Abstract
Translational control plays a crucial role in the regulation of apoptosis, with the EIF4 family serving as one of the mRNA translation factors that modulate the process of mRNA translation based on mRNA characteristics. To address this potential causal role of EIF4 family proteins and breast cancer, Mendelian randomization was employed. The study incorporated four sets of genetics instrumental variables, namely EIF4E, EIF4B, EIF4A, and EIF4EBP2. The outcome variables selected for analysis were the BCAC consortium, which included estrogen receptor positive (ER+) and estrogen receptor negative (ER−) samples. To assess the potential violations of the MR assumption, the primary MR analysis employed inverse variance weighted (IVW), and several sensitivity analyses were conducted. The findings of the two-sample MR analysis indicate that EIF4E has an adverse effect on breast cancer risk (p = 0.028). However, the evidence for the relationship between EIF4E and ER status of breast cancer suggests a weak association with ER+ breast cancer (p = 0.054), but not with ER- breast cancer (p > 0.05). The study findings indicate that EIF4A is not causally linked to the risk of ER+ breast cancer, but is significantly associated with an elevated risk of ER− breast cancer (p = 0.028). However, the evidence is inadequate to support the effects of EIF4B and EIF4EBP2 on breast cancer (p > 0.05). Our results suggest that EIF4 may be a potential factor in the occurrence and development of breast cancer, which may lead to a better understanding of its causes and prevention.
Keywords: Breast cancer, EIF4, Causality, Mendelian randomization
Subject terms: Cancer, Breast cancer, Cancer screening, Tumour biomarkers, Transcription
Introduction
Breast cancer (BC) is one of the most common diseases of cancer mortality among women1. The high incidence and therapy resistance rates lead to cancer relapse and metastasis, which account for the high mortality2. Breast cancer subtypes with different clinical behaviors and molecular properties make treatment and prevention difficult3. Depending on the status of the estrogen receptor (ER), patients with BC can be classified into ER+ and ER− subtypes. 70% or more of all individuals with malignant BC are ER+ patients4. For this subtype of patients, endocrine therapy is an essential adjuvant treatment, including aromatase inhibitors (AIs), selective ER downregulators (SERDs), and selective ER modulators (SERMs)4–6. Therefore, ER + BC patients generally have a better prognosis than those with ER− tumors, whereas ER-negative tumors are insensitive to antiestrogen therapy7,8. It is now increasingly recognized that possible mechanisms underlying the rapid cellular growth, invasiveness, and drug resistance characteristics of breast cancer cells involve the deregulation of signaling pathways and protein synthesis9–11. For example, it is believed that the mTOR pathway represents the core of several signaling pathways that regulate several essential steps in breast cancer cells, among which the eukaryotic initiation factor family is one of its downstream effectors12,13.
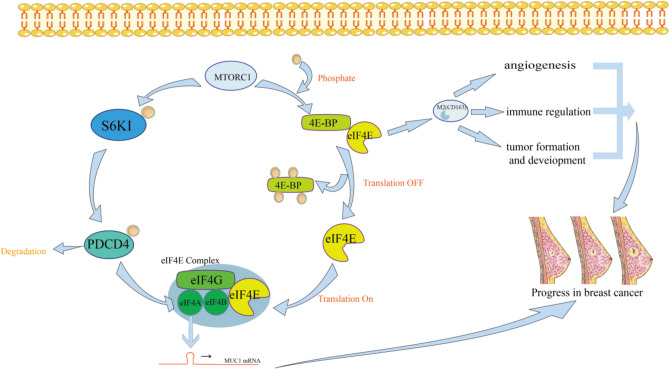
Initiation, elongation, and termination are the 3 steps of mRNA translation14,15. Translational control regulates many fundamental cellular processes in eukaryotes, such as differentiation, apoptosis, and cell multiplication. Protein initiation factor EIF4F binds to the 5′-cap of mRNA to begin eukaryotic translation (Fig. 1)16. The cap-binding protein EIF4E, the scaffolding subunit EIF4G and the RNA helicase EIF4A comprise the EIF4F complex16. EIF4E is responsible for the degree of protein synthesis and retardation. As part of the EIF4F, EIF4E attaches to mRNA's 7-methylguanosine cap17. ATPase and unwinding activities of EIF4A are stimulated by EIF4B through its 7-repeat region18. Multiple mechanisms regulate the amounts and activity of EIF4E, one of them is EIF4E-binding proteins (4E-BPs)13. The EIF4EBP protein exists in three forms: EIF4EBP1, EIF4EBP2, and EIF4EBP3. Since EIF4EBP and EIF4G have a shared binding site, 4E-BPs prevent EIF4E from binding to EIF4G by interacting with EIF4E and restrict the formation of EIF4F13,19,20. EIF4E signaling pathways are frequently aberrantly activated in breast cancer, suggesting potential therapeutic strategies21. However, it has not been reported whether the elevated levels of EIF4E signaling pathway protein play a potential causal role in breast cancer and its subtype susceptibility22.
Fig. 1.
Effects of EIF4 protein family on breast cancer cells and its pathway regulation mechanism. An activated S6K1-PDCD4 pathway promotes the unwinding of EIF4A, and the 4EBP-EIF4E pathway accelerates the release of activated EIF4E, which together promote the formation of the EIF4E complex and increase the abundance of MUC1-C oncoprotein in breast cancer. MUC1-C oncoprotein and activated EIF4E promote tumorigenesis, tumor cell metastasis, cancer cell angiogenesis and vascular invasion.
