Graphical abstract
Keywords: Ventricular septal defect, Persistent left cranial vena cava, Caprine, Congenital heart disease, 3D modeling
Highlights
-
•
Multimodal imaging contributes to understanding of caprine congenital heart disease.
-
•
Echocardiography is useful for identification of DCJA VSD.
-
•
3D heart models aid in assessing vascular anomalies not noted on echocardiography.
Introduction
Congenital cardiac abnormalities in small ruminants are rarely reported in the literature.1,2 This report describes a case of a goat diagnosed with a doubly committed juxta-arterial (DCJA) ventricular septal defect (VSD) and a persistent left cranial vena cava (PLCVC). Doubly committed juxta-arterial VSDs, characterized by the unique location of the VSD, are rarely reported in goats and pose both diagnostic and therapeutic challenges. The concurrent presence of a PLCVC added to the complexity and rarity of the case. Diagnoses of the goat's congenital anomalies relied on the integration of several imaging modalities: thoracic radiography, echocardiography, nongated computed tomographic angiography (CTA), and virtual cardiac three-dimensional (3D) reconstruction software.
Case Presentation
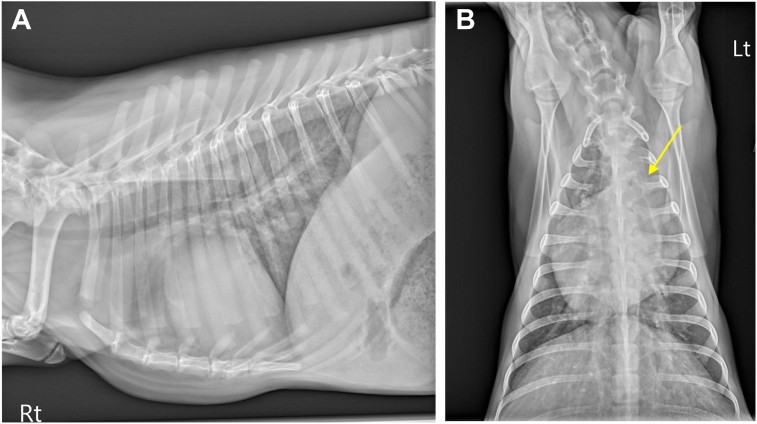
A 4-month-old female intact Nigerian Dwarf goat weighing 6.7 kg was presented to the teaching hospital for evaluation of an incidental heart murmur. The doeling (young female goat) was not reported to have any clinical signs of heart disease; however, she was smaller than her littermates. A grade VI/VI continuous murmur was auscultated by the primary veterinarian shortly after birth. Upon presentation to the teaching hospital, cardiothoracic auscultation revealed a grade V/VI systolic murmur with maximal intensity at the left sternal border. The doeling was tachycardic3 with a heart rate of 160 beats/minute, normal and synchronous femoral pulses, and moist pink mucous membranes. Pulmonary auscultation demonstrated increased bronchovesicular sounds. Thoracic radiography revealed a vertebral heart score of 9.5 vertebral body lengths (reference range, 8.9-10.6)4 with a focal bulging at the main pulmonary artery (MPA). Pulmonary parenchyma and pulmonary vasculature appeared normal (Figure 1).
Figure 1.
Right lateral (A, patient’s head to the left) and dorsoventral (B) thoracic radiographs in this goat. The dorsoventral view demonstrates an enlargement of the MPA (arrow). The pulmonary parenchyma is normal in appearance.
