Abstract
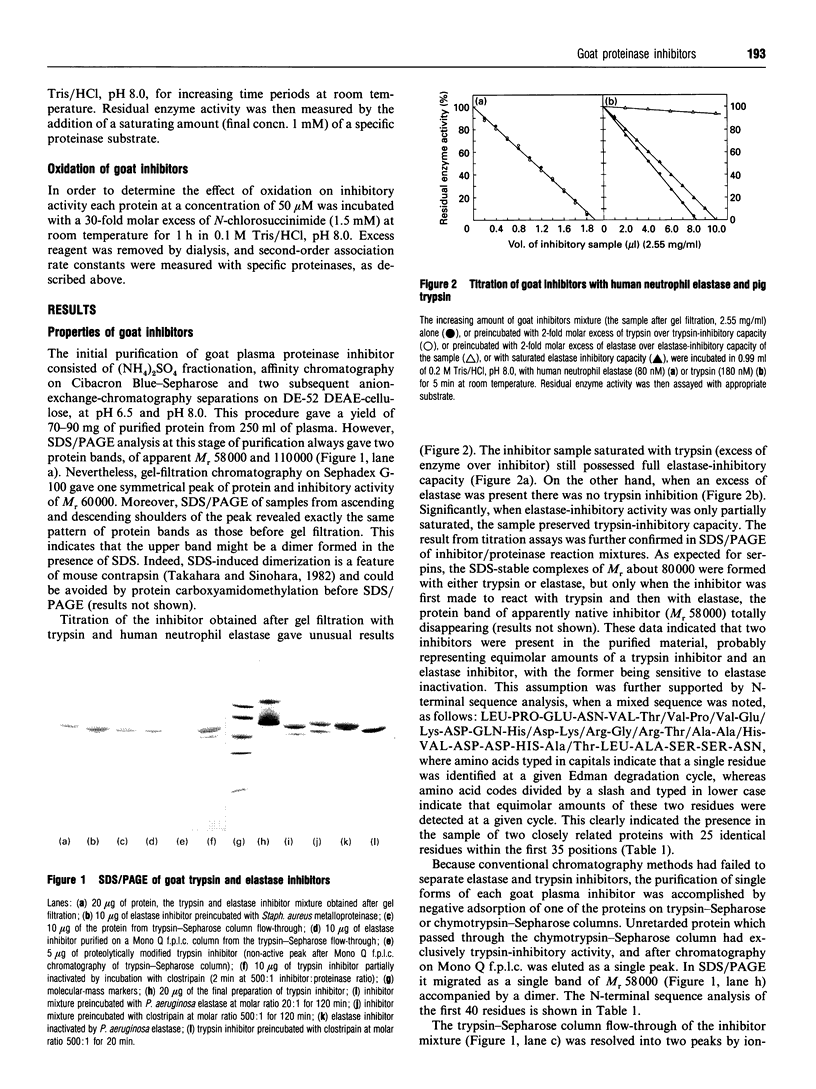
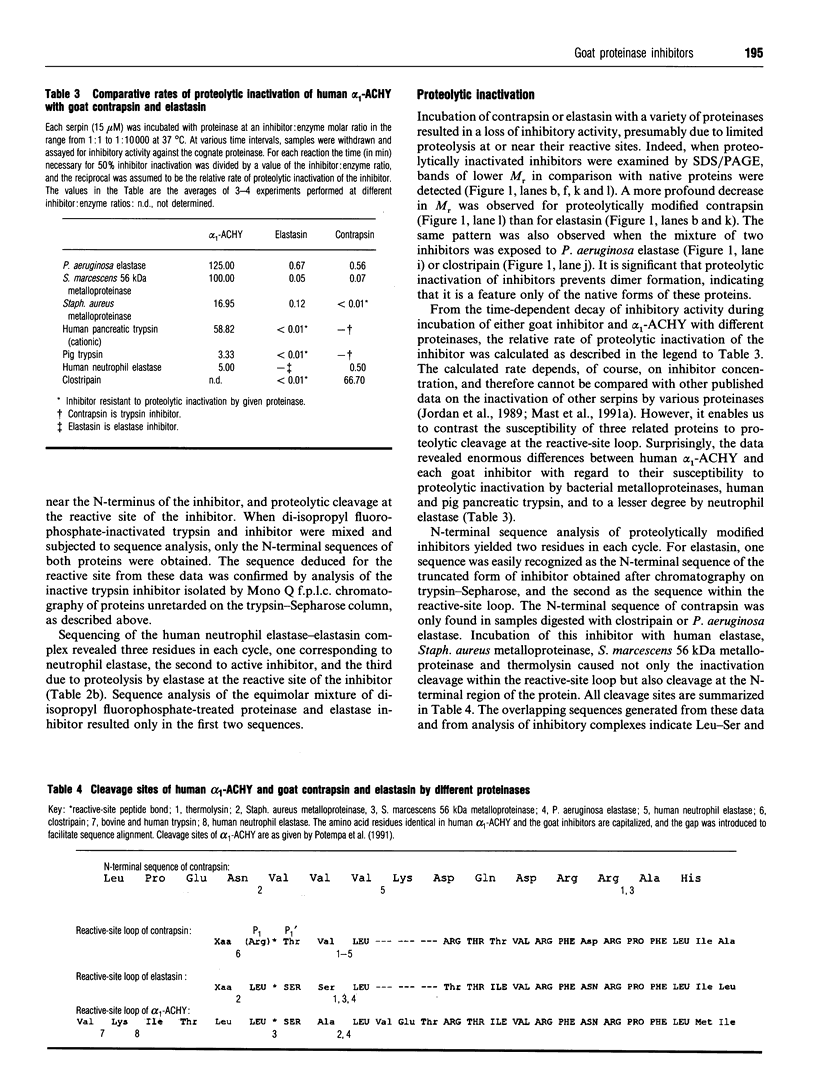
Two primary serine proteinase inhibitors in goat plasma have been isolated and characterized. The N-terminal sequence analysis of the purified proteins revealed that they are closely related to each other and are highly homologous to human alpha 1-anti-chymotrypsin rather than alpha 1-proteinase inhibitor. However, despite structural similarities the inhibitory specificity of the goat inhibitors differed from each other and from that of anti-chymotrypsin. In contrast with human anti-chymotrypsin, one of the goat inhibitors was shown to be a strong and specific inhibitor of trypsin (k(ass.) = 1.9 x 10(6) M-1.s-1), whereas the other was an efficient inhibitor of neutrophil elastase (k(ass.) = 1.5 x 10(6) M-1.S-1). Differences in the inhibitory specificity of each protein could readily be attributed to the amino acid sequence within the reactive site region. The trypsin inhibitor with an assumed arginine residue at the P1 position of the reactive-site peptide bond is referred to as 'contrapsin', and indicates that the occurrence of contrapsins is not restricted to rodents. In contrast, the inhibitory specificity, resistance to oxidative and proteolytic inactivation and the presence of a P1 leucine residue in the elastase inhibitor is unique among inhibitory serpins that have been characterized to date. Because this serpin is apparently the major elastase inhibitor in goat plasma, it is likely to be involved in the control of goat neutrophil elastase. Therefore, we suggest the name 'elastasin', and extend it to any other anti-chymotrypsin related serpins possessing neutrophil-elastase- inhibitory activity.
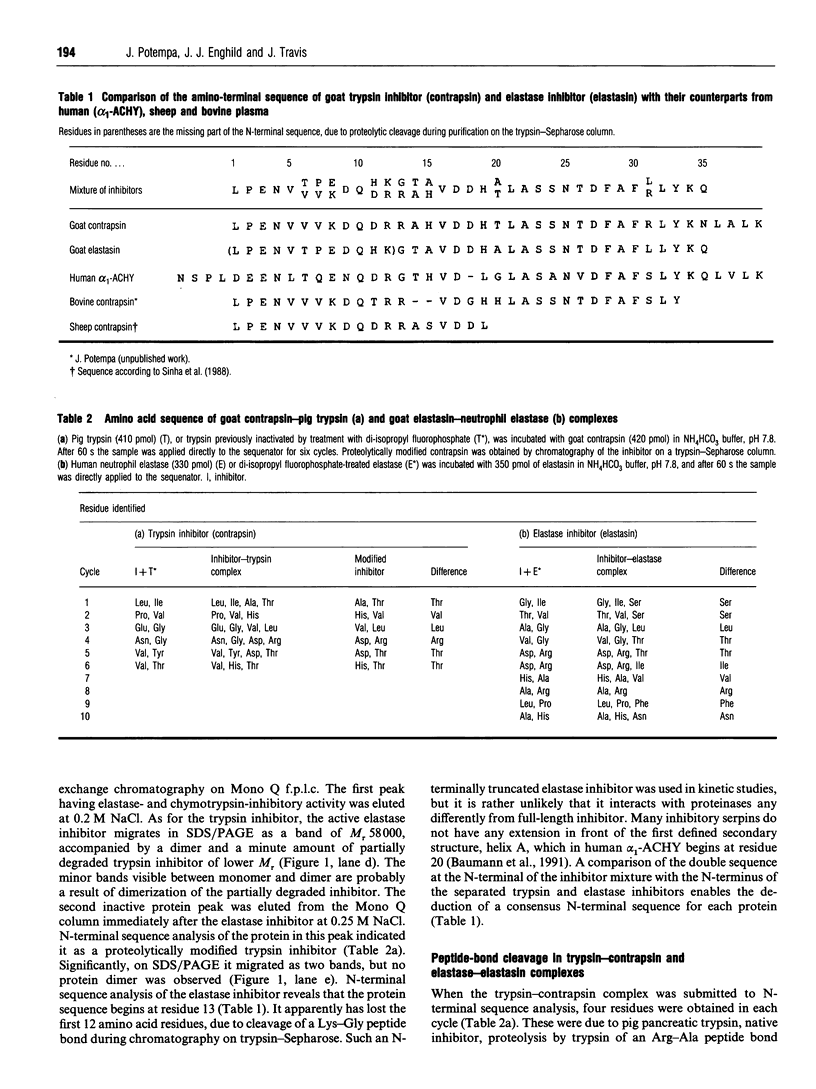
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Baumann U., Huber R., Bode W., Grosse D., Lesjak M., Laurell C. B. Crystal structure of cleaved human alpha 1-antichymotrypsin at 2.7 A resolution and its comparison with other serpins. J Mol Biol. 1991 Apr 5;218(3):595–606. doi: 10.1016/0022-2836(91)90704-a. [DOI] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Bieth J. G. Pathophysiological interpretation of kinetic constants of protease inhibitors. Bull Eur Physiopathol Respir. 1980;16 (Suppl):183–197. doi: 10.1016/b978-0-08-027379-2.50020-x. [DOI] [PubMed] [Google Scholar]