To explore the genetic association between EIF4 family proteins and breast cancer risk, we used two-sample Mendelian randomization analyses (MRs). The research used genome-wide association analysis data from the IN study of 3301 subjects and the BCAC database of 122,977 patients and 105,974 controls. Use five approaches including MR-Egger,inverse-variance weighted (IVW) regression to test the potential causal association of EIF4E, EIF4B, EIF4A, and EIF4EBP2 with breast cancer.
Methods
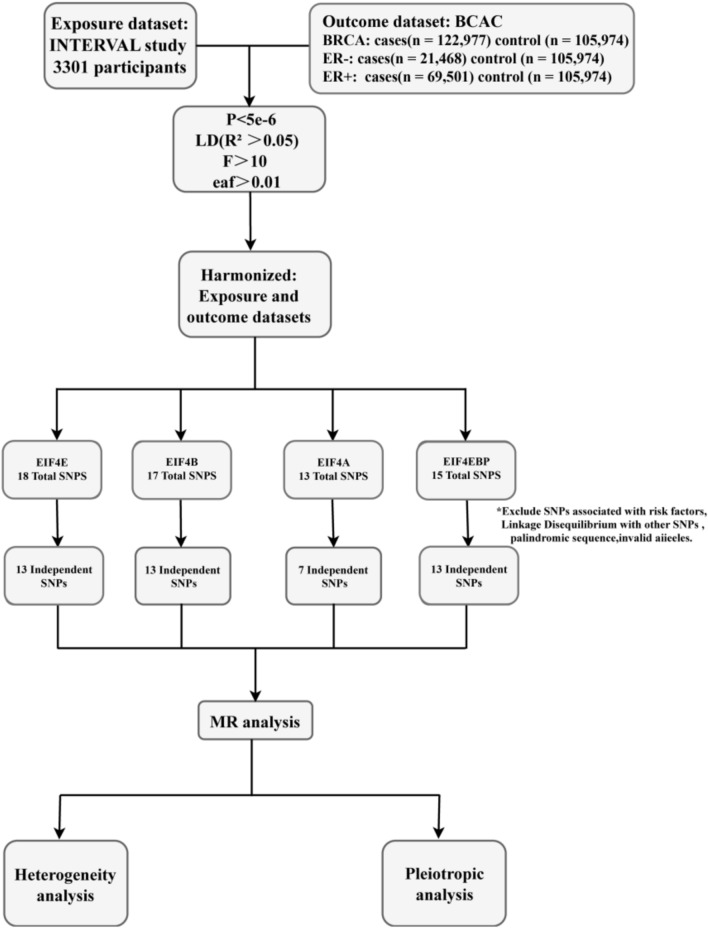
The research design of this paper is shown in Fig. 2. The study was analyzed from a publicly available database without requiring separate ethical approval.
Fig. 2.
Research methods to determine the causal relationship between EIF4 family-related proteins and breast cancer development.
Data source
Heritable data on breast carcinoma were from the Breast Cancer Association Consortium (BCAC), enrolling 122,977 patients and 105,974 controls, estimating the genotypes of approximately 21 M variants from the 1000 Genomes Project. Samples of different estrogen hormone receptor states were contained in the BCAC. These samples were composed of women of European origin. Single nucleotide polymorphisms (SNPs) for ER- breast cancer included 21,468 cases and 105,974 controls. Summary-level data for ER+ breast cancer included 69,501 cases and 105,974 controls. There was a filter for minor allele frequency (MAF) greater than 0.5% and an imputation quality score greater than 0.3 for the data23. Exposure data of EIF4E, EIF4B, EIF4A, and EIF4EBP2 were obtained from the INTERVAL study, which measured approximately 3000 plasma proteins in a genome-wide association studies (GWAS) analysis of 3301 individuals. The INTERVAL study recruited healthy blood donors of European ancestry over the age of 18 at 25 National Health Service (NHS) Blood and Transplant Centers from 2012 to 2014. The researchers used SOMAmers to assess potential off-target cross-reactivity and repeated the experiment to ensure the robustness of the protein measurements. More specific information about the study can be obtained from references to publicly available publications24.
Selection of IVs
The choice of instrumental variables (IVs) determined the reliability of this MR analysis. Valid IVs must satisfy three fundamental assumptions: (i) IVs were not associated with confounders; (ii) IVs are correlated with exposure factors; (iii) IVs are not associated with outcome variables; IVs can only be associated with outcome variables through exposure factors. At the same time, IVs should also exclude linkage disequilibrium (LD), pleiotropy, heterogeneity, and population stratification25. This research screened IVs by satisfying the following statistical standards: (i) a GWAS-correlated P-value of 5 × 10−6, (ii) a linkage disequilibrium r2 of > 0.05, and F statistic for each SNP > 10. LD between SNPs was calculated from European individuals from the 1000 Genomes Project. Proxy SNPs could be found if IVs were unavailable (https://snipa.helmholtz-muenchen.de/snipa3/). The condition of the F-statistic that the included Single nucleotide polymorphisms (SNPs) were powerful tools. We further excluded SNPs with allele frequencies less than 0.01. The research utilized the PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk) website to manually screen IVs for potential confounders.