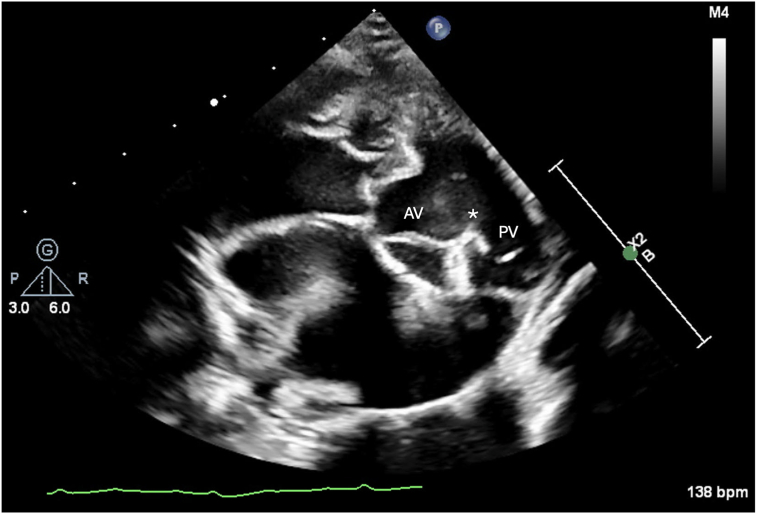
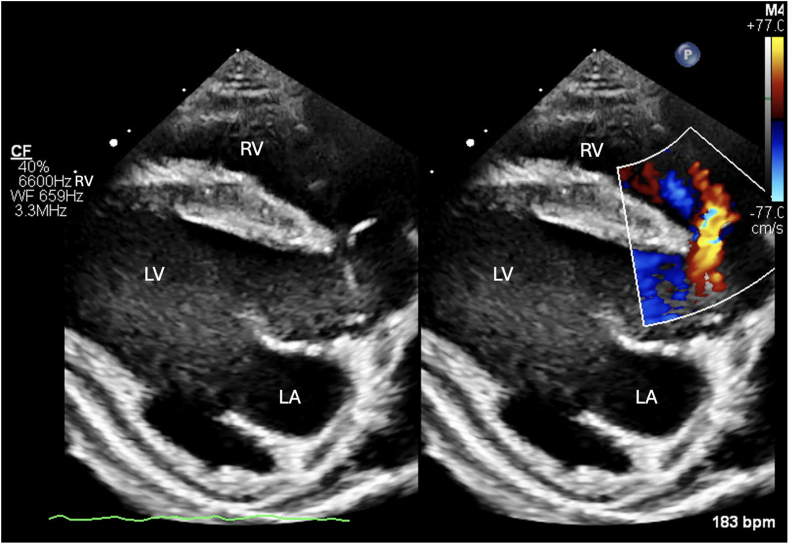
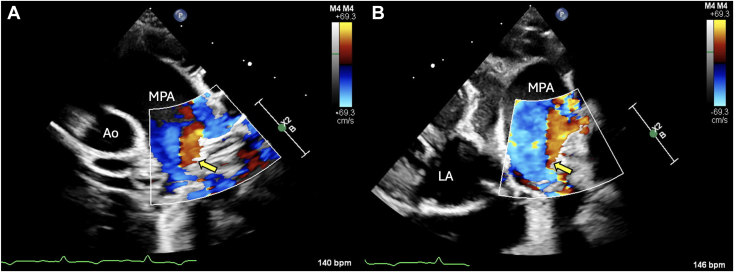
Two-dimensional transthoracic echocardiography (TTE) including Doppler was performed using a 9 MHz probe. The left atrium and ventricle were subjectively moderately enlarged with bowing of the interatrial septum toward the right atrium (Video 1). A DCJA VSD was present, measuring 1.2 cm (Figure 2, Video 2). Color-flow Doppler illustrated blood flow, which was primarily shunting left to right. However, bidirectional flow could not be completely excluded (Figure 3, Videos 3 and 4). The Qp/Qs of the defect could not be calculated because anatomic changes and conformation of the doeling's chest precluded obtaining spectral Doppler transaortic measurements. The aortic valve (AV) and pulmonary valve (PV) were normal in appearance with mild regurgitation. Mild to moderate concentric hypertrophy of the right ventricle and flattening of the interventricular septum during systole were noted (Video 5). The pulmonary trunk (PT) and branch pulmonary arteries (PAs) appeared severely dilated, with a PA:aorta ratio of 1.33 (Video 2). Increased systolic blood flow velocity was present in the right ventricular (RV) outflow tract, with a maximal velocity of 3.77 m/sec, based on continuous-wave Doppler (Video 3). Without obvious stenosis below or at the PV, this increased blood flow was suspected to be a relative pulmonary stenosis secondary to the left-to-right blood flow shunting across the VSD. Color-flow Doppler also revealed turbulent flow in the PT (Figure 4, Video 6); however, given the dilation of the PT combined with chest conformation, the origin of the turbulent flow could not be identified. Differentials for this turbulent flow included color aliasing within the PT due to the turbulence secondary to increased flow from the VSD, flow from a patent ductus arteriosus (PDA) given historically reported continuous murmur, or, less likely, increased flow secondary to other left-to-right shunts not identified in echocardiographic images.
