Abstract
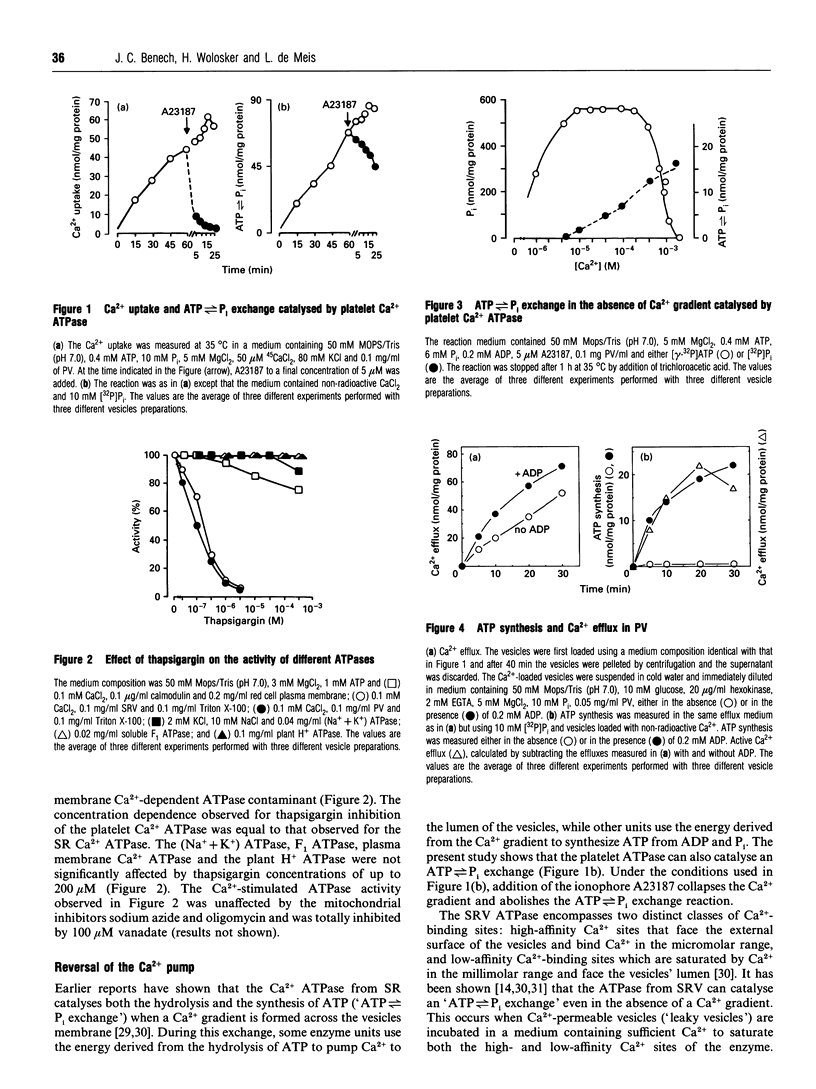
In this study, the endoplasmic Ca2+ transport ATPase of blood platelets was compared with the Ca2+ ATPase of sarcoplasmic reticulum skeletal muscle. Similar to the muscle enzyme, the Ca2+ ATPase from platelets was found to catalyse an ATP<-->P(i) exchange both in the presence and in the absence of a transmembrane Ca2+ gradient. When platelet vesicles are loaded with Ca2+ and diluted in medium containing ADP, P(i) and EGTA, the ATPase catalyses Ca2+ efflux coupled to synthesis of ATP. The stoichiometry between Ca2+ ion released and ATP synthesized by platelet Ca2+ ATPase is 1, while that of skeletal muscle is 2. Thapsigargin, a specific inhibitor of sarcoplasmic/endoplasmic reticulum Ca2+ ATPases, inhibited both the Ca(2+)-dependent ATPase activity and the reversal of the platelet Ca2+ pump. The possibility is discussed that the differences observed between the two transport systems is related to the distinct amino acid sequences of the enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adunyah S. E., Dean W. L. Effects of sulfhydryl reagents and other inhibitors on Ca2+ transport and inositol trisphosphate-induced Ca2+ release from human platelet membranes. J Biol Chem. 1986 Oct 5;261(28):13071–13075. [PubMed] [Google Scholar]
- Barlogie B., Hasselbach W., Makinose M. Activation of calcium efflux by ADP and inorganic phosphate. FEBS Lett. 1971 Jan 30;12(5):267–268. doi: 10.1016/0014-5793(71)80194-1. [DOI] [PubMed] [Google Scholar]
- Bobe R., Bredoux R., Wuytack F., Quarck R., Kovàcs T., Papp B., Corvazier E., Magnier C., Enouf J. The rat platelet 97-kDa Ca2+ATPase isoform is the sarcoendoplasmic reticulum Ca2+ATPase 3 protein. J Biol Chem. 1994 Jan 14;269(2):1417–1424. [PubMed] [Google Scholar]
- Brass L. F., Joseph S. K. A role for inositol triphosphate in intracellular Ca2+ mobilization and granule secretion in platelets. J Biol Chem. 1985 Dec 5;260(28):15172–15179. [PubMed] [Google Scholar]
- Carvalho M. G., de Souza D. G., de Meis L. On a possible mechanism of energy conservation in sarcoplasmic reticulum membrane. J Biol Chem. 1976 Jun 25;251(12):3629–3636. [PubMed] [Google Scholar]
- Chiesi M., Inesi G. The use of quench reagents for resolution of single transport cycles in sarcoplasmic reticulum. J Biol Chem. 1979 Oct 25;254(20):10370–10377. [PubMed] [Google Scholar]
- Dean W. L. Purification and reconstitution of a Ca2+ pump from human platelets. J Biol Chem. 1984 Jun 10;259(11):7343–7348. [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Enouf J., Bredoux R., Bourdeau N., Sarkadi B., Levy-Toledano S. Further characterization of the plasma membrane- and intracellular membrane-associated platelet Ca2+ transport systems. Biochem J. 1989 Oct 15;263(2):547–552. doi: 10.1042/bj2630547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enouf J., Bredoux R., Papp B., Djaffar I., Lompré A. M., Kieffer N., Gayet O., Clemetson K., Wuytack F., Rosa J. P. Human platelets express the SERCA2-b isoform of Ca(2+)-transport ATPase. Biochem J. 1992 Aug 15;286(Pt 1):135–140. doi: 10.1042/bj2860135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A., Sarkadi B., Földes-Papp Z., Monostory S., Gárdos G. Demonstration of two distinct calcium pumps in human platelet membrane vesicles. J Biol Chem. 1986 Jul 15;261(20):9558–9563. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fauvel J., Chap H., Roques V., Levy-Toledano S., Douste-Blazy L. Biochemical characterization of plasma membranes and intracellular membranes isolated from human platelets using Percoll gradients. Biochim Biophys Acta. 1986 Mar 27;856(1):155–164. doi: 10.1016/0005-2736(86)90022-2. [DOI] [PubMed] [Google Scholar]
- Fischer T. H., Campbell K. P., White G. C., 2nd An investigation of functional similarities between the sarcoplasmic reticulum and platelet calcium-dependent adenosinetriphosphatases with the inhibitors quercetin and calmidazolium. Biochemistry. 1987 Dec 1;26(24):8024–8030. doi: 10.1021/bi00398a070. [DOI] [PubMed] [Google Scholar]
- Fischer T. H., Campbell K. P., White G. C., 2nd Evidence that platelet and skeletal sarcoplasmic reticulum Ca2+-ATPase are structurally distinct. J Biol Chem. 1985 Jul 25;260(15):8996–9001. [PubMed] [Google Scholar]
- Javors M. A., Bowden C. L., Ross D. H. Kinetic characterization and substrate requirement for the Ca2+ uptake system in platelet membrane. Biochim Biophys Acta. 1982 Oct 7;691(2):220–226. doi: 10.1016/0005-2736(82)90410-2. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ plus K+ )-ATPase. IV. Estimation of the purity and of the molecular weight and polypeptide content per enzyme unit in preparations from the outer medulla of rabbit kidney. Biochim Biophys Acta. 1974 Jul 12;356(1):53–67. doi: 10.1016/0005-2736(74)90293-4. [DOI] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakábová M., George J. N., Lüscher E. F. Further characterization of calcium-accumulating vesicles from human blood platelets. Biochim Biophys Acta. 1978 Sep 11;512(1):1–12. doi: 10.1016/0005-2736(78)90213-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Peuch C. J., Le Peuch D. A., Katz S., Demaille J. G., Hincke M. T., Bredoux R., Enouf J., Levy-Toledano S., Caen J. Regulation of calcium accumulation and efflux from platelet vesicles. Possible role for cyclic-AMP-dependent phosphorylation and calmodulin. Biochim Biophys Acta. 1983 Jun 23;731(3):456–464. doi: 10.1016/0005-2736(83)90041-x. [DOI] [PubMed] [Google Scholar]
- Makinose M. Calcium efflux dependent formation of ATP from ADP and orthophosphate by the membranes of the sarcoplasmic vesicles. FEBS Lett. 1971 Jan 30;12(5):269–270. doi: 10.1016/0014-5793(71)80195-3. [DOI] [PubMed] [Google Scholar]
- Makinose M. Phosphoprotein formation during osmo-chemical energy conversion in the membrane of the sarcoplasmic reticulum. FEBS Lett. 1972 Sep 1;25(1):113–115. doi: 10.1016/0014-5793(72)80466-6. [DOI] [PubMed] [Google Scholar]