Statistical analyses
We used R (version 0.5.6) software for two-sample MR analyses. First, to make sure that each SNP's alleles were consistent between causes and effects, we harmonized the statistics for SNP-cause and SNP-effect. Subsequently, several statistical methods were combined to explore the connections between causes and effects. A p-value less than 0.05 indicates a strong level of correlation. We primarily used the inverse variance weighting (IVW) method for causality analysis. When polytomous, heterogeneous and weak instrumental variables were not present, the analyses were conducted with IVW as the main decision criterion26,27. IVW is categorized into a random effects model and a fixed effects model. If p > 0.05 showed little heterogeneity, a fixed-effect model was advised to be employed for the following analyses. Otherwise, the random-effect model was applied. Since IVW requires all IVs to be valid to ensure the consistency of the results, we also used MR-egger analysis and weighted median analysis. By using the regression slope and intercept, the MR-Egger regression model produced a result that corrected for the presence of horizontal pleiotropy and offered a reasonably reliable estimate independent of IV validity. The weighted median analysis provided consistent causality estimates with 50% of randomly selected IVs. In addition, Simple Mode and weighted Mode were used to supplement the analysis of the two samples. These two analysis methods provide less results bias and lower type I errors.
Sensitivity analyses
In this work, various methods for sensitivity analysis were conducted. An initial assessment of IV heterogeneity was conducted using Cochrane's Q-statistic. To visualize pleiotropy directly, funnel plots and forest plots were built. Second, we examined the directional pleiotropy of IVs using the MR-Egger intercept approach31. If the p-value < 0.05, the IVW estimate in the MR-Egger test might be biased. Intercepts were calculated based on the average horizontal pleiotropic effect across all SNPs. In contrast to the IVW technique, other methods provided broad confidence intervals (CI)32 and were only used as supplementary techniques. High pleiotropy was evaluated by applying the MR-Egger Regression Model, and final outliers were identified by MR-PRESSO. Otherwise, IVW results took priority. Third, to determine if a single SNP was responsible for the outcomes, we performed a leave-one-out sensitivity test.
Results
Characteristics of exposures and outcomes
