Abstract
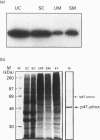
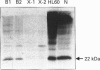
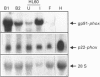
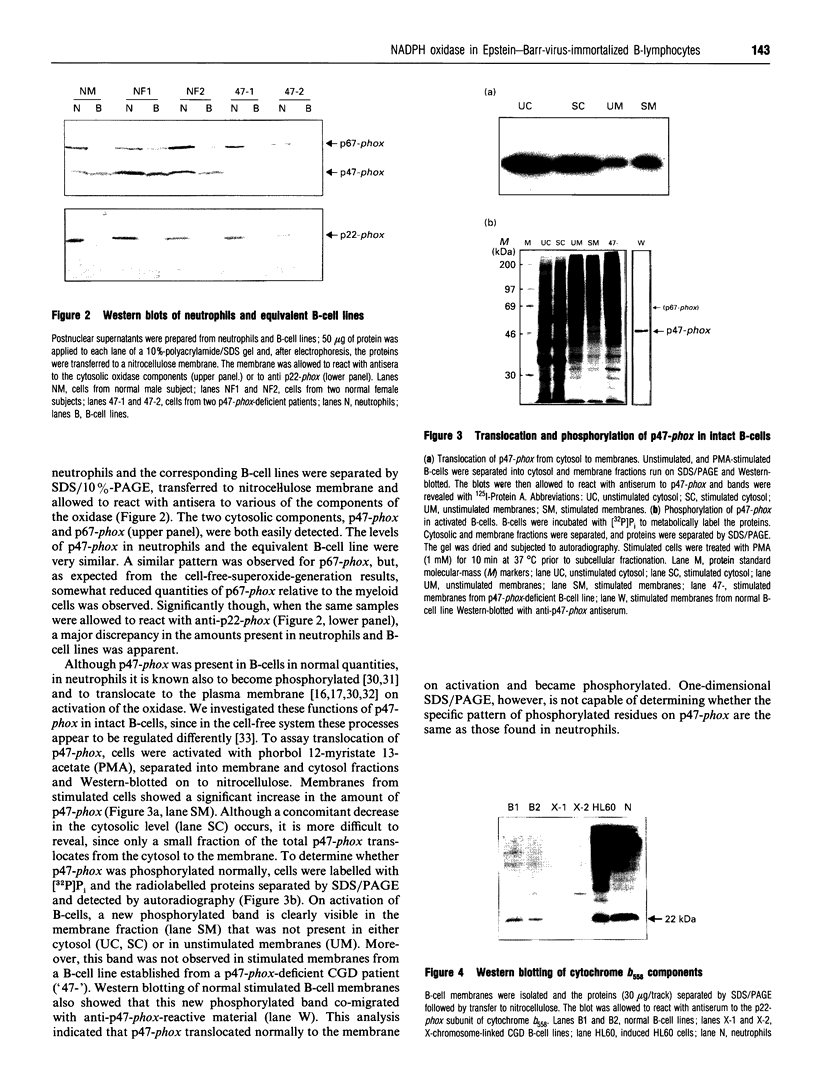
The NADPH oxidase of phagocytes is known to be expressed in Epstein-Barr-virus-transformed B-lymphocytes, albeit at levels only approx. 5% of those found in neutrophils. We have investigated the basis of this low level of expression and find that all four specific components of the NADPH oxidase are expressed in B-lymphocytes, but only p47-phox protein attains levels equivalent with those found in neutrophils. This component was shown to phosphorylate and translocate to the membrane normally on activation. The other cytosolic component, p67-phox, did show a deficit, and by supplementing a B-cell cytosol extract with recombinant p67-phox, this was shown to account for the somewhat reduced activity of B-cell cytosol in a cell-free oxidase system. The cell-free analysis also clearly located the major deficiency in superoxide-generating capacity of B-lymphocytes to the membrane. Western blotting of membrane proteins revealed major reductions in the amount of cytochrome b558. Analysis of the levels of mRNA for both subunits of cytochrome b558, however, showed levels greater than expected. Significantly more mRNA for gp91-phox was present in B-cells than in undifferentiated HL60 cells, although it was not quite as abundant as in differentiated HL60 cells, which are capable of producing large amounts of superoxide. We conclude that the failure of B-lymphocytes to generate amounts of superoxide equivalent to those generated by neutrophils is primarily due to a post-transcriptionally determined block to the accumulation of cytochrome b558.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Boyhan A., West I., Thrasher A. J., Segal A. W. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b-245. J Biol Chem. 1992 Aug 25;267(24):16767–16770. [PubMed] [Google Scholar]
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991 Oct 17;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Abo A., Pick E. Purification and characterization of a third cytosolic component of the superoxide-generating NADPH oxidase of macrophages. J Biol Chem. 1991 Dec 15;266(35):23577–23585. [PubMed] [Google Scholar]
- Casimir C. M., Bu-Ghanim H. N., Rodaway A. R., Bentley D. L., Rowe P., Segal A. W. Autosomal recessive chronic granulomatous disease caused by deletion at a dinucleotide repeat. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2753–2757. doi: 10.1073/pnas.88.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir C., Chetty M., Bohler M. C., Garcia R., Fischer A., Griscelli C., Johnson B., Segal A. W. Identification of the defective NADPH-oxidase component in chronic granulomatous disease: a study of 57 European families. Eur J Clin Invest. 1992 Jun;22(6):403–406. doi: 10.1111/j.1365-2362.1992.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Malech H. L., Gallin J. I., Nunoi H., Volpp B. D., Pearson D. W., Nauseef W. M., Curnutte J. T. Genetic variants of chronic granulomatous disease: prevalence of deficiencies of two cytosolic components of the NADPH oxidase system. N Engl J Med. 1989 Sep 7;321(10):647–652. doi: 10.1056/NEJM198909073211005. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Volpp B. D., Leidal K. G., Nauseef W. M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest. 1990 Mar;85(3):714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Tanugi L., Morel F., Pilloud-Dagher M. C., Seigneurin J. M., Francois P., Bost M., Vignais P. V. Activation of O2(-)-generating oxidase in an heterologous cell-free system derived from Epstein-Barr-virus-transformed human B lymphocytes and bovine neutrophils. Application to the study of defects in cytosolic factors in chronic granulomatous disease. Eur J Biochem. 1991 Dec 5;202(2):649–655. doi: 10.1111/j.1432-1033.1991.tb16419.x. [DOI] [PubMed] [Google Scholar]
