). 10.1007/s10433-023-00752-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komp-Leukkunen, K. Capturing the diversity of working age life-courses: A European perspective on cohorts born before 1945. PLoS ONE14, e0212400. 10.1371/journal.pone.0212400 (2019). 10.1371/journal.pone.0212400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Metzger, S. & Gracia, P. Gender differences in mental health following the transition into parenthood: Longitudinal evidence from the UK. Adv. Life Course Res.56, 100550. 10.1016/j.alcr.2023.100550 (2023). 10.1016/j.alcr.2023.100550 [DOI] [PubMed] [Google Scholar]
- 77.Winslow, S. & Davis, S. N. Gender inequality across the academic life course. Sociol. Compass10, 404–416. 10.1111/soc4.12372 (2016). 10.1111/soc4.12372 [DOI] [Google Scholar]
- 78.Calderone, S. M., McDonald, T., Hill, G. & Derrington, M. L. Life course and gender: Unpacking women’s thoughts about access to the superintendency. Front. Educ.5, 124 (2020). 10.3389/feduc.2020.00124 [DOI] [Google Scholar]
- 79.Christiansen, L. E. et al.Unlocking Female Employment Potential in Europe: Drivers and Benefits (International Monetary Fund, 2016). [Google Scholar]
- 80.Van Winkle, Z. Family trajectories across time and space: Increasing complexity in family life courses in Europe?. Demography55, 135–164. 10.1007/s13524-017-0628-5 (2018). 10.1007/s13524-017-0628-5 [DOI] [PubMed] [Google Scholar]
- 81.Van Winkle, Z. & Fasang, A. Complexity in employment life courses in europe in the twentieth century—large cross-national differences but little change across birth cohorts. Soc. Forces96, 1–30. 10.1093/sf/sox032 (2017). 10.1093/sf/sox032 [DOI] [Google Scholar]
- 82.Bozhar, H. et al. Socio-economic inequality of utilization of cancer testing in Europe: A cross-sectional study. Prev. Med. Rep.26, 101733. 10.1016/j.pmedr.2022.101733 (2022). 10.1016/j.pmedr.2022.101733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardey, D., Maruani, M. & Meron, M. Digital Encyclopedia of European History [URL: https://ehne.fr/en/node/12328] (2000).
- 84.Graeber, D. Bullshit Jobs (Simon & Schuster, 2019). [Google Scholar]
- 85.Siegrist, J. The New Blackwell Companion to Medical Sociology (Wiley-Blackwell, 2010). [Google Scholar]
- 86.Chida, Y., Hamer, M., Wardle, J. & Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival?. Nat. Clin. Pract. Oncol.5, 466–475 (2008). 10.1038/ncponc1134 [DOI] [PubMed] [Google Scholar]
- 87.Kruk, J., Aboul-Enein, B. H., Bernstein, J. & Gronostaj, M. Psychological stress and cellular aging in cancer: A meta-analysis. Oxidative Med. Cell. Longev.2019, 1270397. 10.1155/2019/1270397 (2019). 10.1155/2019/1270397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heikkilä, K. et al. Work stress and risk of cancer: Meta-analysis of 5700 incident cancer events in 116 000 European men and women. BMJ Br. Med. J.346, f165. 10.1136/bmj.f165 (2013). 10.1136/bmj.f165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allison, M. et al. Gendered working environments as a determinant of mental health inequalities: A systematic review of 27 studies. Occup. Environ. Med.78, 147. 10.1136/oemed-2019-106281 (2021). 10.1136/oemed-2019-106281 [DOI] [PubMed] [Google Scholar]