We retrieved IVs that were related to breast cancer (P < 5 × 10−6) and excluded LD (r2 < 0.05, 10,000-kb) from the GWAS. The chosen SNPs' F values were higher than the usual cutoff point of 10, suggesting that they would reduce the bias of causal analysis. In the MR study, a total of 12 SNPs (EIF4E: rs62143198, rs527838288, rs11084300; EIF4B: rs62143197, rs148800371; EIF4A: rs34436714, rs11084300, rs3859507, rs79549584, rs6792693; EIF4EBP2: rs10733789, rs6993770) were excluded for removing the influence of other confounders (breast cancer, immunity, ect). All remaining IVs were aligned to the allelic direction of exposure and outcome. In addition, they were correlated with the exposure factors but not the outcome variables. The final study included 46 instrumental variables (EIF4E: 13 SNPs; EIF4B: 13 SNPs; EIF4A: 7 SNPs; EIF4EBP2: 13 SNPs) for analysis for breast cancer and different ER subtypes (Supplementary Tables 1–4).
Two-sample MR analysis
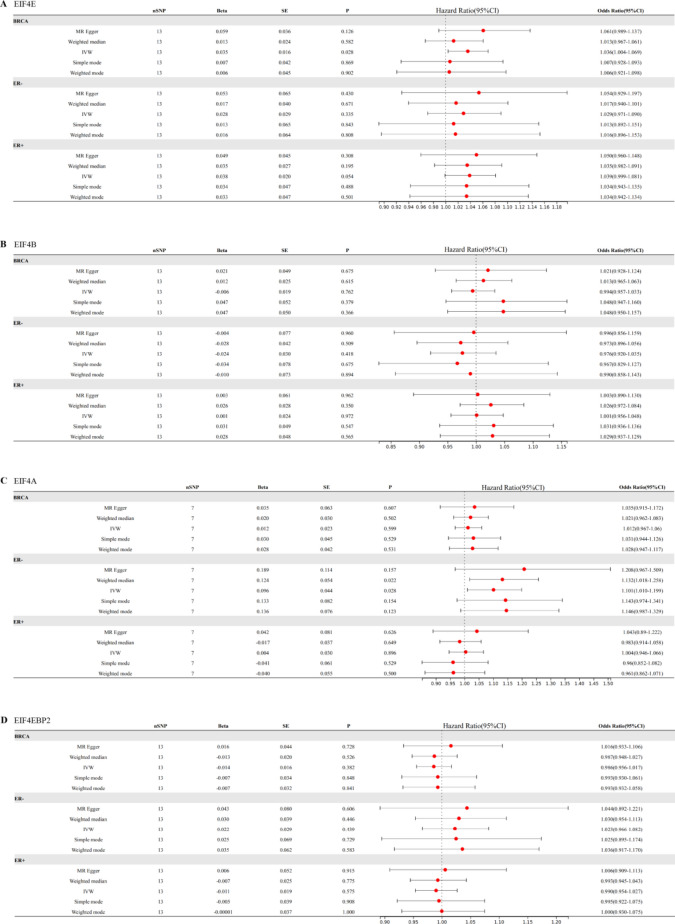
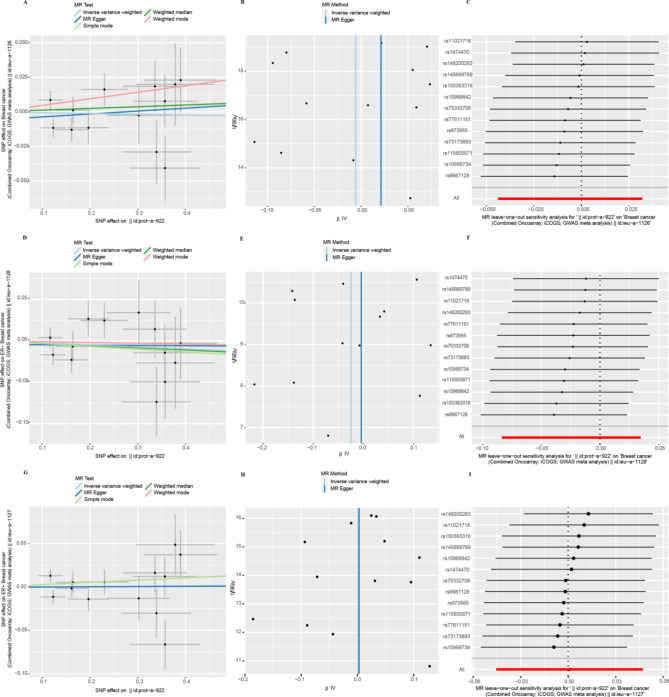
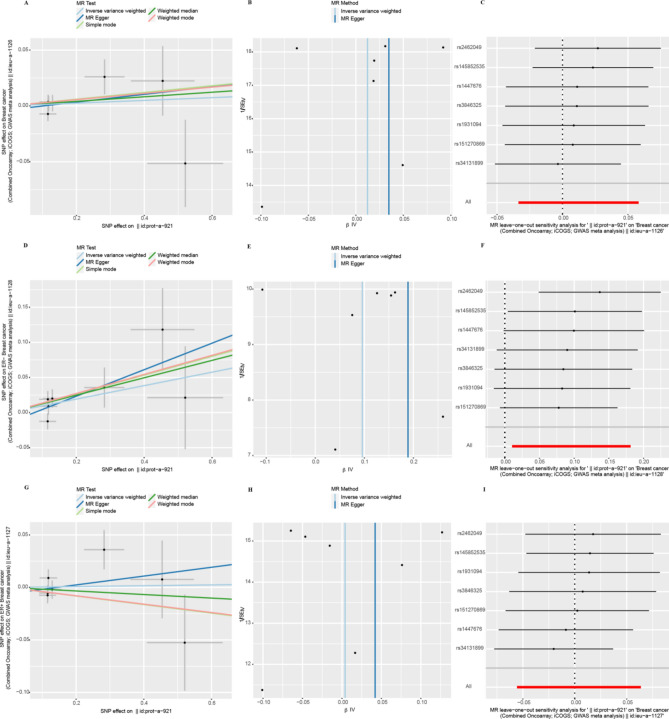
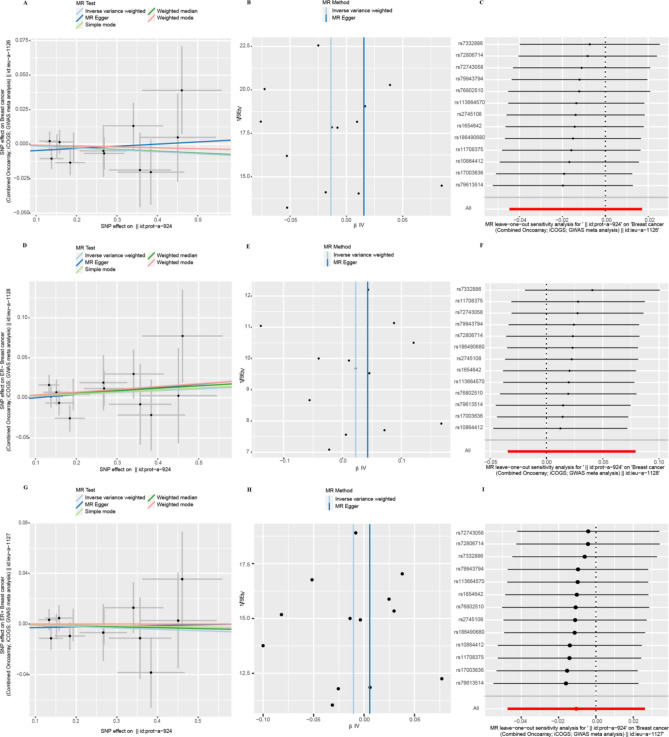
IVW results suggested a direct potential causal relationship between EIF4E and breast cancer (OR 1.04, 95% CI 1.00–1.07, p = 0.028). There was a weak association between EIF4E and ER+ patients (OR 1.04, 95% CI 1.00–1,08, p = 0.054), but not ER− patients (OR 1.03, 95% CI 0.97–1.09, p = 0.34). Results from other methods were in the same direction (Fig. 3A). However, the findings didn't indicate a direct connection between EIF4B and breast cancer (OR 0.99, 95% CI 0.96–1.03, p = 0.76). As an outcome variable, the MR results showed consistent between ER+ patients (OR 1.00, 95% CI 0.96–1.05, p = 0.97) and ER- patients (OR 0.98, 95% CI 0.94–1.04, p = 0.42). The results are shown in Fig. 3B. According to MR study of IVs, EIF4A, and breast cancer were not directly related (OR 1.01, 95% CI 0.97–1.06, p = 0.60). Results consistent with overall breast cancer were found in ER+ patients (OR 1.00, 95% CI 0.95–1.07, p = 0.90). However, we revealed a strong relationship between EIF4A and ER− patients (OR 1.10, 95% CI 1.01–1.20, p = 0.028) (Fig. 3C). We found that EIF4EBP2 didn't contribute to the development of breast cancer (OR 0.99, 95% CI 0.96–1.02, p = 0.38). And there was also no direct association between EIF4EBP2 and the outcome of ER status for breast cancer (ER−: OR 1.02, 95% CI 0.97–1.08, p = 0.44; ER+: OR 0.99, 95% CI 0.95–1.03, p = 0.57) (Fig. 3D).
Fig. 3.
Two-sample Mendelian randomization estimations showing the effect of EIF4E, EIF4B, EIF4A and EIF4EBP on the risk of breast cancer and different status of ER.
