Abstract
It is not clear whether different radiation methods have different effects on enamel. The purpose of this study was to compare the effects of single and fractionated radiation on enamel and caries susceptibility and to provide an experimental basis for further study of radiation‑related caries. Thirty-six caries-free human third molars were collected and randomly divided into three groups (n = 12). Group1 (control group) was not exposed to radiation. Group 2 received single radiation with a cumulative dose of 70 Gy. Group 3 underwent fractionated radiation, receiving 2 Gy/day for 5 days followed by a 2-day rest period, for a total of 7 weeks with a cumulative dose of 70 Gy. Changes in microhardness, roughness, surface morphology, bacterial adhesion and ability of acid resistance of each group were tested. Scanning electron microscope revealed that the enamel surface in both radiation groups exhibited unevenness and cracks. Compared with the control group, microhardness and acid resistance of enamel decreased, while roughness and bacterial adhesion increased in both the single radiation and fractionated radiation groups. Compared with the single radiation group, the enamel surface microhardness and acid resistance decreased in the fractionated radiation group, while roughness and bacterial adhesion increased. Both single radiation and fractionated radiation resulting in changes in the physical and biological properties of enamel, with these changes being more pronounced in the fractionated radiation group. Therefore, fractionated radiation is recommended as a more suitable method for constructing a radiation‑related caries model in vitro.
Keywords: Radiation‑related caries, Caries susceptibility, Radiotherapy, Head and neck cancer
Subject terms: Oral cancer, Dental conditions, Dental radiology, Dental public health
Introduction
Cancer ranks among the leading causes of death worldwide, second only to heart disease1. Among them, head and neck cancer (HNC) stands as the seventh most common cancer globally2,3, with approximately 900,000 new cases and over 480,000 deaths reported in 2022 (source: https://gco.iarc.fr/today/en). Furthermore, both its incidence and mortality rates are steadily rising, with a projected 30% increase by 2030, and HPV-associated oral cancers continue to rise at a rate of 2% per year1,4. Squamous cell carcinoma accounts for 90% of cases of head and neck cancer, profoundly impacting patients’ quality of life5. Risk factors include smoking, alcohol consumption, betel chewing, and human papillomavirus infection2,5. Currently, although numerous new therapeutic strategies exist, the primary treatment for head and neck cancer generally involves multi-disciplinary team (MDT) therapy, which encompasses surgery, radiotherapy (RT), and chemotherapy6,7. Despite advancements in treatment modalities, the global 5-year survival rate for HNC averages at 50%2,8,9. Generally, early-stage HNSCC can be effectively managed through surgical intervention or radiotherapy, with 5-year survival rates ranging from 70 to 90%10.
It is evident that radiotherapy plays an indispensable role in the treatment of head and neck cancer. Although the concept of precision radiation therapy has emerged in recent years11, and intensity-modulated radiation therapy (IMRT) can mitigate the risk of radiation exposure to surrounding tissues to some extent12, radiotherapy can still lead to various complications in patients. These complications include, but are not limited to, oral symptoms such as dry mouth, caries, jaw damage, hearing impairment, and vision impairment13. Oral complications arising from radiotherapy for head and neck cancer have garnered significant attention due to their profound impact on patients’ daily lives, ranging from reduced salivary secretion in the early stages to the late-stage development of radiation-related caries (RRC)13–15.
Radiation-related caries is well-documented as one of the most common complications, affecting approximately 29–37% of post-radiotherapy head and neck cancer patients, with its incidence tending to increase with the dose of radiotherapy16–18. RRC manifests as a serious, rapid, and progressive destruction of the hard tissue of the tooth following radiation therapy. In comparison to conventional caries, it exhibits characteristics of rapid progression, wide accumulation range, and concentration in the tooth cervical areas and the dental cusp19. Furthermore, the development of radiation-related caries poses challenges in clinical treatment due to patients’ diminished physical condition post-radiotherapy, reduced oral saliva, and the high cost of treatment20. Moreover, research on RRC is hindered by the difficulty of obtaining dental specimens and the lack of an in vitro model. Therefore, the objective of this study is to assess the effects of different radiation approaches on enamel structure and caries susceptibility, aiming to establish a reference model of RRC in vitro. This may serve as a foundation for further research on the prevention and management of RRC.
Methods
Tooth collection and sample preparation
The extracted tooth samples were obtained from third molars scheduled for removal for preventive reasons at the School and Hospital of Stomatology, Fujian Medical University. Patients aged between 18 and 25 years provided informed consent for the use of their extracted teeth. Exclusion criteria comprised teeth with immature roots, caries, those subjected to root canal treatment, restorations and visible chalky demineralization of enamel, and cracks observed under a stereomicroscope (10×) (ZEISS; Stemi508; Carl Zeiss AG; Oberkochen; GER).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study received approval from the local Ethics Committee (Ethics Committee of the School and Hospital of Stomatology, Fujian Medical University, approval number 2021-FJMUSS-062). Also, written informed consent was obtained from participants.
A total of 36 teeth were collected and cleaned on their outer surfaces before being immersed in normal saline (0.9% NaCl; Kelun Co. Ltd.; Chengdu, China) and stored in a refrigerator at 4 °C, for no longer than 1 month post-extraction. The samples were randomly allocated into three groups (n = 12): Group 1 (control group), Group 2 (single radiation 70 Gy group), and Group 3 (fractionated radiation 70 Gy group).
Sample size calculation
The sample size for the study was calculated using G*Power 3.1.9.7 (University of Düsseldorf, GER), based on an a priori analysis of variance (ANOVA) for fixed effects, omnibus, one-way design. The calculation aimed to detect a large effect size (f = 0.65) with an alpha level of 0.05 and a power of 85%. This effect size was derived from previous studies21, which quantified the impact of radiation on dentin microhardness, indicating significant decreases in microhardness compared to control. The analysis indicated that a total sample size of 30 was required to achieve these parameters, assuming equal distribution across the three groups. Considering potential sample loss and to ensure a robust analysis, a total of 36 samples (12 per group) were ultimately collected.