Figure 2.
Two-dimensional TTE, right parasternal short-axis diastolic view, demonstrates fibrous continuity (asterisk) between the AV and PV, consistent with a DCJA VSD.
Figure 3.
Two-dimensional TTE, right parasternal long-axis diastolic view without (left) and with (right) color-flow Doppler, demonstrates left-to-right shunting through the VSD. LA, Left atrium; LV, left ventricle; RV, right ventricle.
Figure 4.
Two-dimensional TTE, left parasternal short-axis systolic (A) and off-angle left parasternal short-axis systolic view (B) demonstrates abnormal color-flow Doppler (arrow) indicating either turbulent or reflected flow in the MPA. Ao, Aorta; LA, left atrium.
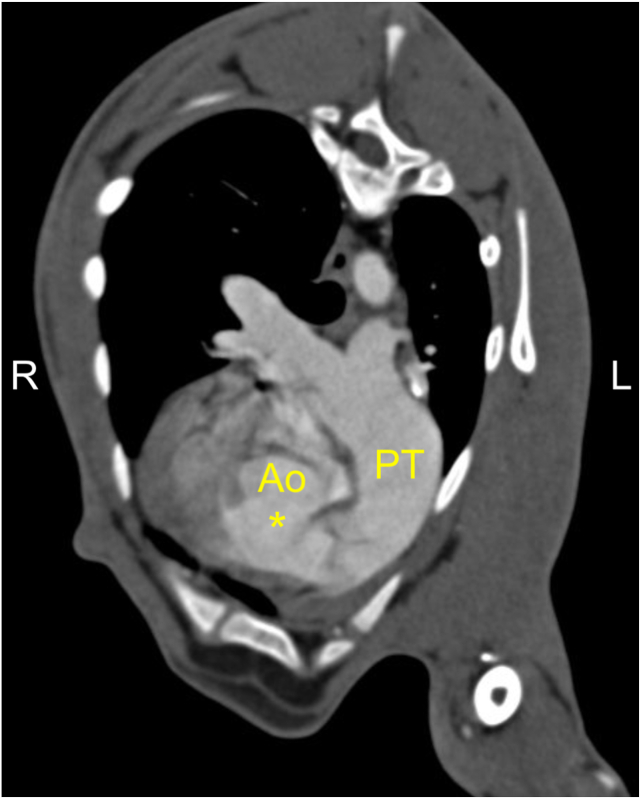
Nongated CTA was performed to further characterize the congenital heart disease. The goat was anesthetized and positioned in sternal recumbency on the couch of a 64-slice computed tomography scanner. Images of the thorax were acquired using 120 kVp, 300 mAs, and a pitch of 0.8 and reconstructed into 0.6 mm to 2 mm thick slices using a soft tissue algorithm. Noncontrast images of the thorax were acquired. Iohexol at a dose of 600 mgI/kg was delivered intravenously via power injector at a rate of 3.5 mL/sec, followed by a 10 mL saline flush at the same rate. Postcontrast arterial, venous, and delayed-phase CTA images of the thorax were then acquired after contrast injection using the same scan protocol as used in the noncontrast scan. Bolus tracking was utilized with a region of interest on the aortic arch, and the arterial phase was acquired using a threshold of 80 HU. The MPA was severely dilated (Figure 5) along the entirety of its length, and the left and right PAs were moderately dilated, raising concern for a degree of pulmonary hypertension (PH) given the absence of stenosis of the RV outflow tract. Aberrant systemic vascular connection to the MPA was not identified in the study. The presence of the previously diagnosed DCJA VSD was appreciated in the study (Figure 5). A PLCVC was incidentally discovered, and the right cranial vena cava was absent.