Sensitivity analyses
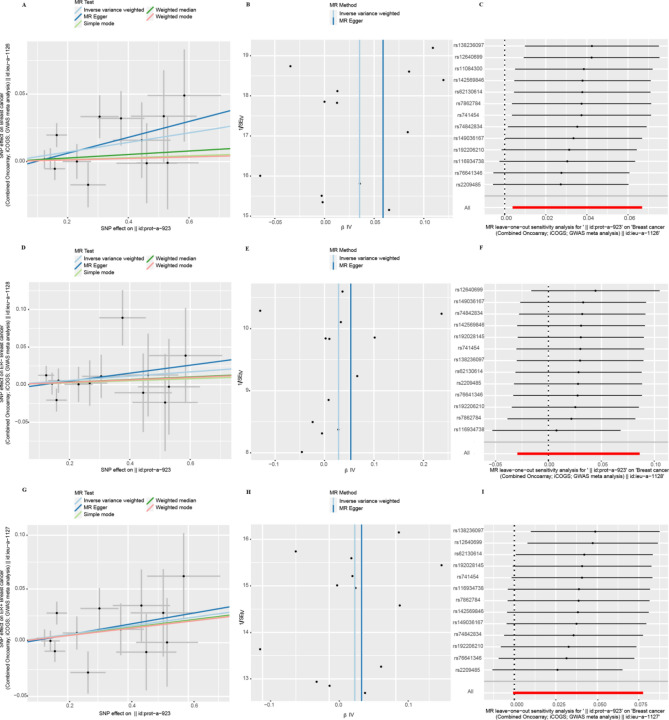
The heterogeneity test showed that the p-values of Cochran's Q statistics were all greater than 0.05, which indicated that there was no heterogeneity among these SNPs (Table 1). As a result, this MR study was primarily analyzed using the IVW approach. According to MR-Egger regression intercepts, the IVs of different causes with breast cancer showed only weak evidence of pleiotropy (Table 1). Additionally, the leave-one-out test indicated that the potential causal relationship between breast cancer risk and different causes did not depend on a single SNP. The scatter plots and the forest plots are shown in Figs. 4, 5, 6 and 7.
Table 1.
The estimations of heterogeneity and horizontal pleiotropy for MR results.
| Exposure | Outcome | Number of IVs | Pleiotropy test | Heterogeneity test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MR-Egger | MR-Egger | Inverse-variance weighted | |||||||||
| Intercept | SE | p | Q | Q_df | Q_pval | Q | Q_df | Q_pval | |||
| EIF4E | BRCA | 13 | − 5.65E−03 | 0.01 | 0.47 | 11.26 | 11 | 0.42 | 11.83 | 12 | 0.46 |
| ER− | − 5.89E−03 | 0.01 | 0.68 | 8.44 | 11 | 0.67 | 8.63 | 12 | 0.73 | ||
| ER+ | − 2.44E−03 | 0.01 | 0.81 | 13.04 | 11 | 0.29 | 13.11 | 12 | 0.36 | ||
| EIF4B | BRCA | 13 | − 5.76E−03 | 0.01 | 0.56 | 16.08 | 11 | 0.14 | 16.61 | 12 | 0.16 |
| ER− | − 4.36E−03 | 0.02 | 0.78 | 11.90 | 11 | 0.37 | 11.99 | 12 | 0.45 | ||
| ER+ | − 4.62E−04 | 0.01 | 0.97 | 17.27 | 11 | 0.10 | 17.27 | 12 | 0.14 | ||
| EIF4A | BRCA | 7 | − 3.55E−03 | 0.01 | 0.72 | 6.35 | 5 | 0.27 | 6.53 | 6 | 0.37 |
| ER− | − 1.45E−02 | 0.02 | 0.41 | 5.86 | 5 | 0.32 | 6.80 | 6 | 0.34 | ||
| ER+ | − 6.03E−03 | 0.01 | 0.63 | 7.40 | 5 | 0.19 | 7.79 | 6 | 0.25 | ||
| EIF4EBP2 | BRCA | 13 | − 6.20E−03 | 0.01 | 0.49 | 6.70 | 11 | 0.82 | 7.21 | 12 | 0.84 |
| ER− | − 4.27E−03 | 0.02 | 0.79 | 7.25 | 11 | 0.78 | 7.33 | 12 | 0.84 | ||
| ER+ | − 3.42E−03 | 0.01 | 0.74 | 5.71 | 11 | 0.89 | 5.82 | 12 | 0.92 | ||
Fig. 4.
Heterogeneity and horizontal pleiotropy testing of the effect of EIF4E on breast cancer and its subtypes (ER−, ER+) risk. (A) Scatter plot showing estimates of the causal effect of EIF4E on breast cancer. The slopes of the lines correspond to causal assessments using different methods. (B) Funnel plots showed estimates of EIF4E for breast cancer, using each SNP as a tool, the inverse of the standard error of the causal estimate. Vertical lines show the results of IVW or MR Egger methods using all SNPs. (C) Leave-one-out analysis. Determine if any single SNP leads to a direct causal relationship between EIF4E and breast cancer. The horizontal line indicates the 95% CI. (D) Scatter plot, (E) funnel plot, and (F) leave-one-out analysis from genetically predicted the effect of EIF4E on ER- breast cancer. (G) Scatter plot, (H) funnel plot, and (I) leave-one-out analysis from genetically predicted the effect of EIF4E on ER+ breast cancer.
Fig. 5.