Radiation model of isolated teeth
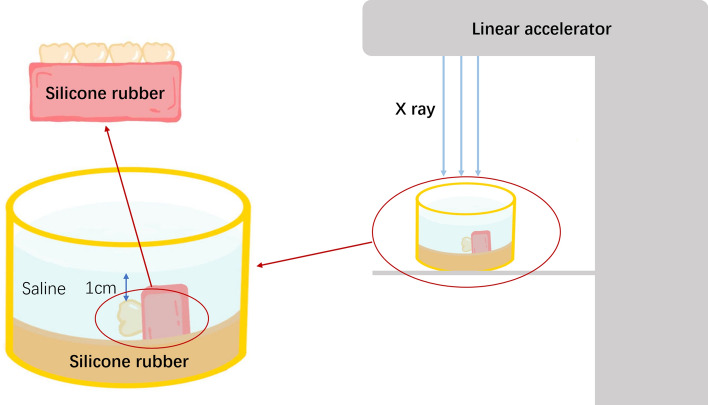
Following fixation of the isolated tooth with silicone rubber (3M Deutschlan GmbH; MDSA-V02; Neuss; GER), the buccal side of the tooth was positioned upward, and normal saline was applied to soak approximately 1 cm above the tooth surface to simulate the moist oral environment (shown in Fig. 1). The tooth samples were directly exposed to radiation using a linear accelerator (Varian Clinac 23EX; Clina; California; USA) to replicate clinical therapy for head-and-neck cancer patients.
Fig. 1.
Schematic diagram of radiation mode of isolated teeth.
Radiation approaches: In the single radiation group, a radiation dose of 70 Gy was administered. In the fractionated radiation group, a dose of 2 Gy/day was continuously delivered for 5 days, followed by a 2-day rest period, for a total of 7 weeks. The cumulative radiation dose reached 70 Gy.
Scanning electron microscopy (SEM)
Teeth were sectioned with a water cooled diamond disc (Buehler; IsoMet low speed; Buehler An ITW Company; Chicago; USA) to obtain specimens sized 4 mm × 3 mm × 2 mm. Following ultrasonic cleaning (Supmile; KQ-500DE; Kunshan Jiang, China) for 20 min, the samples were sequentially dehydrated using ethanol gradients of 30%, 50%, 70%, 80%, 90%, and 100%, and subsequently gold-coated using an ion sputtering coater (Gevee; GVC-1000; Beijing KYKY Co. Ltd.; Beijing, China) after air drying. Surface morphology of the specimens was examined using scanning electron microscopy (Quanta 250; FEI; Hillsboro; USA).
Roughness detection
The isolated teeth of each group underwent ultrasonic cleaning with deionized water for 20 min. Following drying, a roughness meter (Kosaka; EF680; Kosaka Laboratory Ltd.; Tokyo; JPN) was utilized to assess the surface roughness of each group. The cut-off value is 0.8 mm, the evaluation length is 3.2 mm, and the measurement speed is 0.1 mm/s. Three positions were randomly measured for each sample, and the average value was recorded as the roughness value of the sample.
Surface microhardness test
Teeth were sectioned along the buccal and lingual aspects using a water cooled diamond disc, fixed with acrylic resin (New century; Shanghai New century Dental Materials Co. Ltd.; Shanghai, China), and the microhardness of enamel on the buccal or lingual sides, dentin at the dentin–enamel junction, and dentin at the cemento-enamel junction was measured post-ultrasonic swaging. The microhardness tester (Buehler; VH1202; Buehler An ITW Company; Chicago; USA) was set with a test force of 200 gf, a loading time of 15 s, and diamond indentation was observed under 600× magnification, adjusted to the center of the field of view. The surface of the sample was clearly imprinted, and the diagonal length of the diamond (d1, d2) was measured using a measuring instrument. The Vickers microhardness value (HV) of each sample was automatically calculated by the instrument. Three points were measured for each sample, approximately 200 μm apart, and the average HV value was recorded as the Surface Microhardness (SMH) value of the sample.
Bacterial culture
Streptococcus. Mutans (S. Mutans) ATCC 25175 strains were cultured on Brain–Heart Infusion (BHI; OXOID; CM1135B; Thermo Fisher; Hampshire; UK) agar (Biofroxx; 8211GR500; NeoFroxx; GER) and incubated at 37 °C in CO2 incubator (Jing Hong; DNP-9082; Shanghai Jing Hong Laboratory Instrument Co. Ltd.; Shanghai, China).
Specimen preparation
Teeth were sectioned using a water cooled diamond disc to obtain specimens sized 4 mm × 3 mm × 2 mm. The back and sides were coated with silicone rubber (3M Deutschlan GmbH; MDSA-V02; Neuss; GER), leaving only the radiated side exposed. After ultrasonic washing (Supmile; KQ-500DE; Kunshan Jiang, China), ethylene oxide sterilization (SAN QIANG MEDICAL; SQ-H40; Henan, China) was performed, and specimens were stored in sterile normal saline. Sterilized dental blocks were placed into 24-well plates (Corning; DWG01261; New York; USA) with one block per well, radiated side facing up. Diluted S. mutans solution (1 × 106 CFU/mL) was inoculated into the wells at 1 mL per well and incubated in a constant temperature incubator at 37 °C for 48 h.
Adhesion test
After removal, teeth were gently washed three times with PBS buffer (Beijing Dingguo Changsheng Biotechnology Co. Ltd.; Beijing, China). Each tooth was placed in a 1.5 mL centrifuge tube (Axygen; MCT-150-C; New York; USA), one sample per tube, and 200 μL PBS was added to each tube. Teeth were vortex-shaken for 2 min with Vortex oscillator (Maxi Mix; Thermo Fisher Scientific; Massachusetts; USA). The bacterial suspension in the centrifuge tube was diluted to 10−4 using the double dilution method. Twenty microliters of the diluted solution were inoculated onto BHI (OXOID; CM1135B; Thermo Fisher; Hampshire; UK) agar (Biofroxx; 8211GR500; NeoFroxx; GER) plates and incubated at 37 °C for 48 h. The number of bacterial colonies was counted, and the concentration of the original bacterial solution was calculated based on the colony count.