Figure 5.
Computed tomographic angiography multiplanar reconstruction from the postcontrast arterial phase demonstrates the DCJA VSD (∗) and dilation of the MPA. PT, Pulmonary trunk.
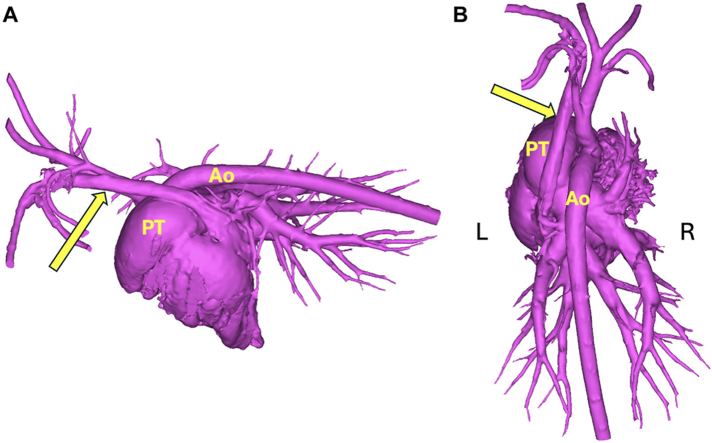
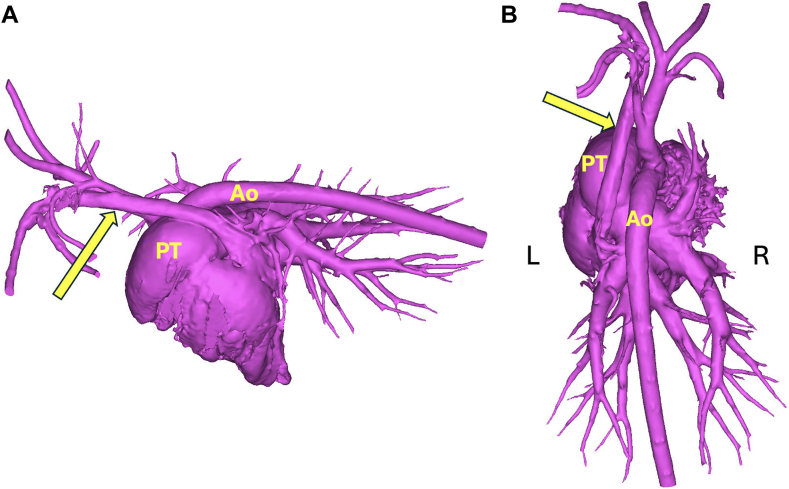
The CTA datasets were uploaded into cardiac 3D modeling software, and virtual models of the doeling's heart were generated to further assess the cardiovascular morphology. The PLCVC was found to drain into the right atrium via an enlarged coronary sinus (Figure 6). Aberrant systemic-to-pulmonary vascular connections were not present. The turbulent flow in the MPA seen on TTE was concluded to be secondary to increased flow from the DCJA VSD.
Figure 6.