Heterogeneity and horizontal pleiotropy testing of the effect of EIF4B on breast cancer and its subtypes (ER−, ER+) risk. (A) Scatter plot, (B) funnel plot, and (C) leave-one-out analysis from genetically predicted the effect of EIF4B on breast cancer. (D) Scatter plot, (E) funnel plot, and (F) leave-one-out analysis from genetically predicted the effect of EIF4B on ER− breast cancer. (G) Scatter plot, (H) funnel plot, and (I) leave-one-out analysis from genetically predicted the effect of EIF4B on ER+ breast cancer.
Fig. 6.
Heterogeneity and horizontal pleiotropy testing of the effect of EIF4A on breast cancer and its subtypes (ER−, ER+) risk. (A) Scatter plot, (B) funnel plot, and (C) leave-one-out analysis from genetically predicted the effect of EIF4A on breast cancer. (D) Scatter plot, (E) funnel plot, and (F) leave-one-out analysis from genetically predicted the effect of EIF4A on ER− breast cancer. (G) Scatter plot, (H) funnel plot, and (I) leave-one-out analysis from genetically predicted the effect of EIF4A on ER+ breast cancer.
Fig. 7.
Heterogeneity and horizontal pleiotropy testing of the effect of EIF4BP2 on breast cancer and its subtypes (ER−, ER+) risk. (A) Scatter plot, (B) funnel plot, and (C) leave-one-out analysis from genetically predicted the effect of EIF4BP2 on breast cancer. (D) Scatter plot, (E) funnel plot, and (F) leave-one-out analysis from genetically predicted the effect of EIF4BP2 on ER− breast cancer. (G) Scatter plot, (H) funnel plot, and (I) leave-one-out analysis from genetically predicted the effect of EIF4BP2 on ER+ breast cancer.
Discussion
This is the first study to leverage MR to determine the potential causal association of EIF4E, EIF4B, EIF4A, and EIF4EBP2 circulating levels with breast cancer in humans. The data suggest that increased circulating levels of EIF4E can increase the risk of breast cancer and that patients with ER+ breast cancer can be minimally affected. Furthermore, high levels of EIF4A are associated with an increased risk of ER− breast cancer. However, we did not find evidence that EIF4A levels directly influence breast cancer incidence or the ER+ subtype. EIF4B and EIF4EBP2 circulating levels were not related to breast cancer risk.
As a restriction factor involved in translation initiation, EIF4E promotes the recruitment of ribosomal submission to control the translation process. And given that EIF4E is the regulatory intersection of multiple cancer-related pathways, such as PI3K/AKT, mTOR, and Ras/MAPK/Mnk, its overactivity was an important hallmark of many cancers33,34. The study from Fan Li et al. revealed that the high expression of EIF4E may cause poor prognosis in cancer patients by affecting immune cells such as macrophages in breast cancer tissue22. In addition, several studies have proved that EIF4E also promotes metastasis of tumor cells, cancer cell angiogenesis, and vascular invasion35,36. These clinical and mechanistic studies are consistent with our findings that EIF4E has a direct potential causal relationship with breast cancer pathogenesis and disease progression. Given the notion that EIF4E is one of the intersections of major signaling pathways associated with cancer, there have been a lot of studies to understand how targeted EIF4E can treat breast cancer36. There are both direct and indirect approaches to target EIF4E. One direct approach involves inhibiting the translation of EIF4E mRNA using specific antisense oligonucleotides (ASOs) or 7-benzyl guanosine monophosphate (Bn7GMP)13. Indirect strategies, on the other hand, involve targeting the EIF4E phosphorylation pathway or inhibiting the binding of EIF4E to other components using inhibitors such as 4EGI-1 and 4E1RCat13. Inhibitors of the mTOR pathway such as everolimus have also been used to indirectly target EIF4E in breast cancer37.
EIF4EBP is generally regarded as a translational repressor that is regulated by phosphorylation, with hypophosphorylated EIF4EBP able to bind to and inhibit EIF4E13,20. The rapamycin (mTOR)-4EBP1 pathway is one of the major signaling pathways that regulates the initiation of mammalian processes38. In the course of cancer development, activation of mTORC1 hyperphosphorylates 4EBP, releasing active EIF4E and stimulating transcription in cancer39. Several researches have confirmed that 4EBP can be used as a predictive marker during breast cancer treatment40. Analysis of the TCGA database also reveals a significant association between decreased expression of 4EBP1 and an unfavorable survival outcome38. Due to the lack of GWAS data for EIF4EBP, EIF4EBP1, and EIF4EBP3, only EIF4EBP2 was included in our study. Our investigation has revealed that EIF4EBP2 does not exert a direct effect on breast cancer. This observation may be attributed to the fact that, as a member of the 4EBP family of proteins, EIF4EBP2 may not be fully representative of the entire EIF4EBP protein family, thereby limiting its impact on breast cancer.
Eukaryotic initiation factor 4A (EIF4A) is a DEAD-box RNA helicase, which is characterized by high conservation41. It has been demonstrated that EIF4A activity is downregulated in breast cancer xenograft models, accelerating cell death and reducing angiogenesis42. EIF4B can stimulate the RNA helicase activity of EIF4A18. Nevertheless, Our results indicate that EIF4A does not have a direct association with breast cancer, likely because of the complex subtypes of the disease. However, we found an association between EIF4A and ER- breast cancer, which is consistent with some previous studies. It has been found that the independent predictors of adverse prognosis of ER-negative diseases include EIF4A1, EIF4B, and EIF4E42. The results regarding EIF4B and EIF4E are inconsistent with our study, but fewer studies have examined the association between EIF4B and breast cancer, which deserves deeper investigation. In addition, several studies in recent years have focused on the therapeutic strategy of targeting EIF4A in breast cancer, including the study by Cencic et al., which also suggested that because EIF4A is overexpressed in human ER-negative breast cancers, EIF4A is an attractive therapeutic target for the treatment of TNBC and validated the orally bioavailable rocaglate based molecule, MG-002, was shown to be effective in inhibiting mRNA translation and primary TNBC tumor growth by targeting and inhibiting EIF4A activity43. Na Zhao found that the protein level of EIF4A1 was higher in TNBC than in non-TNBC and verified that targeting EIF4A triggered an interferon response that synergized with chemotherapy and inhibited triple-negative breast cancer44. Recent studies have focused on the crucial role of targeting EIF4A in TNBC, which deserves further investigation.