Crystal violet staining
After removal, teeth were gently washed three times with PBS buffer. PBS was blotted, and 1 mL of 4% paraformaldehyde was added per well for fixation for 15 min. Then, 1 mL of 0.1% crystal violet (Beyotime; C0121; Beyotime Biotechnology; Shanghai, China) was added for staining for 15 min. The tooth block was removed from the silicone rubber, rinsed with PBS, and dried. Each well received 1 mL of 95% ethanol for decolorization, placed on a shaker (MIX-1500; Shenzhen Huinuo Biotechnology Co. Ltd.; Shenzhen, China) for complete dissolution for 30 min, and 200 μL from each well was transferred to a 96-well plate for optical density measurement at a wavelength of 595 nm with microcoder (SpectraMax iD3; Molecular Devices; Sunnyvale; USA).
Artificial enamel caries formation
The selected teeth were immersed in demineralizing solution (2.2 mM KH2PO4, 2.2 mM CaCl2, 50 mM acetic acid, pH 4.422) at 37 °C for 96 h to produce artificial enamel caries under continuous, incubator shaker (100 rpm; MIULAB; ES-60; MIULAB; Hanzhou, China). The tooth surface was painted with 2 layers of acid-resistant nail varnish, leaving an enamel-exposed window of only 4 mm × 3 mm. Then the demineralization of tooth surface was observed with stereomicroscope (ZEISS; Stemi508; Carl Zeiss AG; Oberkochen; GER), and test Vickers microhardness value (HV) calculate △SMH% = (SMH0 − SMH1)/SMH0 * 100%.
Detection of demineralization by confocal microscopy
The dental plates were stained with 0.1 mmol/L Rhodamine B (Macklin; R817226; Shanghai Macklin Biochemical Technology Co. Ltd.; Shanghai, China) solution, and the fluorescence penetration was observed under laser confocal microscopy (Olympus; FV3000; Olympus Corporation; Tokyo; JPN) (the red part represents the demineralized penetration area). Keep the shooting conditions of the measured images consistent, image-J software (National Institutes of Health; USA) was used to measure the average fluorescence density.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software (IBM; New York; USA). If the data followed a normal distribution and variances were equal, One-way ANOVA was employed for inter-group comparison, with the LSD test used for multiple comparisons. If the data did not follow a normal distribution or variances were unequal, the non-parametric Kruskal–Wallis H test was used for between-group comparisons. The significance level for all tests was set at bilateral α = 0.05.
Results
Different radiation approaches resulted in varying degrees of enamel surface destruction
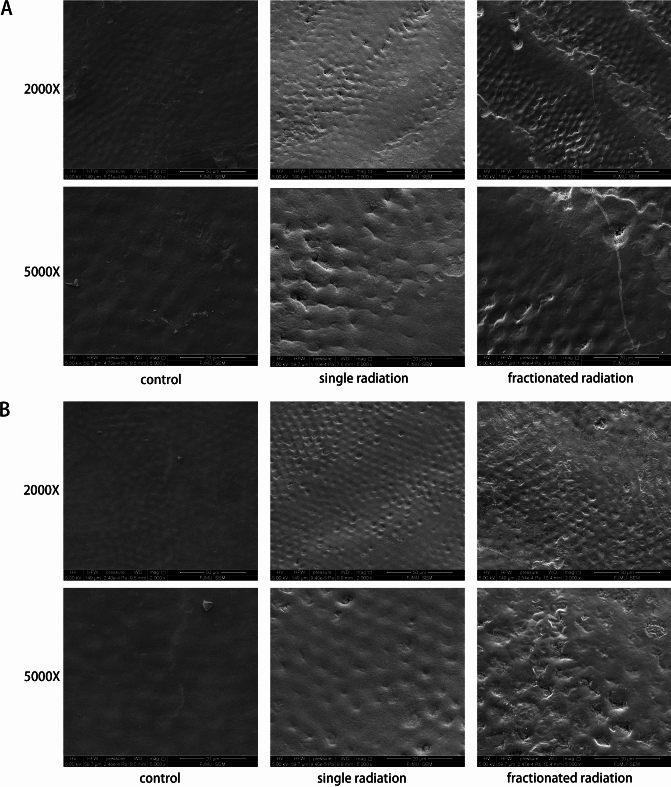
Upon examination of enamel surfaces using SEM after exposure to different radiation approaches, it was observed that the enamel surfaces exhibited damage in the radiated groups compared to the control group (shown in Fig. 2). The enamel surfaces appeared smoothest in the control group, while both the single and fractionated radiation groups showed surface irregularities characterized by numerous small pits and cracks. In the fractionated radiation group, the number of pits and cracks was higher compared to the single radiation group, regardless of whether they were on the buccal or lingual surfaces.
Fig. 2.
Scanning electron microscopy (SEM) images showing enamel surfaces. (A) Buccal enamel in different radiation approaches. (B) Lingual enamel in different radiation approaches.
Radiation induced surface roughness of enamel
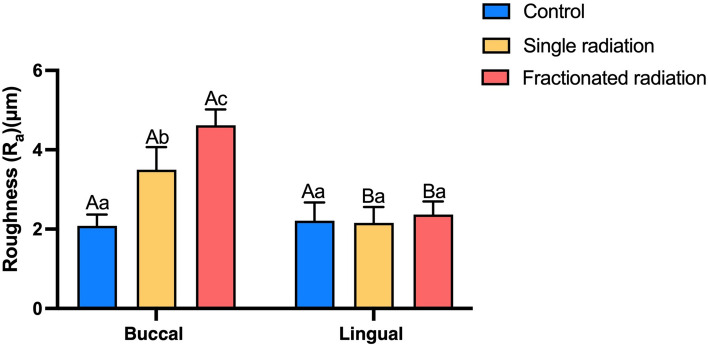
The buccal surfaces exhibited increased roughness in both the single and fractionated radiation groups compared to the control group. Furthermore, the fractionated radiation group showed a higher mean value of Ra than the single radiation group. Following radiation, the Ra value of buccal surfaces of enamel was higher than that of lingual surfaces. Significant differences in Ra were observed among the different groups (shown in Fig. 3). These findings indicate that radiation induced roughening of buccal surfaces more prominently than lingual surfaces, with fractionated radiation resulting in the roughest enamel surfaces on the buccal aspect.