- Mauco G., Dajeans P., Chap H., Douste-Blazy L. Subcellular localization of inositol lipids in blood platelets as deduced from the use of labelled precursors. Biochem J. 1987 Jun 15;244(3):757–761. doi: 10.1042/bj2440757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashi S., Davis C., Crawford N. Calcium uptake associated with an intracellular membrane fraction prepared from human blood platelets by high-voltage, free-flow electrophoresis. FEBS Lett. 1982 Apr 19;140(2):298–302. doi: 10.1016/0014-5793(82)80918-6. [DOI] [PubMed] [Google Scholar]
- Rossi B., de Assis Leone F., Gache C., Lazdunski M. Pseudosubstrates of the sarcoplasmic Ca2+-ATPase as tools to study the coupling between substrate hydrolysis and Ca2+ transport. J Biol Chem. 1979 Apr 10;254(7):2302–2307. [PubMed] [Google Scholar]
- Sagara Y., Fernandez-Belda F., de Meis L., Inesi G. Characterization of the inhibition of intracellular Ca2+ transport ATPases by thapsigargin. J Biol Chem. 1992 Jun 25;267(18):12606–12613. [PubMed] [Google Scholar]
- Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989 Jan;69(1):58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Tuena De Gómez-Puyou M., Gómez-Puyou A. A simple method of purification of a soluble oligomycin-insensitive mitochondrial ATPase. Arch Biochem Biophys. 1977 Jul;182(1):82–86. doi: 10.1016/0003-9861(77)90285-5. [DOI] [PubMed] [Google Scholar]
- Wolosker H., Pacheco A. G., de Meis L. Local anesthetics induce fast Ca2+ efflux through a nonenergized state of the sarcoplasmic reticulum Ca(2+)-ATPase. J Biol Chem. 1992 Mar 25;267(9):5785–5789. [PubMed] [Google Scholar]
- Wuytack F., Papp B., Verboomen H., Raeymaekers L., Dode L., Bobe R., Enouf J., Bokkala S., Authi K. S., Casteels R. A sarco/endoplasmic reticulum Ca(2+)-ATPase 3-type Ca2+ pump is expressed in platelets, in lymphoid cells, and in mast cells. J Biol Chem. 1994 Jan 14;269(2):1410–1416. [PubMed] [Google Scholar]
- de Meis L. Approaches to studying the mechanisms of ATP synthesis in sarcoplasmic reticulum. Methods Enzymol. 1988;157:190–206. doi: 10.1016/0076-6879(88)57075-1. [DOI] [PubMed] [Google Scholar]
- de Meis L. Fast efflux of Ca2+ mediated by the sarcoplasmic reticulum Ca2(+)-ATPase. J Biol Chem. 1991 Mar 25;266(9):5736–5742. [PubMed] [Google Scholar]
- de Meis L., Inesi G. Functional evidence of a transmembrane channel within the Ca2+ transport ATPase of sarcoplasmic reticulum. FEBS Lett. 1992 Mar 24;299(1):33–35. doi: 10.1016/0014-5793(92)80093-v. [DOI] [PubMed] [Google Scholar]
- de Meis L., Suzano V. A., Inesi G. Functional interactions of catalytic site and transmembrane channel in the sarcoplasmic reticulum ATPase. J Biol Chem. 1990 Nov 5;265(31):18848–18851. [PubMed] [Google Scholar]
- de Meis L., Vianna A. L. Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]