The strength of our study is that MR studies can effectively avoid confounding biases in the random assignment of SNPs at conception. MR can also prevent the reverse causal effect compared to other observational studies. Establishing a potential causal link between EIF4E and breast cancer has helped us better understand that EIF4E signaling would enhance the consideration of EIF4E as a target for developing new therapies.
Although in contrast to other observational studies, the MR study we conducted was effective in avoiding confounding bias in randomly assigned SNPs at conception and preventing reverse causal effects. The study still has many limitations. Firstly, our research specifically examined the genetic determinants of EIF4 families and did not explore other variables or potential interactions with additional risk factors for breast cancer. Secondly, all GWAS data are derived from European populations and the population is not representative. Thirdly, only EIF4EBP2 was studied, it cannot represent EIF4EBP.
Supplementary Information
Author contributions
J.S.: Conceptualization, writing-reviewing and editing. R.W.: Methodology, data curation. J.C.: Writing-original draft preparation. Y.F.: Investigation. Y.Z.: Data curation. S.H.: Visualization. Y.X.: Software. J.W.: Supervision, writing-reviewing and editing. Y.Z.: Supervision, writing-reviewing and editing.
Funding
This work was funded by the Science and Technology Commission of Shanxi province (Grant number 201901D111428).
Data availability
The datasets supporting the conclusions of this article are available in the IEU open gwas project repository (https://gwas.mrcieu.ac.uk/).
Competing interests
The authors declare no competing interests.
Ethics statement
As this study solely utilizes secondary data from published studies and contains no patient-identifiable information, ethics approval was not deemed necessary.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jin-Yu Shi and Rui Wen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71059-1.
References
- 1.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021). 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Conde, I., Ribeiro, A. S. & Paredes, J. Breast cancer stem cell membrane biomarkers: Therapy targeting and clinical implications. Cells.11(6), 934 (2022). 10.3390/cells11060934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waks, A. G. & Winer, E. P. Breast cancer treatment: A review. JAMA.321(3), 288–300 (2019). 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- 4.Nunnery, S. E. & Mayer, I. A. Targeting the PI3K/AKT/mTOR pathway in hormone-positive breast cancer. Drugs.80(16), 1685–1697 (2020). 10.1007/s40265-020-01394-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbeck, N. & Gnant, M. Breast cancer. Lancet.389(10074), 1134–1150 (2017). 10.1016/S0140-6736(16)31891-8 [DOI] [PubMed] [Google Scholar]
- 6.Hanker, A. B., Sudhan, D. R. & Arteaga, C. L. Overcoming endocrine resistance in breast cancer. Cancer Cell.37(4), 496–513 (2020). 10.1016/j.ccell.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng, Y. et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis.5(2), 77–106 (2018). 10.1016/j.gendis.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segovia-Mendoza, M. & Morales-Montor, J. Immune tumor microenvironment in breast cancer and the participation of estrogen and its receptors in cancer physiopathology. Front. Immunol.10, 348 (2019). 10.3389/fimmu.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naeem, M. et al. A review of twenty years of research on the regulation of signaling pathways by natural products in breast cancer. Molecules.27(11), 3412 (2022). 10.3390/molecules27113412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sannino, S. & Brodsky, J. L. Targeting protein quality control pathways in breast cancer. BMC Biol.15(1), 109 (2017). 10.1186/s12915-017-0449-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong, C. et al. Phosphorylation independent eIF4E translational reprogramming of selective mRNAs determines tamoxifen resistance in breast cancer. Oncogene.39(15), 3206–3217 (2020). 10.1038/s41388-020-1210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell.168(6), 960–976 (2017). 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romagnoli, A. et al. Control of the eIF4E activity: Structural insights and pharmacological implications. Cell Mol. Life Sci.78(21–22), 6869–6885 (2021). 10.1007/s00018-021-03938-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubio, A., Garland, G. D., Sfakianos, A., Harvey, R. F. & Willis, A. E. Aberrant protein synthesis and cancer development: The role of canonical eukaryotic initiation, elongation and termination factors in tumorigenesis. Semin. Cancer Biol.86(Pt 3), 151–165 (2022). 10.1016/j.semcancer.2022.04.006 [DOI] [PubMed] [Google Scholar]
- 15.Zhang, L. et al. Translational regulation by eIFs and RNA modifications in cancer. Genes (Basel).13(11), 2050 (2022). 10.3390/genes13112050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genuth, N. R. & Barna, M. Heterogeneity and specialized functions of translation machinery: From genes to organisms. Nat. Rev. Genet.19(7), 431–452 (2018). 10.1038/s41576-018-0008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batool, A., Aashaq, S. & Andrabi, K. I. Eukaryotic initiation factor 4E (eIF4E): A recap of the cap-binding protein. J. Cell Biochem.120(9), 14201–14212 (2019). 10.1002/jcb.28851 [DOI] [PubMed] [Google Scholar]