- 90.Krieger, N. Embodiment: A conceptual glossary for epidemiology. J. Epidemiol. Community Health59, 350–355. 10.1136/jech.2004.024562 (2005). 10.1136/jech.2004.024562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olvera Alvarez, H. A., Kubzansky, L. D., Campen, M. J. & Slavich, G. M. Early life stress, air pollution, inflammation, and disease: An integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci. Biobehav. Rev.92, 226–242. 10.1016/j.neubiorev.2018.06.002 (2018). 10.1016/j.neubiorev.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muscatell, K. A., Brosso, S. N. & Humphreys, K. L. Socioeconomic status and inflammation: A meta-analysis. Mol. Psychiatry25, 2189–2199. 10.1038/s41380-018-0259-2 (2020). 10.1038/s41380-018-0259-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fiorito, G. et al. Social adversity and epigenetic aging: A multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Sci. Rep.7, 16266. 10.1038/s41598-017-16391-5 (2017). 10.1038/s41598-017-16391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hughes, A. et al. Socioeconomic position and DNA methylation age acceleration across the life course. Am. J. Epidemiol.187, 2346–2354. 10.1093/aje/kwy155 (2018). 10.1093/aje/kwy155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Obeng-Gyasi, E., Tarver, W. & Obeng-Gyasi, S. Allostatic load and breast cancer: A systematic review of the literature. Curr. Breast Cancer Rep.14, 180–191. 10.1007/s12609-022-00455-1 (2022). 10.1007/s12609-022-00455-1 [DOI] [Google Scholar]
- 96.Mathew, A. et al. Allostatic load in cancer: A systematic review and mini meta-analysis. Biol. Res. Nurs.23, 341–361. 10.1177/1099800420969898 (2021). 10.1177/1099800420969898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weber, M. Economy and Society (University of California Press, 1922). [Google Scholar]
- 98.Galobardes, B., Shaw, M., Lawlor, D. A., Lynch, J. W. & Davey Smith, G. Indicators of socioeconomic position (part 1). J. Epidemiol. Community Health60, 7–12. 10.1136/jech.2004.023531 (2006). 10.1136/jech.2004.023531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewin-Epstein, N. & Yuchtman-Yaar, E. Health risks of self-employment. Work Occup.18, 291–312. 10.1177/0730888491018003003 (1991). 10.1177/0730888491018003003 [DOI] [Google Scholar]
- 100.Willeke, K. et al. Occurrence of mental illness and mental health risks among the self-employed: A systematic review. Int. J. Environ. Res. Public Health18, 8617 (2021). 10.3390/ijerph18168617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desai, M. M., Bruce, M. L., Desai, R. A. & Druss, B. G. Validity of self-reported cancer history: A comparison of health interview data and cancer registry records. Am. J. Epidemiol.153, 299–306. 10.1093/aje/153.3.299 (2001). 10.1093/aje/153.3.299 [DOI] [PubMed] [Google Scholar]
- 102.Loh, V. et al. The validity of self-reported cancer in an Australian population study. Aust. N. Z. J. Public Health38, 35–38. 10.1111/1753-6405.12164 (2014). 10.1111/1753-6405.12164 [DOI] [PubMed] [Google Scholar]
- 103.Malvezzi, M. et al. European cancer mortality predictions for the year 2015: Does lung cancer have the highest death rate in EU women?. Ann. Oncol.26, 779–786. 10.1093/annonc/mdv001 (2015). 10.1093/annonc/mdv001 [DOI] [PubMed] [Google Scholar]
- 104.Potischman, N., Troisi, R. & Vatten, L. A Life Course Approach to Chronic Disease Epidemiology (Oxford University Press, 2004). [Google Scholar]
- 105.Bergmann, M., Kneip, T., De Luca, G. & Scherpenzeel, A. Survey participation in the survey of health, ageing and retirement in Europe (SHARE), Wave 1–7. (Munich Center for the Economics of Aging, Working Paper Series 41–2019, Munich, 2019).