Fig. 3.
Bar graph illustrating surface roughness levels of enamel (Ra μm). The superscript lowercase letter indicates intra-group comparison, and the capital letter indicates buccal and lingual comparison of the same radiation method. Superscripts with different letters (i.e., a–b, b–c, A–B) indicate significant differences between groups at P < 0.05.
Radiation led to increased enamel cracks compared to the control
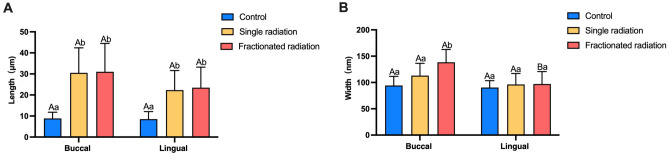
SEM observations post-radiation revealed that the enamel surface became more uneven with the appearance of small pits and cracks, accompanied by a significant increase in the average Ra value compared to the control group. Hence, quantitative data reflecting the degree of enamel surface damage were sought. The length and width of cracks on the enamel surface were measured under SEM to quantify the degree of damage. Following two different radiation methods, the length of cracks on both buccal and lingual enamel surfaces was significantly longer than those in the control group, with statistically significant differences observed. No significant difference in enamel crack length was found between the two radiation methods (shown in Fig. 4A).
Fig. 4.
Histogram depicting enamel crack length and width measured under SEM. (A) Histogram of enamel crack length (μm). (B) Histogram of enamel crack width (nm). The superscript lowercase letter indicates intra-group comparison, and the capital letter indicates buccal and lingual comparison of the same radiation method. Superscripts with different letters (i.e., a–b, b–c, A–B) indicate significant differences between groups at P < 0.05.
Comparison of enamel surface crack width revealed that the buccal enamel crack width in the fractionated radiation group was significantly wider than that in the single radiation group and the control group, with statistically significant differences observed (shown in Fig. 4B). Additionally, the buccal enamel cracks were significantly wider than the lingual cracks in the fractionated radiation group. These results underscore the damaging effect of radiation on enamel surfaces, with the most significant damage observed in the buccal enamel of the fractionated radiation group.
Radiation decreased the microhardness of enamel
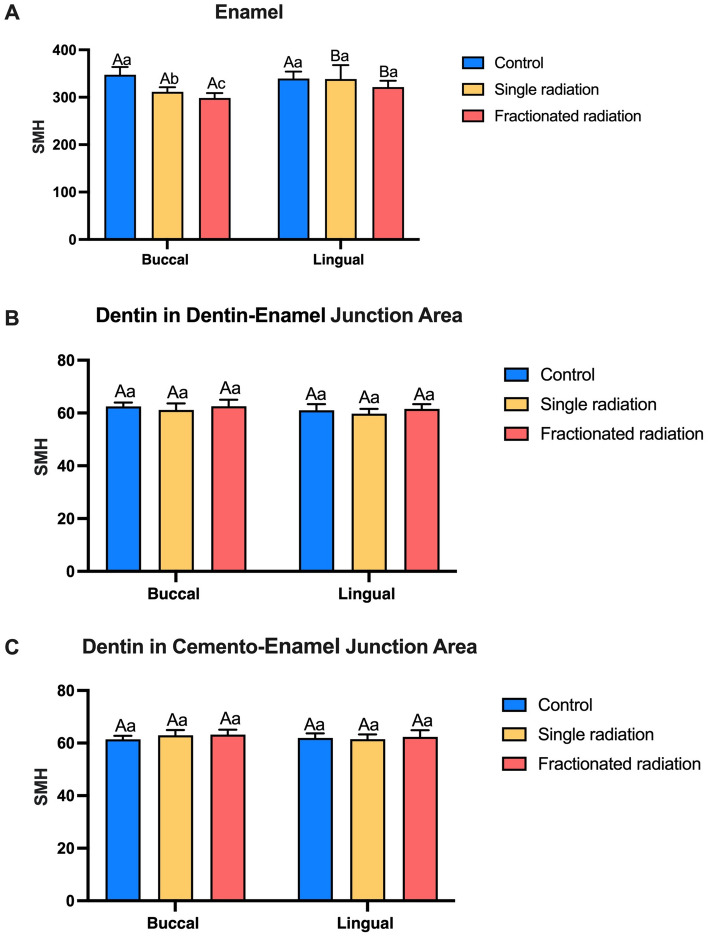
Tooth hardness is a crucial indicator of their resistance to external factors. The structural integrity of tooth was assessed through microhardness testing using a Vickers indenter at various locations, including enamel, dentin in dentin-enamel junction area, and dentin in cemento-enamel junction area. Reduction in microhardness was noted in the buccal surfaces of enamel after radiation. Furthermore, the microhardness of buccal enamel in the fractionated radiation group was lower than that in the single radiation group. Following radiation, the microhardness of buccal enamel was lower than that of lingual enamel (shown in Fig. 5A). No significant difference in microhardness was observed between dentin in dentin-enamel junction area, and dentin in cemento-enamel junction area (shown in Fig. 5B,C). Therefore, radiation significantly decreases the surface microhardness of buccal enamel but has minimal impact on the tooth interior, with the microhardness of buccal enamel being the lowest in the fractionated radiation group.
Fig. 5.
Vickers microhardness in different areas of teeth. (A) Microhardness of enamel. (B) Microhardness of dentin in dentin-enamel junction. (C) Microhardness of dentin in cemento-enamel junction. The superscript lowercase letter indicates intra-group comparison, and the capital letter indicates buccal and lingual comparison of the same radiation method. Superscripts with different letters (i.e., a–b, b–c, A–B) indicate significant differences between groups at P < 0.05.
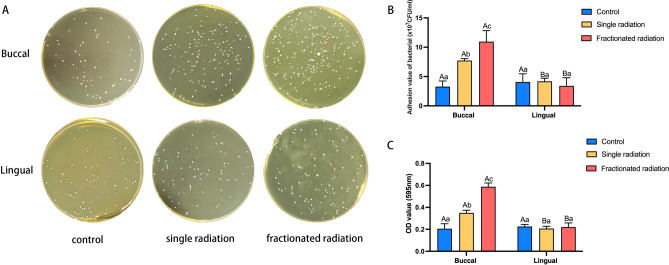
Radiation increased the adhesion of Streptococcus mutans on enamel surfaces
In addition to structural changes such as roughness, microhardness, and acid resistance of enamel, another crucial factor contributing to caries development is the adhesion of cariogenic bacteria. Hence, we further investigated whether enamel surface alterations due to radiation affected bacterial adhesion. The adhesion of Streptococcus mutans on enamel after radiation was examined.