- 18.Andreou, A. Z., Harms, U. & Klostermeier, D. eIF4B stimulates eIF4A ATPase and unwinding activities by direct interaction through its 7-repeats region. RNA Biol.14(1), 113–123 (2017). 10.1080/15476286.2016.1259782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, L. J. et al. Cancer plasticity: The role of mRNA translation. Trends Cancer.7(2), 134–145 (2021). 10.1016/j.trecan.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruner, S. et al. Structural motifs in eIF4G and 4E-BPs modulate their binding to eIF4E to regulate translation initiation in yeast. Nucleic Acids Res.46(13), 6893–6908 (2018). 10.1093/nar/gky542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu, Y. et al. Galeterone sensitizes breast cancer to chemotherapy via targeting MNK/eIF4E and beta-catenin. Cancer Chemother. Pharmacol.87(1), 85–93 (2021). 10.1007/s00280-020-04195-w [DOI] [PubMed] [Google Scholar]
- 22.Li, F. et al. High expression of eIF4E is associated with tumor macrophage infiltration and leads to poor prognosis in breast cancer. BMC Cancer.21(1), 1305 (2021). 10.1186/s12885-021-09010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michailidou, K. et al. Association analysis identifies 65 new breast cancer risk loci. Nature.551(7678), 92–94 (2017). 10.1038/nature24284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun, B. B. et al. Genomic atlas of the human plasma proteome. Nature.558(7708), 73–79 (2018). 10.1038/s41586-018-0175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess, S., Foley, C. N., Allara, E., Staley, J. R. & Howson, J. M. M. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat. Commun.11(1), 376 (2020). 10.1038/s41467-019-14156-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sproviero, W. et al. High blood pressure and risk of dementia: A two-sample Mendelian randomization study in the UK biobank. Biol. Psychiatry.89(8), 817–824 (2021). 10.1016/j.biopsych.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 27.Lin, Z., Deng, Y. & Pan, W. Combining the strengths of inverse-variance weighting and Egger regression in Mendelian randomization using a mixture of regressions model. PLoS Genet.17(11), e1009922 (2021). 10.1371/journal.pgen.1009922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden, J., Hemani, G. & Davey, S. G. Invited commentary: Detecting individual and global horizontal pleiotropy in mendelian randomization-a job for the humble heterogeneity statistic?. Am. J. Epidemiol.187(12), 2681–2685 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol.32(5), 377–389 (2017). 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol.40(4), 304–314 (2016). 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol.44(2), 512–525 (2015). 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slob, E. A. W. & Burgess, S. A comparison of robust Mendelian randomization methods using summary data. Genet. Epidemiol.44(4), 313–329 (2020). 10.1002/gepi.22295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romagnoli, A., Maracci, C., D’Agostino, M., Teana, A. & Marino, D. D. Targeting mTOR and eIF4E: A feasible scenario in ovarian cancer therapy. Cancer Drug Resist.4(3), 596–606 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui, N. & Sonenberg, N. Signalling to eIF4E in cancer. Biochem. Soc. Trans.43(5), 763–772 (2015). 10.1042/BST20150126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo, Q. et al. The MNK1/2-eIF4E axis supports immune suppression and metastasis in postpartum breast cancer. Cancer Res.81(14), 3876–3889 (2021). 10.1158/0008-5472.CAN-20-3143 [DOI] [PubMed] [Google Scholar]
- 36.Yang, X., Zhong, W. & Cao, R. Phosphorylation of the mRNA cap-binding protein eIF4E and cancer. Cell Signal.73, 109689 (2020). 10.1016/j.cellsig.2020.109689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maracci, C. et al. The mTOR/4E-BP1/eIF4E signalling pathway as a source of cancer drug targets. Curr. Med. Chem.29(20), 3501–3529 (2022). 10.2174/0929867329666220224112042 [DOI] [PubMed] [Google Scholar]
- 38.Sunavala-Dossabhoy, G. Disorder at the start: The contribution of dysregulated translation initiation to cancer therapy resistance. Front. Oral. Health.2, 765931 (2021). 10.3389/froh.2021.765931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, M., Lu, Y., Piao, W. & Jin, H. The translational regulation in mTOR pathway. Biomolecules.12(6), 802 (2022). 10.3390/biom12060802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darb-Esfahani, S. et al. Phospho-mTOR and phospho-4EBP1 in endometrial adenocarcinoma: Association with stage and grade in vivo and link with response to rapamycin treatment in vitro. J. Cancer Res. Clin. Oncol.135(7), 933–941 (2009). 10.1007/s00432-008-0529-5 [DOI] [PubMed] [Google Scholar]
- 41.Jia, X. & Zhou, H. Small-molecule inhibitors targeting eIF4A in leukemia. Curr. Protein Pept. Sci.22(7), 559–566 (2021). 10.2174/1389203722666210526155808 [DOI] [PubMed] [Google Scholar]
- 42.Modelska, A. et al. The malignant phenotype in breast cancer is driven by eIF4A1-mediated changes in the translational landscape. Cell Death Dis.6(1), e1603 (2015). 10.1038/cddis.2014.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cencic, R. et al. A second-generation eIF4A RNA helicase inhibitor exploits translational reprogramming as a vulnerability in triple-negative breast cancer. Proc. Natl. Acad. Sci. U. S. A.121(4), e2318093121 (2024). 10.1073/pnas.2318093121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, N. et al. Targeting eIF4A triggers an interferon response to synergize with chemotherapy and suppress triple-negative breast cancer. J. Clin. Investig.133(24), e172503 (2023). 10.1172/JCI172503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are available in the IEU open gwas project repository (https://gwas.mrcieu.ac.uk/).