- Dinauer M. C., Curnutte J. T., Rosen H., Orkin S. H. A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J Clin Invest. 1989 Dec;84(6):2012–2016. doi: 10.1172/JCI114393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Pierce E. A., Bruns G. A., Curnutte J. T., Orkin S. H. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990 Nov;86(5):1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusi S., Della Bianca V., Grzeskowiak M., Rossi F. Relationship between phosphorylation and translocation to the plasma membrane of p47phox and p67phox and activation of the NADPH oxidase in normal and Ca(2+)-depleted human neutrophils. Biochem J. 1993 Feb 15;290(Pt 1):173–178. doi: 10.1042/bj2900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A. M., Chaplin M. F., Segal A. W. Cytochrome b-245 from human neutrophils is a glycoprotein. Biochem J. 1985 May 1;227(3):783–788. doi: 10.1042/bj2270783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Curnutte J. T., Nauseef W. M., Volpp B. D., Pearson D. W., Rosen H., Clark R. A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J Clin Invest. 1991 Jan;87(1):352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Shrimpton C. F., Segal A. W. Localization of the 47 kDa phosphoprotein involved in the respiratory-burst NADPH oxidase of phagocytic cells. Biochem J. 1989 May 15;260(1):243–248. doi: 10.1042/bj2600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Evans T., Curnutte J. T., Bokoch G. M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991 Dec 6;254(5037):1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- Levy R., Rotrosen D., Nagauker O., Leto T. L., Malech H. L. Induction of the respiratory burst in HL-60 cells. Correlation of function and protein expression. J Immunol. 1990 Oct 15;145(8):2595–2601. [PubMed] [Google Scholar]
- Maly F. E., Cross A. R., Jones O. T., Wolf-Vorbeck G., Walker C., Dahinden C. A., De Weck A. L. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J Immunol. 1988 Apr 1;140(7):2334–2339. [PubMed] [Google Scholar]
- Maly F. E., Nakamura M., Gauchat J. F., Urwyler A., Walker C., Dahinden C. A., Cross A. R., Jones O. T., de Weck A. L. Superoxide-dependent nitroblue tetrazolium reduction and expression of cytochrome b-245 components by human tonsillar B lymphocytes and B cell lines. J Immunol. 1989 Feb 15;142(4):1260–1267. [PubMed] [Google Scholar]
- Minegishi N., Nakamura M., Suzaki K., Terasawa M., Minegishi M., Tsuchiya S., Konno T. Chronic granulomatous disease with neutrophil membrane cytochrome b deficiency: demonstration by immunochemical staining with monoclonal antibody. Tohoku J Exp Med. 1988 Feb;154(2):143–148. doi: 10.1620/tjem.154.143. [DOI] [PubMed] [Google Scholar]
- Morel F., Cohen Tanugi Cholley L., Brandolin G., Dianoux A. C., Martel C., Champelovier P., Seigneurin J. M., Francois P., Bost M., Vignais P. V. The O2- generating oxidase of B lymphocytes: Epstein-Barr virus-immortalized B lymphocytes as a tool for the identification of defective components of the oxidase in chronic granulomatous disease. Biochim Biophys Acta. 1993 Aug 4;1182(1):101–109. doi: 10.1016/0925-4439(93)90159-x. [DOI] [PubMed] [Google Scholar]