As depicted in Fig. 6A,B, the adhesion count of S. mutans on buccal enamel after radiation was higher than that in the control group, with no significant difference in the adhesion count of S. mutans on lingual enamel between the radiation group and the control group. The adhesion count of S. mutans on buccal enamel was higher than that on lingual enamel after radiation, with the highest count observed in the fractionated radiation group. Additionally, the fractionated radiation group exhibited the greatest number of S. mutans adhesion.
Fig. 6.
Adhesion ability of Streptococcus mutans changed post-radiation. (A) Representative image of S. mutans cloning. (B) Histogram of adhesion values. (C) Histogram of OD values of Crystal violet staining. The superscript lowercase letter indicates intra-group comparison, and the capital letter indicates buccal and lingual comparison of the same radiation method. Superscripts with different letters (i.e., a–b, b–c, A–B) indicate significant differences between groups at P < 0.05.
In addition to clonal culture of eluted bacterial solution, we further performed crystal violet staining to detect changes in S. mutans adhesion and measure the OD value of the eluent. Consistently, we observed a similar trend: the OD value of buccal enamel was highest in the fractionated radiation group, followed by the single radiation group. Moreover, the OD value of buccal enamel was higher than that of lingual enamel (shown in Fig. 6C). Therefore, we hypothesize that radiation enhances the adhesion ability of S. mutans, with fractionated radiation exerting the greatest effect on the adhesion of buccal enamel.
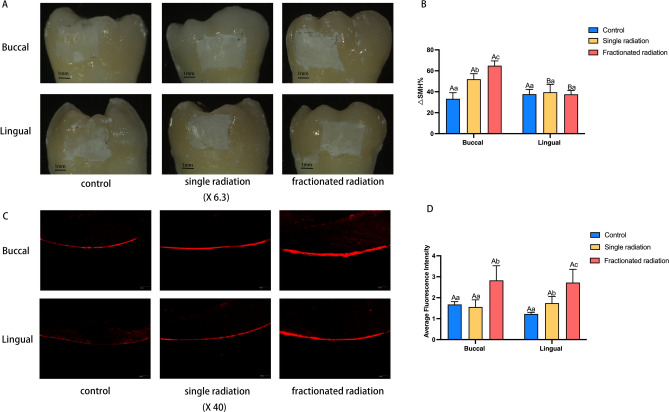
Radiation exposure can decrease the acid resistance of enamel and increase enamel demineralization
Apart from factors such as surface roughness, surface hardness, and bacterial adhesion ability, the ability to resist acid is also a crucial characteristic affecting susceptibility to dental caries. Hence, following immersion of teeth from each group in demineralization solution for 96 h and subsequent observation under a stereomicroscope, it was noted that both the single radiation and fractionated radiation group exhibited larger areas of white spots compared to the control group. This trend was particularly evident on the buccal enamel (shown in Fig. 7A). Upon staining with Rhodamine B to observe demineralization depth, it was observed that the average fluorescence intensity significantly increased post-irradiation, with the fractionated radiation group displaying the highest average fluorescence intensity. However, the difference in enamel between buccal and lingual sides was not significant (shown in Fig. 7C,D). This suggests that teeth become significantly less resistant to acid after irradiation, with radiation therapy rendering enamel more susceptible to demineralization under acidic conditions, a trend more pronounced in the fractionated radiation group. Further comparison of changes in microhardness reduction rates among the groups revealed that the reduction rate of microhardness on buccal enamel post-irradiation was higher than that in the control group, with the fractionated radiation group showing the highest enamel reduction rate. While there was no significant difference in the reduction rate of microhardness on lingual enamel among the groups, it was lower than the reduction rate of microhardness on the buccal side (shown in Fig. 7B).
Fig. 7.
The impact of radiation on enamel acid resistance. (A) Surface demineralization of teeth under stereomicroscope (×6.3). (B) Reduction rate of microhardness. (C) Representative micrographs of enamel demineralization fluorescence under confocal laser microscope (×40). (D) Bar graph of average fluorescence intensity. The superscript lowercase letter indicates intra-group comparison, and the capital letter indicates buccal and lingual comparison of the same radiation method. Superscripts with different letters (i.e., a–b, b–c, A–B) indicate significant differences between groups at P < 0.05.
Discussion
The treatment of head and neck cancer typically involves a combination of surgery, radiotherapy, and chemotherapy. Radiotherapy, being minimally invasive and effective, has become the primary treatment modality for most head and neck cancers23,24. Despite advancements in radiotherapy technology, such as the application of intensity-modulated radiotherapy in clinical practice, the inherent toxic side effects of radiation on surrounding tissues remain a concern25. Given the anatomical proximity of teeth to the treatment area, direct damage to dental hard tissues due to radiation exposure is difficult to avoid.
