Abstract
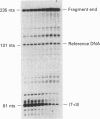
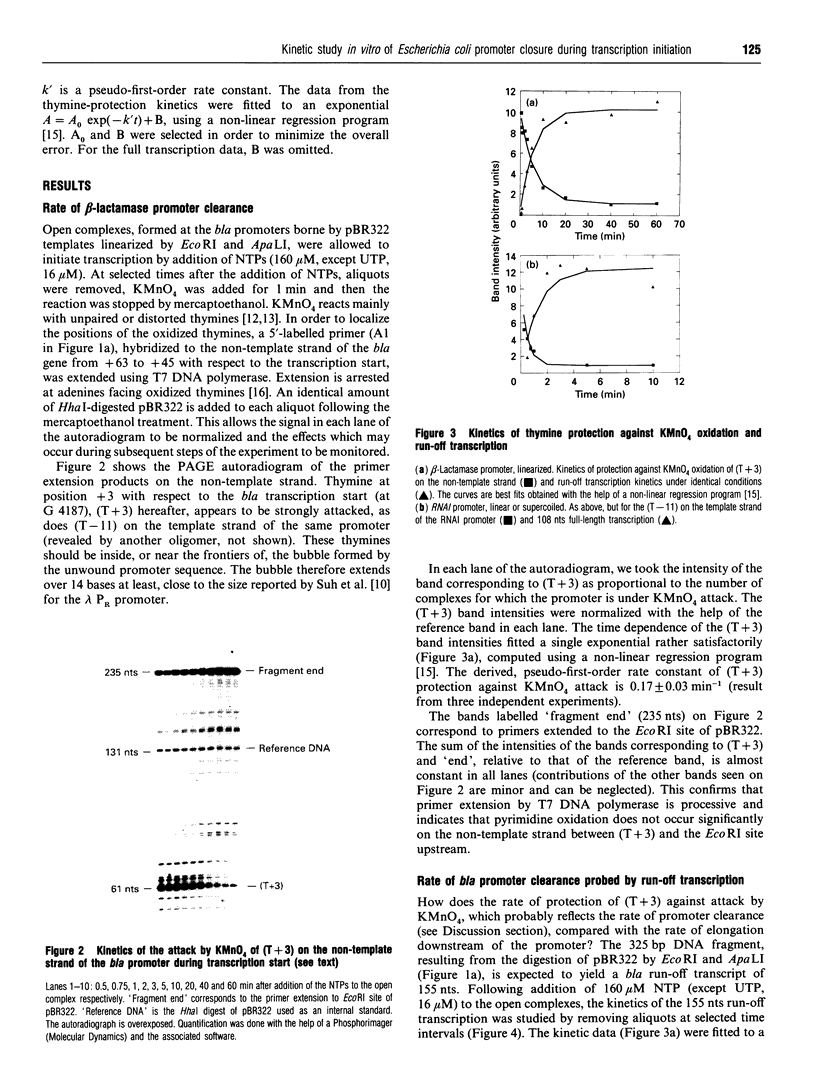
The rate of closure of two Escherichia coli promoters borne by plasmid pBR322, following transcription initiation from the open complex, was probed in vitro by the protection of unpaired thymines in the open complex against oxidation by KMnO4. Run-off transcription kinetics were also studied under identical conditions. Closure of the open promoter appears to be by far the rate-limiting step of transcription initiation and elongation for the linearized beta-lactamase gene, and is strongly dependent on template topology for the RNAI gene. It is suggested that the corresponding signals are deposited 30 bases at least downstream of transcription initiation and that promoter closure, and its clearance by elongating RNA polymerase, may occur almost simultaneously.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borowiec J. A., Zhang L., Sasse-Dwight S., Gralla J. D. DNA supercoiling promotes formation of a bent repression loop in lac DNA. J Mol Biol. 1987 Jul 5;196(1):101–111. doi: 10.1016/0022-2836(87)90513-4. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Functional effects of cis-thymine glycol lesions on DNA synthesis in vitro. Biochemistry. 1987 Aug 25;26(17):5398–5403. doi: 10.1021/bi00391a027. [DOI] [PubMed] [Google Scholar]
- Deuschle U., Kammerer W., Gentz R., Bujard H. Promoters of Escherichia coli: a hierarchy of in vivo strength indicates alternate structures. EMBO J. 1986 Nov;5(11):2987–2994. doi: 10.1002/j.1460-2075.1986.tb04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G. A nonlinear regression program for small computers. Anal Biochem. 1981 Jan 1;110(1):9–18. doi: 10.1016/0003-2697(81)90104-4. [DOI] [PubMed] [Google Scholar]
- Duval-Valentin G., Ehrlich R. Dynamic and structural characterisation of multiple steps during complex formation between E. coli RNA polymerase and the tetR promoter from pSC101. Nucleic Acids Res. 1987 Jan 26;15(2):575–594. doi: 10.1093/nar/15.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Valentin G., Ehrlich R. Far upstream sequences of the bla promoter from TN3 are involved in complexation with E. coli RNA-polymerase. Nucleic Acids Res. 1988 Mar 25;16(5):2031–2044. doi: 10.1093/nar/16.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Valentin G., Schmitt B., Ehrlich R. A second RNA-polymerase can bind specifically to the bla promoter of Tn3, repressing transcription initiation. Nucleic Acids Res. 1988 Jun 24;16(12):5277–5290. doi: 10.1093/nar/16.12.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R., Larousse A., Jacquet M. A., Marin M., Reiss C. In vitro transcription initiation from three different Escherichia coli promoters. Effect of supercoiling. Eur J Biochem. 1985 Apr 15;148(2):293–298. doi: 10.1111/j.1432-1033.1985.tb08838.x. [DOI] [PubMed] [Google Scholar]