- Brockway W. J., Castellino F. J. Measurement of the binding of antifibrinolytic amino acids to various plasminogens. Arch Biochem Biophys. 1972 Jul;151(1):194–199. doi: 10.1016/0003-9861(72)90488-2. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Dziegielewska K. M., Foreman R. C., Saunders N. R., Wu Y. Nucleotide and deduced amino acid sequence of sheep alpha 1 antitrypsin. Nucleic Acids Res. 1989 Aug 11;17(15):6398–6398. doi: 10.1093/nar/17.15.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Bussolino F., Baglioni C. Synthesis and release of platelet-activating factor is inhibited by plasma alpha 1-proteinase inhibitor or alpha 1-antichymotrypsin and is stimulated by proteinases. J Exp Med. 1988 Oct 1;168(4):1293–1306. doi: 10.1084/jem.168.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Owen M. C. Plakalbumin, alpha 1-antitrypsin, antithrombin and the mechanism of inflammatory thrombosis. Nature. 1985 Oct 24;317(6039):730–732. doi: 10.1038/317730a0. [DOI] [PubMed] [Google Scholar]
- Chai K. X., Chen L. M., Chao J., Chao L. Kallistatin: a novel human serine proteinase inhibitor. Molecular cloning, tissue distribution, and expression in Escherichia coli. J Biol Chem. 1993 Nov 15;268(32):24498–24505. [PubMed] [Google Scholar]
- Chen Z., Potempa J., Polanowski A., Wikstrom M., Travis J. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. J Biol Chem. 1992 Sep 15;267(26):18896–18901. [PubMed] [Google Scholar]
- Christensson A., Laurell C. B., Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990 Dec 27;194(3):755–763. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- Coughlin P. B., Tetaz T., Salem H. H. Identification and purification of a novel serine proteinase inhibitor. J Biol Chem. 1993 May 5;268(13):9541–9547. [PubMed] [Google Scholar]
- Davril M., Laine A., Hayem A. Studies on the interactions of human pancreatic elastase 2 with human alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin. Biochem J. 1987 Aug 1;245(3):699–704. doi: 10.1042/bj2450699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers P. E., Jeffrey J. J., Weiss S. J. Interstitial collagenase (matrix metalloproteinase-1) expresses serpinase activity. J Clin Invest. 