In order to better understand the mechanisms underlying radioactive caries, it is imperative to establish in vitro models. Conventional radiotherapy for head and neck cancers typically involves daily fractionated doses of 2 Gy, administered five days a week, totaling 64–70 Gy26. Presently, most scholars prefer fractionated radiation simulation to closely mimic clinical conditions, with only a few studies employing single radiation due to logistical challenges and potential treatment delays27,28. It is still unclear whether the selection of radiation method in vitro model needs to follow the clinical radiotherapy process and whether a single radiation can achieve similar effects. Evidence suggests that enamel microstructure undergoes significant changes at doses of 30 Gy in single radiation, with increasing doses exacerbating enamel damage29. Similarly, changes were observed at doses of 40 Gy in fractionated radiation, with increasing doses leading to aggravated enamel damage and the appearance of enamel cracks21,30. The dose of single radiation and fractionated radiation that can affect the tooth surface is different. So we hypothesized that a single dose at a relatively low level such as 30 Gy may induce tooth surface damage, which becomes more pronounced with cumulative doses in fractionated radiation such as 40 Gy23,31–33. Sever et al.34 utilized single radiation and fractionated radiation protocols cumulative exposure dose reached 70 Gy and demonstrated both protocols could reduce enamel microhardness and increased roughness post-radiation. These results were consistent with ours. However, they emphasized that neither protocol caused damage to dental hard tissues and did not conduct a horizontal comparison of the two radiation approaches. Therefore, it was necessary to investigate the effects of different radiation methods on tooth structure and caries susceptibility. Our experiment compared the effects of single and fractionated radiation on dental hard tissues, revealing that surface roughness and bacterial adherence were lower with single radiation than with fractionated radiation. This difference may be attributed to single radiation inducing more pronounced thermal effects, melting enamel surfaces and filling some demineralization-induced micropores, thereby reducing roughness and bacterial adherence. Additionally, radiation primarily affects the organic portion of enamel, causing organic component denaturation35, decreasing the protein-mineral ratio, and relatively increasing mineral content36. The effects of single radiation are more pronounced than those of fractionated radiation, resulting in a less significant reduction in surface microhardness.
In addition to the radiation method, the amount of radiation dose also has a direct effect on the hard tissue of the teeth. Research found that radiation-induced damage to dental hard tissues correlates positively with radiation dose, with the most significant decrease in enamel mechanical properties observed at doses ranging from 30 to 50 Gy37. The risk of tooth destruction increases 2–3 times at doses between 30–60 Gy, with doses exceeding 60 Gy increasing the risk by tenfold, primarily attributed to direct radiation damage to dental tissues36. Studies by Deniz et al.38 found that radiation-induced damage to dental tissues becomes apparent only at doses exceeding 30 Gy, with increasing doses resulting in widening of enamel prism gaps and doses exceeding 40 Gy leading to severe loss of tooth mineral volume39. It is speculated that when the accumulated radiation dose surpasses a certain threshold such as 60 Gy, tooth damage exacerbates. The radiation dose of the two radiation methods in this paper exceeds 60 Gy and reaches 70 Gy, our study demonstrating that both single and fractionated radiation induce damage to dental surfaces, with fractionated radiation causing more severe damage. Furthermore, the mechanical properties of lingual dental tissues in the radiation group did not exhibit significant decline, possibly due to their distance from the radiation source and the protective effect of buccal dental hard tissues, resulting in reduced radiation exposure and negligible enamel damage.
It has been observed that radiation can alter the surface microstructure of dental hard tissue, leading to a reduction in its microhardness40. Similarly, Kudkuli et al.’s study39 found that tooth enamel microhardness decreased post-radiation, accompanied by the presence of micropores and surface roughness. In our experiment, scanning electron microscopy of teeth post-radiation revealed uneven surfaces on buccal enamel, characterized by honeycomb pores and micro-cracks, accompanied by decreased microhardness and increased roughness, consistent with findings from previous studies. Radiation-induced damage to dental hard tissues shares similarities with the demineralization process41. Following radiation, dental hard tissue surfaces undergo demineralization and a reduction in mineral content, which correlates with alterations in their physical properties21. This demineralization process may explain the observed decrease in dental tissue microhardness post-radiation exposure. Additionally, demineralization can lead to focal pits and surface roughening of enamel, increasing surface roughness and subsequently affecting bacterial adherence, thereby increasing susceptibility to caries. Scholars speculate that radiation, through radiolysis, decomposes water within enamel, leading to dehydration42. Simultaneously, radiation acts on organic matter, inducing decarboxylation of carboxyl groups on collagen side chains, weakening the interaction between hydroxyapatite crystals and the organic matrix, resulting in enamel embrittlement, microcrack formation, decreased microhardness, and increased surface roughness37,43. Lu et al.40 compared calcium-phosphorus content in microcracks and adjacent crack-free dental surfaces post-irradiation, revealing significant differences, with lower calcium-phosphorus content in the crack sites indicating mineral loss. Dentin, a highly mineralized hard tissue located between enamel and dental bone, plays a crucial protective role in supporting enamel and safeguarding the pulp. Studies found no significant difference in microhardness of dentin near the enamel-dentin junction before and after irradiation40,44, consistent with the results of our experiment. Higher surface roughness provides more niches for bacterial colonization, thus enhancing bacterial adhesion45. The threshold roughness value for significant bacterial adhesion has been reported as approximately 0.2 µm46. In our study, since the enamel surface is unpolished, the roughness values of enamel surfaces in all groups exceeded this threshold. Radiation increased demineralization of the enamel surface, further increasing the surface area for bacterial adhesion. Which likely contributed to the observed increase in Streptococcus mutans adhesion. Early signs of caries involve enamel demineralization; our study found that post-fractionated radiation, enamel is more prone to demineralization, particularly on the buccal side. Many scholars have also observed increased susceptibility to demineralization in dental hard tissues post-irradiation47,48. Thus, post-radiation, increased enamel surface roughness and bacterial adherence, coupled with reduced acid resistance, collectively increase the risk of radiation-related caries.
Limitations
The limitations of our experiment, which cannot fully replicate the effects of radiation therapy for head and neck tumors on dental hard tissues. Due to the protection of maxillofacial soft tissue and bone in the actual radiotherapy, the radiation dose that may remain in the teeth may be less than that in the study. There is no animal experiment in the study to better simulated the course of radiotherapy and radiation-associated caries. Its strengths lie in comparing the damage caused by two radiation methods and their impact on susceptibility to caries, providing valuable insights for establishing future in vitro experimental models.
Conclusions
Both single and fractionated radiation can cause damage to dental hard tissues, leading to a decrease in their physical and biological properties. However, differences exist between the damage caused by single and fractionated radiation and their effects on susceptibility to enamel caries. Compared to single-dose radiation, fractionated radiation induces more severe damage to buccal enamel and increases bacterial plaque adherence while decreasing acid resistance.
Acknowledgements
Part of the teeth samples were provided by Dr. Shixian Zhang from School and Hospital of Stomatology, Fujian Medical University. We appreciate Dr. Zhang contribution.