- Nugent J. H., Gratzer W., Segal A. W. Identification of the haem-binding subunit of cytochrome b-245. Biochem J. 1989 Dec 15;264(3):921–924. doi: 10.1042/bj2640921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C. A., Dinauer M. C., Jesaitis A. J., Orkin S. H., Curnutte J. T. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989 May 1;73(6):1416–1420. [PubMed] [Google Scholar]
- Parkos C. A., Dinauer M. C., Walker L. E., Allen R. A., Jesaitis A. J., Orkin S. H. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci U S A. 1988 May;85(10):3319–3323. doi: 10.1073/pnas.85.10.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. D., Parkar M. H., Collins M. K., Levinsky R. J., Kinnon C. Superoxide production by normal and chronic granulomatous disease (CGD) patient-derived EBV-transformed B cell lines measured by chemiluminescence-based assays. J Immunol Methods. 1992 Nov 5;155(2):151–157. doi: 10.1016/0022-1759(92)90281-w. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Mullen M. L., Jesaitis A. J. Human neutrophil cytochrome b contains multiple hemes. Evidence for heme associated with both subunits. J Biol Chem. 1992 Apr 15;267(11):7303–7309. [PubMed] [Google Scholar]
- Rotrosen D., Leto T. L. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor. Translocation to membrane is associated with distinct phosphorylation events. J Biol Chem. 1990 Nov 15;265(32):19910–19915. [PubMed] [Google Scholar]
- Rotrosen D., Yeung C. L., Katkin J. P. Production of recombinant cytochrome b558 allows reconstitution of the phagocyte NADPH oxidase solely from recombinant proteins. J Biol Chem. 1993 Jul 5;268(19):14256–14260. [PubMed] [Google Scholar]
- Rotrosen D., Yeung C. L., Leto T. L., Malech H. L., Kwong C. H. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992 Jun 5;256(5062):1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993 Feb;18(2):43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985 Aug 8;316(6028):547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature. 1978 Nov 30;276(5687):515–517. doi: 10.1038/276515a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., West I., Wientjes F., Nugent J. H., Chavan A. J., Haley B., Garcia R. C., Rosen H., Scrace G. Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem J. 1992 Jun 15;284(Pt 3):781–788. doi: 10.1042/bj2840781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teahan C., Rowe P., Parker P., Totty N., Segal A. W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. 1987 Jun 25-Jul 1Nature. 327(6124):720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Thrasher A., Chetty M., Casimir C., Segal A. W. Restoration of superoxide generation to a chronic granulomatous disease-derived B-cell line by retrovirus mediated gene transfer. Blood. 1992 Sep 1;80(5):1125–1129. [PubMed] [Google Scholar]
- Tyagi S. R., Neckelmann N., Uhlinger D. J., Burnham D. N., Lambeth J. D. Cell-free translocation of recombinant p47-phox, a component of the neutrophil NADPH oxidase: effects of guanosine 5'-O-(3-thiotriphosphate), diacylglycerol, and an anionic amphiphile. Biochemistry. 1992 Mar 17;31(10):2765–2774. doi: 10.1021/bi00125a017. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Clark R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988 Dec 2;242(4883):1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]