1991 Jun;87(6):2258–2265. doi: 10.1172/JCI115262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers P. E., Mookhtiar K., Van Wart H. E., Hasty K. A., Weiss S. J. Proteolytic inactivation of alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin by oxidatively activated human neutrophil metalloproteinases. J Biol Chem. 1992 Mar 5;267(7):5005–5012. [PubMed] [Google Scholar]
- Drapeau G. R. Role of metalloprotease in activation of the precursor of staphylococcal protease. J Bacteriol. 1978 Nov;136(2):607–613. doi: 10.1128/jb.136.2.607-613.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin A., Potempa J., Travis J. Isolation of nine human plasma proteinase inhibitors by sequential affinity chromatography. Prep Biochem. 1990;20(1):63–74. doi: 10.1080/00327489008050177. [DOI] [PubMed] [Google Scholar]
- Gilles A. M., Imhoff J. M., Keil B. alpha-Clostripain. Chemical characterization, activity, and thiol content of the highly active form of clostripain. J Biol Chem. 1979 Mar 10;254(5):1462–1468. [PubMed] [Google Scholar]
- Hercz A. Isolation and characterization of goat serum alpha 1-globulin protease inhibitors. Can J Biochem. 1982 Jan;60(1):28–35. doi: 10.1139/o82-005. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Shaw P. H., Boyd P. A., Baumann H., Hastie N. D. Plasma protease inhibitors in mouse and man: divergence within the reactive centre regions. Nature. 1984 Sep 13;311(5982):175–177. doi: 10.1038/311175a0. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Purviance R. T., Azaryan A. V., Hubbard G., Krieger T. J. Purification and characterization of alpha 1-antichymotrypsin-like protease inhibitor that regulates prohormone thiol protease involved in enkephalin precursor processing. J Biol Chem. 1993 Sep 25;268(27):20570–20577. [PubMed] [Google Scholar]
- Hudig D., Haverty T., Fulcher C., Redelman D., Mendelsohn J. Inhibition of human natural cytotoxicity by macromolecular antiproteases. J Immunol. 1981 Apr;126(4):1569–1574. [PubMed] [Google Scholar]
- Jallat S., Carvallo D., Tessier L. H., Roecklin D., Roitsch C., Ogushi F., Crystal R. G., Courtney M. Altered specificities of genetically engineered alpha 1 antitrypsin variants. Protein Eng. 1986 Oct-Nov;1(1):29–35. doi: 10.1093/protein/1.1.29. [DOI] [PubMed] [Google Scholar]