Author contributions
RG, LL, BW, YL contributed to conception, design, in charge of data acquisition, controlled the quality of data and algorithms, analyzed and interpreted the data. RG and LL had a major contributor in writing the manuscript. DS, FT, GN had a major contributor in sample collection and radiation treatment. DZ contributed to conception, interpretation and critically revised manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was sponsored by Fujian Provincial Health Technology Project (Grant number 2021GGA055; Grant number 2020GGB036). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Competing interests
The authors declare no competing interests.
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study received approval from the local Ethics Committee (Ethics Committee of the School and Hospital of Stomatology, Fujian Medical University, approval number 2021-FJMUSS-062). Also, written informed consent was obtained from participants.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rui-huan Gan and Li-qing Lan.
Contributor Information
Bin Wang, Email: wangbinkq@163.com.
You-guang Lu, Email: fjlyg63@fjmu.edu.cn.
References
- 1.Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin.74(1), 12–49 (2024). 10.3322/caac.21820 [DOI] [PubMed] [Google Scholar]
- 2.Gormley, M., Creaney, G., Schache, A., Ingarfield, K. & Conway, D. I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J.233(9), 780–786 (2022). 10.1038/s41415-022-5166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021). 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 4.Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin.70(1), 7–30 (2020). 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 5.De Martel, C., Georges, D., Bray, F., Ferlay, J. & Clifford, G. M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health8(2), e180–e190 (2020). 10.1016/S2214-109X(19)30488-7 [DOI] [PubMed] [Google Scholar]
- 6.Chow, L. Q. M. Head and neck cancer. N. Engl. J. Med.382(1), 60–72 (2020). 10.1056/NEJMra1715715 [DOI] [PubMed] [Google Scholar]
- 7.Mody, M. D., Rocco, J. W., Yom, S. S., Haddad, R. I. & Saba, N. F. Head and neck cancer. Lancet398(10318), 2289–2299 (2021). 10.1016/S0140-6736(21)01550-6 [DOI] [PubMed] [Google Scholar]
- 8.Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol.45(4–5), 309–316 (2009). 10.1016/j.oraloncology.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 9.Eberly, H. W., Sciscent, B. Y., Lorenz, F. J., Rettig, E. M. & Goyal, N. Current and emerging diagnostic, prognostic, and predictive biomarkers in head and neck cancer. Biomedicines.10.3390/biomedicines12020415 (2024). 10.3390/biomedicines12020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano, M. et al. Head and neck cancer: The role of anti-EGFR agents in the era of immunotherapy. Ther. Adv. Med. Oncol.13, 1758835920949418. 10.1177/1758835920949418 (2021). 10.1177/1758835920949418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aboagye, E. O., Barwick, T. D. & Haberkorn, U. Radiotheranostics in oncology: Making precision medicine possible. CA Cancer J. Clin.73(3), 255–274 (2023). 10.3322/caac.21768 [DOI] [PubMed] [Google Scholar]
- 12.Chin, A. L., Lin, A., Anamalayil, S. & Teo, B. K. Feasibility and limitations of bulk density assignment in MRI for head and neck IMRT treatment planning. J. Appl. Clin. Med. Phys.15(5), 4851. 10.1120/jacmp.v15i5.4851 (2014). 10.1120/jacmp.v15i5.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strojan, P. et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev.59, 79–92 (2017). 10.1016/j.ctrv.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge, X. et al. Radiotherapy-related quality of life in patients with head and neck cancers: A meta-analysis. Support. Care Cancer28(6), 2701–2712 (2020). 10.1007/s00520-019-05077-5 [DOI] [PubMed] [Google Scholar]
- 15.Kielbassa, A. M., Hinkelbein, W., Hellwig, E. & Meyer-Luckel, H. Radiation-related damage to dentition. Lancet Oncol.7(4), 326–335 (2006). 10.1016/S1470-2045(06)70658-1 [DOI] [PubMed] [Google Scholar]
- 16.Moore, C. et al. Dental caries following radiotherapy for head and neck cancer: A systematic review. Oral Oncol.100, 104484. 10.1016/j.oraloncology.2019.104484 (2020). 10.1016/j.oraloncology.2019.104484 [DOI] [PubMed] [Google Scholar]
- 17.Chopra, A. et al. Indices for the assessment of radiation-related caries. J. Conserv. Dent.25(5), 481–486 (2022). 10.4103/jcd.jcd_237_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almstahl, A., Finizia, C., Carlen, A., Fagerberg-Mohlin, B. & Alstad, T. Explorative study on mucosal and major salivary secretion rates, caries and plaque microflora in head and neck cancer patients. Int. J. Dent. Hyg.16(4), 450–458 (2018). 10.1111/idh.12338 [DOI] [PubMed] [Google Scholar]
- 19.Sroussi, H. Y. et al. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med.6(12), 2918–2931 (2017). 10.1002/cam4.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmier, N. R. et al. Radiation-related caries: Current diagnostic, prognostic, and management paradigms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol.130(1), 52–62 (2020). 10.1016/j.oooo.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 21.Gonçalves, L. M. et al. Radiation therapy alters microhardness and microstructure of enamel and dentin of permanent human teeth. J. Dent.42(8), 986–992 (2014). 10.1016/j.jdent.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 22.Ten Cate, J. M. & Duijsters, P. P. Influence of fluoride in solution on tooth demineralization. II. Microradiographic data. Caries Res.17(6), 513–519 (1983). 10.1159/000260711 [DOI] [PubMed] [Google Scholar]
