Abstract
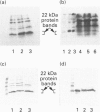
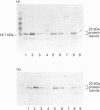
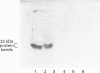
1. Earlier studies have shown that exposure of fat-cells to insulin results in the rapid increased phosphorylation of an acid-soluble protein which migrates as a doublet on SDS/PAGE with an apparent molecular mass of close to 22 kDa; agents such as isoprenaline, which increase cell concentrations of cyclic AMP, also increase phosphorylation, but to a lesser extent [Belsham, Brownsey, Hughes and Denton (1980) Diabetologia 18, 307-312; Diggle and Denton (1992) Biochem. J. 282, 729-736]. 2. The protein has been purified from rat epididymal adipose tissue, and the sequences of six tryptic peptides were determined. All six peptides are present in the deduced sequence of a protein of similar properties, designated PHAS-I by Hu, Pang, Kong, Velleca and Lawrence [(1994) Proc. Natl. Acad. Sci. U.S.A. 91, 3730-3734]. Hence the proteins are the same or extremely similar. 3. A rabbit anti-peptide antibody has been raised against one of the peptides (AGGDESQFEMD). The antibody was found to be highly specific for the phosphorylated and non-phosphorylated forms of the acid-soluble 22 kDa protein in Western blots and by immunoprecipitation. Studies with the antibody preparation have shown that both phosphorylated and non-phosphorylated forms of the protein appear to be exclusively located in the cytoplasm, and that exposure of cells to isoprenaline causes increased phosphorylation of the same acid-soluble 22 kDa protein as does insulin treatment. 4. Western blots carried out with the antibody preparation indicate that the protein is also present in other insulin-sensitive tissues, including liver, skeletal muscle, heart and brown adipose tissue. The protein was also detected in lung and spleen, but not brain and kidney. It is concluded that the protein may play an important role in some of the actions of insulin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belsham G. J., Brownsey R. W., Denton R. M. Reversibility of the insulin-stimulated phosphorylation of ATP citrate lyase and a cytoplasmic protein of subunit Mr 22000 in adipose tissue. Biochem J. 1982 Apr 15;204(1):345–352. doi: 10.1042/bj2040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G. J., Brownsey R. W., Hughes W. A., Denton R. M. Anti-insulin receptor antibodies mimic the effects of insulin on the activities of pyruvate dehydrogenase and acetylCoA carboxylase and on specific protein phosphorylation in rat epididymal fat cells. Diabetologia. 1980 Apr;18(4):307–312. doi: 10.1007/BF00251011. [DOI] [PubMed] [Google Scholar]
- Belsham G. J., Denton R. M. The effect of insulin and adrenaline on the phosphorylation of a 22 000-molecular weight protein within isolated fat cells; possible identification as the inhibitor-1 of the 'general phosphatase' [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):382–383. doi: 10.1042/bst0080382. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nemenoff R. A., Avruch J. Insulin and growth factors stimulate the phosphorylation of a Mr-22000 protein in 3T3-L1 adipocytes. Biochem J. 1983 Jul 15;214(1):11–19. doi: 10.1042/bj2140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Nemenoff R. A., Avruch J. Preliminary characterization of a heat-stable protein from rat adipose tissue whose phosphorylation is stimulated by insulin. Biochem J. 1982 Jun 15;204(3):817–824. doi: 10.1042/bj2040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Borthwick A. C., Edgell N. J., Denton R. M. Protein-serine kinase from rat epididymal adipose tissue which phosphorylates and activates acetyl-CoA carboxylase. Possible role in insulin action. Biochem J. 1990 Sep 15;270(3):795–801. doi: 10.1042/bj2700795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownsey R. W., Hughes W. A., Denton R. M. Adrenaline and the regulation of acetyl-coenzyme A carboxylase in rat epididymal adipose tissue. Inactivation of the enzyme is associated with phosphorylation and can be reversed on dephosphorylation. Biochem J. 1979 Oct 15;184(1):23–32. doi: 10.1042/bj1840023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M. Early events in insulin actions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:293–341. [PubMed] [Google Scholar]
- Diggle T. A., Denton R. M. Comparison of the effects of insulin and adrenergic agonists on the phosphorylation of an acid-soluble 22 kDa protein in rat epididymal fat-pads and isolated fat-cells. Biochem J. 1992 Mar 15;282(Pt 3):729–736. doi: 10.1042/bj2820729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle T. A., Schmitz-Peiffer C., Borthwick A. C., Welsh G. I., Denton R. M. Evidence that insulin activates casein kinase 2 in rat epididymal fat-cells and that this may result in the increased phosphorylation of an acid-soluble 22 kDa protein. Biochem J. 1991 Oct 15;279(Pt 2):545–551. doi: 10.1042/bj2790545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Hu C., Pang S., Kong X., Velleca M., Lawrence J. C., Jr Molecular cloning and tissue distribution of PHAS-I, an intracellular target for insulin and growth factors. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3730–3734. doi: 10.1073/pnas.91.9.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. A., Brownsey R. W., Denton R. M. Studies on the incorporation of [32P]phosphate into pyruvate dehydrogenase in intact rat fat-cells. Effects of insulin. Biochem J. 1980 Nov 15;192(2):469–481. doi: 10.1042/bj1920469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin T. A., Kong X., Haystead T. A., Pause A., Belsham G., Sonenberg N., Lawrence J. C., Jr PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 1994 Oct 28;266(5185):653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- Palfreyman R. W., Clark A. E., Denton R. M., Holman G. D., Kozka I. J. Kinetic resolution of the separate GLUT1 and GLUT4 glucose transport activities in 3T3-L1 cells. Biochem J. 1992 May 15;284(Pt 1):275–282. doi: 10.1042/bj2840275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994 Oct 27;371(6500):762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Vargas A. M., Halestrap A. P., Denton R. M. The effects of glucagon, phenylephrine and insulin on the phosphorylation of cytoplasmic, mitochondrial and membrane-bound proteins of intact liver cells from starved rats. Biochem J. 1982 Oct 15;208(1):221–229. doi: 10.1042/bj2080221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Watts T. D., Gould G. W., Lienhard G. E., Gibbs E. M. Regulation of protein phosphorylation by insulin and an insulinomimetic oligosaccharide in 3T3-L1 adipocytes and Fao hepatoma cells. Biochem Biophys Res Commun. 1988 Jun 30;153(3):992–998. doi: 10.1016/s0006-291x(88)81326-3. [DOI] [PubMed] [Google Scholar]
- Zhang B., Tavaré J. M., Ellis L., Roth R. A. The regulatory role of known tyrosine autophosphorylation sites of the insulin receptor kinase domain. An assessment by replacement with neutral and negatively charged amino acids. J Biol Chem. 1991 Jan 15;266(2):990–996. [PubMed] [Google Scholar]