Three-dimensional left lateral (A) and dorsal (B) reconstructions using CTA images of the heart and major vessels. The PLCVC (arrows) drains into the right heart through the enlarged coronary sinus. No aberrant vascular connection to the MPA is identified.
Due to the absence of clinical signs, the proximity of the VSD to the AV, and the presence of bidirectional flow, interventional closure was not recommended. Pulmonary artery banding (PAB)5 was considered; however, given the lack of clinical signs and concern for possible untreated PH affecting the goat's anesthetic candidacy, serial echocardiographic examinations to monitor for progression of disease were elected. Two hundred twenty days after initial referral, the doeling was still free of clinical signs.
Discussion
Congenital cardiac anomalies in goats are rarely reported in the veterinary literature. In a study of 1,886 goat autopsies at one veterinary center, the prevalence of cardiac malformations was 1.5%, with the most common malformation being a VSD.1 Most VSDs reported in the study were perimembranous in location, with only 1 found to be DCJA.1 The presence of VSD with a concurrent cardiac malformation, most commonly a patent foramen ovale or PDA, was present in 31% of cases.1
Ventricular septal defects, as described in human literature, are classified based on the location of the defect and the bordering cardiac structures.6 A DCJA VSD, characterized by the absence of formation of a completely muscular infundibulum, results in alignment of the PV and AV and fibrous continuity between the leaflets of these 2 valves.7, 8, 9 Perimembranous defects are bordered by the area of fibrous continuity between the AV and the tricuspid valve.6 Long-axis echocardiographic images of the goat described herein demonstrated a defect high in the interventricular septum, most consistent with either a perimembranous or DCJA VSD; however, the short-axis images demonstrated PV and AV fibrous continuity, identifying the goat's defect as DCJA (Figure 2).
Complications from a DCJA VSD may occur as the AV tissue is mechanically weakened by the increased velocities from the left-to-right shunting through the VSD. The so-called Venturi effect created by rapid flow from the shunt may cause prolapse of the right coronary AV leaflet into the defect.7, 8, 9 As a result, the severity of left-to-right shunting may vary with restriction of flow resulting from prolapse of the AV into the defect.8 Possible sequelae of AV prolapse, investigated in human studies, include functional closure of the VSD and aortic regurgitation (AR).8,9 At the time of diagnosis, the doeling had no evidence of AV prolapse and exhibited only mild AR.
In humans, spontaneous closure of a DCJA defect is uncommon and surgical intervention to repair a hemodynamically significant defect is indicated to prevent AV prolapse, AR, or in severe cases of left-to-right shunting.7, 8, 9 The hemodynamic significance of a VSD in both humans and animals can be evaluated via echocardiographically derived Qp/Qs, ventricular dimensions, and pulmonary arterial pressures.10 In domestic animals, VSD closure or occlusion is recommended if left heart remodeling is present and the left-to-right shunt is hemodynamically significant.11 Closure of a VSD that is reversed or partially reversed may result in RV overload secondary to PH, which puts the patient at higher risk for right-sided congestive heart failure and acute myocardial failure.5,8,11 Additionally, the defect’s proximity to the AV complicates surgical closure and increases the risk of postoperative iatrogenic AR.8,9
Some degree of PH was suspected because of the patient’s mild to moderate concentric hypertrophy of the right ventricle, septal flattening, possibility of some degree of right-to-left flow across the VSD, and severe dilation of the MPA noted on both TTE and CTA. Characterization of the severity of RV hypertrophy was subjective due to the lack of established weight/age/breed reference ranges in goats. The patient’s RV concentric hypertrophy was thought to be an adaptive response to increased workload, secondary to the patient’s bidirectional VSD and some degree of PH.11 However, a prominent right heart in a pediatric goat could not be excluded as a cause for this finding.