- 23.De Barros Da Cunha, S. R. et al. Effects of different radiation doses on the microhardness, superficial morphology, and mineral components of human enamel. Arch. Oral Biol.80, 130–135 (2017). 10.1016/j.archoralbio.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 24.Abed, H. Dental considerations for head and neck cancer: A clinical review. Saudi Dent. J.35(5), 476–486 (2023). 10.1016/j.sdentj.2023.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen, Y. P. et al. Nasopharyngeal carcinoma. Lancet394(10192), 64–80 (2019). 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 26.Bourhis, J., Etessami, A. & Lusinchi, A. New trends in radiotherapy for head and neck cancer. Ann. Oncol.16(Suppl 2), ii255–ii257 (2005). 10.1093/annonc/mdi736 [DOI] [PubMed] [Google Scholar]
- 27.Joyston-Bechal, S. The effect of X-radiation on the susceptibility of enamel to an artificial caries-like attack in vitro. J. Dent.13(1), 41–44 (1985). 10.1016/0300-5712(85)90061-2 [DOI] [PubMed] [Google Scholar]
- 28.Douchy, L. et al. The effect of therapeutic radiation on dental enamel and dentin: A systematic review. Dent. Mater.38(7), e181–e201 (2022). 10.1016/j.dental.2022.04.014 [DOI] [PubMed] [Google Scholar]
- 29.Demirkan, I. et al. Acoustic diagnosis of elastic properties of human tooth by 320 MHz scanning acoustic microscopy after radiotherapy treatment for head and neck cancer. Radiat. Oncol.15(1), 38. 10.1186/s13014-020-01486-7 (2020). 10.1186/s13014-020-01486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arid, J. et al. Radiotherapy impairs adhesive bonding in permanent teeth. Support. Care Cancer28(1), 239–247 (2020). 10.1007/s00520-019-04782-5 [DOI] [PubMed] [Google Scholar]
- 31.Paredes, W. E. B., Geraldo, A. B. C. & De Andrade, D. A. ATR-FTIR Assessment of the biochemical composition and micro hardness of the hard tissues of oral cavity submitted to gamma irradiation. J. Cancer Sci. Ther.10.4172/1948-5956.1000446 (2017). 10.4172/1948-5956.1000446 [DOI] [Google Scholar]
- 32.Hegde, M. N., Hegde, N. D., Kumari, S., Sanjeev, G. & Priya,. Radiation effect on structure and mechanical properties of teeth-an in vitro study. Eur. J. Pharm. Med. Res.2(3294–3211), 788–800 (2015). [Google Scholar]
- 33.Siripamitdul, P. et al. The effects of radiotherapy on microhardness and mineral composition of tooth structures. Eur. J. Dent.17(2), 357–364 (2023). 10.1055/s-0042-1746414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klaric Sever, E., Tarle, A., Vukelja, J., Soce, M. & Grego, T. Direct induced effects of standard and modified radiotherapy protocol on surface structure of hard dental tissue. Acta Stomatol. Croat.55(4), 334–345 (2021). 10.15644/asc55/4/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Siqueira Mellara, T. et al. The effect of radiation therapy on the mechanical and morphological properties of the enamel and dentin of deciduous teeth—An in vitro study. Radiat. Oncol.9, 30. 10.1186/1748-717X-9-30 (2014). 10.1186/1748-717X-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed, R. et al. Radiotherapy effect on nano-mechanical properties and chemical composition of enamel and dentine. Arch. Oral Biol.60(5), 690–697 (2015). 10.1016/j.archoralbio.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang, X., Zhang, J. Y., Cheng, I. K. & Li, J. Y. Effect of high energy X-ray irradiation on the nano-mechanical properties of human enamel and dentine. Braz. Oral Res.10.1590/1807-3107BOR-2016.vol30.0009 (2016). 10.1590/1807-3107BOR-2016.vol30.0009 [DOI] [PubMed] [Google Scholar]
- 38.Deniz, Y., Aktas, C., Misilli, T. & Carikcioglu, B. Effects of radiotherapeutic X-ray irradiation on cervical enamel. Int. J. Radiat. Biol.97(12), 1667–1674 (2021). 10.1080/09553002.2021.1987560 [DOI] [PubMed] [Google Scholar]
- 39.Kudkuli, J. et al. Demineralization of tooth enamel following radiation therapy; An in vitro microstructure and microhardness analysis. J. Cancer Res. Ther.16(3), 612–618 (2020). 10.4103/jcrt.JCRT_8_19 [DOI] [PubMed] [Google Scholar]
- 40.Lu, H. et al. Direct radiation-induced effects on dental hard tissue. Radiat. Oncol.14(1), 5. 10.1186/s13014-019-1208-1 (2019). 10.1186/s13014-019-1208-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franzel, W., Gerlach, R., Hein, H. J. & Schaller, H. G. Effect of tumor therapeutic irradiation on the mechanical properties of teeth tissue. Z. Med. Phys.16(2), 148–154 (2006). 10.1078/0939-3889-00307 [DOI] [PubMed] [Google Scholar]
- 42.Santin, G. C. et al. Physical and adhesive properties of dental enamel after radiotherapy and bonding of metal and ceramic brackets. Am. J. Orthod. Dentofac. Orthop.148(2), 283–292 (2015). 10.1016/j.ajodo.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 43.Madrid, C. C. et al. Structural analysis of enamel in teeth from head-and-neck cancer patients who underwent radiotherapy. Caries Res.51(2), 119–128 (2017). 10.1159/000452866 [DOI] [PubMed] [Google Scholar]
- 44.Duruk, G., Acar, B. & Temelli, O. Effect of different doses of radiation on morphological, mechanical and chemical properties of primary and permanent teeth-an in vitro study. BMC Oral Health20(1), 242. 10.1186/s12903-020-01222-3 (2020). 10.1186/s12903-020-01222-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song, F., Koo, H. & Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res.94(8), 1027–1034 (2015). 10.1177/0022034515587690 [DOI] [PubMed] [Google Scholar]
- 46.Ferreira, I. et al. Influence of post-processing on the adhesion of dual-species biofilm on polylactic acid obtained by additive manufacturing. Saudi Dent. J.36(5), 733–739 (2024). 10.1016/j.sdentj.2024.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdalla, R., Niazy, M. A., Jamil, W. E., Hazzaa, H. A. & Elbatouti, A. A. The role of fluoride and chlorhexidine in preserving hardness and mineralization of enamel and cementum after gamma irradiation. Radiat. Environ. Biophys.56(2), 187–192 (2017). 10.1007/s00411-017-0690-9 [DOI] [PubMed] [Google Scholar]
- 48.Abdalla, R., Omar, A. & Eid, K. Detecting demineralization of enamel and cementum after gamma irradiation using radiographic densitometry. Radiat. Environ. Biophys.57(3), 293–299 (2018). 10.1007/s00411-018-0749-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.