- 106.Lugo, A. et al. Dose–response relationship between cigarette smoking and site-specific cancer risk: Protocol for a systematic review with an original design combining umbrella and traditional reviews. BMJ Open7, e018930. 10.1136/bmjopen-2017-018930 (2017). 10.1136/bmjopen-2017-018930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hermelink, R. et al. Sedentary behavior and cancer–an umbrella review and meta-analysis. Eur. J. Epidemiol.37, 447–460. 10.1007/s10654-022-00873-6 (2022). 10.1007/s10654-022-00873-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lauby-Secretan, B. et al. Body fatness and cancer—viewpoint of the IARC working group. N. Engl. J. Med.375, 794–798. 10.1056/NEJMsr1606602 (2016). 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kyrgiou, M. et al. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ356, j477. 10.1136/bmj.j477 (2017). 10.1136/bmj.j477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morselli, D. & Berchtold, A. Withstanding Vulnerability throughout Adult Life: Dynamics of Stressors, Resources, and Reserves (Springer Nature Singapore, 2023). [Google Scholar]
- 111.Cullati, S. et al.Withstanding Vulnerability throughout Adult Life: Dynamics of Stressors, Resources, and Reserves (Springer Nature Singapore, 2023). [Google Scholar]
- 112.Giddens, A. Modernity and Self-Identity. Self and Society in the Late Modern Age (Stanford University Press, 1991). [Google Scholar]
- 113.OECD. Understanding the socio-economic divide in Europe. (OECD Centre for Opportunity and Equality, URL: https://www.oecd.org/els/soc/cope-divide-europe-2017-background-report.pdf, Paris, 2017).
- 114.van Hedel, K. et al. Work-family trajectories and the higher cardiovascular risk of american women relative to women in 13 European countries. Am. J. Public Health106, 1449–1456. 10.2105/AJPH.2016.303264 (2016). 10.2105/AJPH.2016.303264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Börsch-Supan, A. et al. Data resource profile: The survey of health, ageing and retirement in Europe (SHARE). Int. J. Epidemiol.42, 992–1001. 10.1093/ije/dyt088 (2013). 10.1093/ije/dyt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Börsch-Supan, A., Brandt, M. & Schröder, M. SHARELIFE—one century of life histories in Europe. Adv. Life Course Res.18, 1–4. 10.1016/j.alcr.2012.10.009 (2013). 10.1016/j.alcr.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 117.Studer, M. & Ritschard, G. What matters in differences between life trajectories: A comparative review of sequence dissimilarity measures. J. Royal Stat. Soc. Ser. A Stat. Soc.179, 481–511. 10.1111/rssa.12125 (2016). 10.1111/rssa.12125 [DOI] [Google Scholar]
- 118.Gabadinho, A., Ritschard, G., Müller, N. S. & Studer, M. Analyzing and visualizing state sequences in R with TraMineR. J. Stat. Softw.40, 1–37. 10.18637/jss.v040.i04 (2011). 10.18637/jss.v040.i04 [DOI] [Google Scholar]
- 119.Studer, M. Weighted Cluster Library Manual: A Practical Guide to Creating Typologies of Trajectories in the Social Sciences with R (LIVES Working Papers, 2013). [Google Scholar]
- 120.Kaufman, L. & Rousseeuw, P. Finding Groups in Data: An Introduction to Cluster Analysis (Wiley, 1990). [Google Scholar]
- 121.R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, <https://www.R-project.org/>, 2023).
- 122.Jolidon, V. Gender inequality and mammography screening: Does living with a partner improve women’s mammography uptake?. Soc. Sci. Med.10.1016/j.socscimed.2022.114875 (2022). 10.1016/j.socscimed.2022.114875 [DOI] [PubMed] [Google Scholar]
- 123.Jolidon, V. et al. Revisiting the effects of organized mammography programs on inequalities in breast screening uptake: A multilevel analysis of nationwide data from 1997 to 2017. Front. Public Health10.3389/fpubh.2022.812776 (2022). 10.3389/fpubh.2022.812776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jolidon, V., De Prez, V., Bracke, P., Cullati, S. & Burton-Jeangros, C. Lack of social support, gender and colorectal cancer screening participation across Europe: How do screening programmes mitigate the effect of social support for men and women?. Sociol. Health Illn.10.1111/1467-9566.13791 (2024). 10.1111/1467-9566.13791 [DOI] [PubMed] [Google Scholar]
- 125.Latino-Martel, P. et al. Alcoholic beverages, obesity, physical activity and other nutritional factors, and cancer risk: A review of the evidence. Crit. Rev. Oncol. Hematol.99, 308–323. 10.1016/j.critrevonc.2016.01.002 (2016). 10.1016/j.critrevonc.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 126.Dinu, M., Pagliai, G., Casini, A. & Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr.72, 30–43. 10.1038/ejcn.2017.58 (2018). 10.1038/ejcn.2017.58 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This SHARE dataset is available at http://www.share-project.org/data-access.html.