Computed tomography angiography was performed to further evaluate the congenital heart disease and confirm any anomalies not identified on TTE. The CTA study revealed severe dilation of the PT. Initial differentials of the severe MPA dilation included PH resulting from anomalous vascular connection to the MPA or due to altered cardiac hemodynamics from the VSD. Further evidence of PH, such as dilation of peripheral pulmonary vessels or dilated bronchial arteries, was not appreciated. The origin of the turbulent flow within the MPA could not be identified via TTE, and a virtual 3D reconstruction of the heart and associated vasculature was created from the CTA datasets. The 3D model confirmed no evidence of PDA and the absence of other aberrant systemic-to-pulmonary communication. Therefore, the turbulent flow and dilation within the PT were considered to be secondary to increased blood flow from the previously described DCJA VSD.
Initially introduced as a palliative procedure in human children with large left-to-right shunts, PAB was considered to slow the progression of PH and prolong the onset of congestive heart failure.5 It has also been successfully performed in animals diagnosed with hemodynamically significant VSDs.5 In anatomically immature animals, such as the doeling in this case, accounting for growth while determining optimal band tightness during surgery is challenging. Utilizing a dilatable band prevents excessive band tightening and subsequent shunt reversal as the animal matures.5 Due to a lack of literature regarding percutaneous balloon dilation of a PA band in animals and this patient’s lack of clinical signs, surgical correction and/or medical management for PH was not initiated at the time of diagnosis. In the future, the patient will be monitored with serial TTE and packed cell volume testing to assess for worsening disease, which could warrant further diagnostics or intervention, such as diagnostic catheterization, a sildenafil trial, or PAB with concurrent transcatheter RV pressure monitoring.
Incidentally, CTA and subsequent 3D reconstruction of the doeling also identified the presence of a PLCVC draining to the right atrium through an enlarged coronary sinus (Figure 6). A PLCVC, resulting from the failure of regression of the left anterior cardinal vein during embryonic development, is the most commonly reported congenital thoracic venous malformation of humans and dogs, with a prevalence of 4.4% in humans with cardiac disease and up to 5% in dogs with other cardiovascular abnormalities.2,12 There are limited reports of a PLCVC in goats; diagnosis is often made incidentally during investigation of more clinically significant cardiac malformations such as cardiac septal defects.2,12 However, identification of a PLCVC can affect the planning of many surgical or interventional procedures if the left superior venous approach is considered.2,12 If surgical intervention is indicated in the future for this case, the presence and connection of the PLCVC will be considered in surgical planning.
Conclusion
This patient highlights the use of multiple imaging modalities, including thoracic radiography, TTE, and CTA, to characterize congenital heart disease in a goat. In addition, virtual 3D models of the patient's cardiovascular system provided further insight into the patient's anatomy.
Ethics Statement
The authors declare that the work described here has been carried out in accordance with the ethical policies, animal use approval, and with appropriate owner permissions by the University of Georgia Veterinary Teaching Hospital.
Consent Statement
The authors declare that since this was a non-interventional, retrospective, observational study utilizing de-identified data, informed consent was not required from the patient under an IRB exemption status.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Acknowledgments
We thank Drs. Robson Giglio and Michael Perlini for providing their insight in image interpretation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2024.05.004.
Supplementary Materials
Two-dimensional TTE, right parasternal four-chamber long-axis view, demonstrates moderately enlarged left atrium and left ventricle and a dilated right branch PA.
Two-dimensional TTE, right parasternal short-axis view, demonstrates a DCJA VSD and dilation of the PT and right branch PA.
Two-dimensional TTE, right parasternal 5-chamber long-axis view without (left) and with (right) color-flow Doppler demonstrates bidirectional shunting through the VSD.
Two-dimensional TTE, right parasternal short-axis view without (left) and with (right) color-flow Doppler demonstrates bidirectional shunting through the VSD and turbulent flow in the RV outflow tract.
Two-dimensional TTE, right parasternal transverse view demonstrates systolic septal flattening.
Two-dimensional TTE, left parasternal short-axis view with color flow Doppler demonstrating flow in the distal PA.