- Gralla J. D. Promoter recognition and mRNA initiation by Escherichia coli E sigma 70. Methods Enzymol. 1990;185:37–54. doi: 10.1016/0076-6879(90)85006-a. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Ukita T. The selective degradation of pyrimidines in nucleic acids by permanganate oxidation. Biochem Biophys Res Commun. 1967 Nov 30;29(4):556–561. doi: 10.1016/0006-291x(67)90521-9. [DOI] [PubMed] [Google Scholar]
- Heumann H., Ricchetti M., Werel W. DNA-dependent RNA polymerase of Escherichia coli induces bending or an increased flexibility of DNA by specific complex formation. EMBO J. 1988 Dec 20;7(13):4379–4381. doi: 10.1002/j.1460-2075.1988.tb03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet M. A., Reiss C. Transcription in vivo directed by consensus sequences of E.coli promoters: their context heavily affects efficiencies and start sites. Nucleic Acids Res. 1990 Mar 11;18(5):1137–1143. doi: 10.1093/nar/18.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer W., Deuschle U., Gentz R., Bujard H. Functional dissection of Escherichia coli promoters: information in the transcribed region is involved in late steps of the overall process. EMBO J. 1986 Nov;5(11):2995–3000. doi: 10.1002/j.1460-2075.1986.tb04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel B., Chamberlin M. J. RNA chain initiation by Escherichia coli RNA polymerase. Structural transitions of the enzyme in early ternary complexes. Biochemistry. 1989 Sep 19;28(19):7829–7842. doi: 10.1021/bi00445a045. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Metzger W., Schickor P., Heumann H. A cinematographic view of Escherichia coli RNA polymerase translocation. EMBO J. 1989 Sep;8(9):2745–2754. doi: 10.1002/j.1460-2075.1989.tb08416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R., Bermúdez-Cruz R. M., Chamberlin M. J. Parameters affecting transcription termination by Escherichia coli RNA polymerase. I. Analysis of 13 rho-independent terminators. J Mol Biol. 1992 Mar 5;224(1):31–51. doi: 10.1016/0022-2836(92)90574-4. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. KMnO4 as a probe for lac promoter DNA melting and mechanism in vivo. J Biol Chem. 1989 May 15;264(14):8074–8081. [PubMed] [Google Scholar]
- Straney D. C., Crothers D. M. A stressed intermediate in the formation of stably initiated RNA chains at the Escherichia coli lac UV5 promoter. J Mol Biol. 1987 Jan 20;193(2):267–278. doi: 10.1016/0022-2836(87)90218-x. [DOI] [PubMed] [Google Scholar]
- Suh W. C., Ross W., Record M. T., Jr Two open complexes and a requirement for Mg2+ to open the lambda PR transcription start site. Science. 1993 Jan 15;259(5093):358–361. doi: 10.1126/science.8420002. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Structure and function of E. coli promoter DNA. CRC Crit Rev Biochem. 1987;22(3):181–219. doi: 10.3109/10409238709101483. [DOI] [PubMed] [Google Scholar]
- Wang F. J., Reiss C. Transcription pausing signal detected by sense/antisense transcription. Biochem Mol Biol Int. 1993 Aug;30(5):983–994. [PubMed] [Google Scholar]
- Watson N. A new revision of the sequence of plasmid pBR322. Gene. 1988 Oct 30;70(2):399–403. doi: 10.1016/0378-1119(88)90212-0. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]