References
- 1.Haake C., Kovacs S.L., Choi E.A. A retrospective study of congenital cardiac malformations in 29 goats. J Vet Diagn Invest. 2023;35:405–409. doi: 10.1177/10406387231171568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranjan R., Dosdall D., Norlund L., et al. Diagnostic imaging and pacemaker implantation in a domestic goat with persistent left cranial vena cava. J Vet Cardiol. 2014;16:45–50. doi: 10.1016/j.jvc.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy D.W., Pugh D.G. Sheep and Goat Medicine (Second Edition) Saunders/Elselvier; Maryland Heights, MO: 2012. Handling and examining sheep and goats; pp. 1–17. chap 1. [Google Scholar]
- 4.Ukaha R.O., Kene R.O.C., Gbonko O.E. Vertebral scale system to measure heart size in thoracic radiographs of west African Dwarf goats. Niger Vet J. 2015;34:912–916. [Google Scholar]
- 5.Sutherland B.J., Pierce K.V., Gagnon A.L., Scansen B.A., Orton E.C. Dilatable pulmonary artery banding for ventricular septal defect: surgical technique and case report of three cats. J Vet Cardiol. 2019;25:32–40. doi: 10.1016/j.jvc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Lopez L., Houyel L., Colan S.D., et al. Classification of ventricular septal defects for the eleventh iteration of the international classification of diseases—striving for consensus: a report from the international society for nomenclature of paediatric and congenital heart disease. Ann Thorac Surg. 2018;106:1578–1589. doi: 10.1016/j.athoracsur.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy K.P., Ho S.R., Anderson R.H. Ventricular septal defects: morphology of the doubly committed juxtaarterial and muscular variants. Images Paediatr Cardiol. 2000;2:5–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Devlin P.J., Russell H.M., Mongé M.C., et al. Doubly committed and juxtaarterial ventricular septal defect: outcomes of the aortic and pulmonary valves. Ann Thorac Surg. 2014;97:2134–2141. doi: 10.1016/j.athoracsur.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 9.Griffin M.L., Sullivan I.D., Anderson R.H., Macartney F.J. Doubly committed subarterial ventricular septal defect:new morphological criteria with echocardiographic and angiocardiographic correlation. Br Heart J. 1988;59:474–479. doi: 10.1136/hrt.59.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serres F., Chetboul V., Tissier R., et al. Quantification of pulmonary to systemic flow ratio by a Doppler echocardiographic method in the normal dog: repeatability, reproducibility, and reference ranges. J Vet Cardiol. 2009;11:23–29. doi: 10.1016/j.jvc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Reinero C., Visser L.C., Kellihan H.B., Masseau I., Rozanski E., Clercx C., et al. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J Vet Intern Med. 2020;34:549–573. doi: 10.1111/jvim.15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S., Song Y., Lee Y., Choi H. Imaging characteristics of persistent left cranial vena cava incidentally diagnosed with computed tomography in dogs. J Vet Med Sci. 2016;78:1601–1606. doi: 10.1292/jvms.15-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE, right parasternal four-chamber long-axis view, demonstrates moderately enlarged left atrium and left ventricle and a dilated right branch PA.
Two-dimensional TTE, right parasternal short-axis view, demonstrates a DCJA VSD and dilation of the PT and right branch PA.
Two-dimensional TTE, right parasternal 5-chamber long-axis view without (left) and with (right) color-flow Doppler demonstrates bidirectional shunting through the VSD.
Two-dimensional TTE, right parasternal short-axis view without (left) and with (right) color-flow Doppler demonstrates bidirectional shunting through the VSD and turbulent flow in the RV outflow tract.
Two-dimensional TTE, right parasternal transverse view demonstrates systolic septal flattening.
Two-dimensional TTE, left parasternal short-axis view with color flow Doppler demonstrating flow in the distal PA.