- Janoff A., Carp H. Possible mechanisms of emphysema in smokers: cigarette smoke condensate suppresses protease inhibition in vitro. Am Rev Respir Dis. 1977 Jul;116(1):65–72. doi: 10.1164/arrd.1977.116.1.65. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Travis J. Rapid purification of human trypsin and chymotrypsin I. Anal Biochem. 1976 May 7;72:573–576. doi: 10.1016/0003-2697(76)90568-6. [DOI] [PubMed] [Google Scholar]
- Jordan R. E., Nelson R. M., Kilpatrick J., Newgren J. O., Esmon P. C., Fournel M. A. Inactivation of human antithrombin by neutrophil elastase. Kinetics of the heparin-dependent reaction. J Biol Chem. 1989 Jun 25;264(18):10493–10500. [PubMed] [Google Scholar]
- Kilpatrick L., Johnson J. L., Nickbarg E. B., Wang Z. M., Clifford T. F., Banach M., Cooperman B. S., Douglas S. D., Rubin H. Inhibition of human neutrophil superoxide generation by alpha 1-antichymotrypsin. J Immunol. 1991 Apr 1;146(7):2388–2393. [PubMed] [Google Scholar]
- Kress L. F. Inactivation of human plasma serine proteinase inhibitors (serpins) by limited proteolysis of the reactive site loop with snake venom and bacterial metalloproteinases. J Cell Biochem. 1986;32(1):51–58. doi: 10.1002/jcb.240320106. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Evolutionary relationships among the serpins. Philos Trans R Soc Lond B Biol Sci. 1993 Oct 29;342(1300):101–119. doi: 10.1098/rstb.1993.0141. [DOI] [PubMed] [Google Scholar]
- Mast A. E., Enghild J. J., Nagase H., Suzuki K., Pizzo S. V., Salvesen G. Kinetics and physiologic relevance of the inactivation of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, and antithrombin III by matrix metalloproteinases-1 (tissue collagenase), -2 (72-kDa gelatinase/type IV collagenase), and -3 (stromelysin). J Biol Chem. 1991 Aug 25;266(24):15810–15816. [PubMed] [Google Scholar]
- Mast A. E., Enghild J. J., Pizzo S. V., Salvesen G. Analysis of the plasma elimination kinetics and conformational stabilities of native, proteinase-complexed, and reactive site cleaved serpins: comparison of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, antithrombin III, alpha 2-antiplasmin, angiotensinogen, and ovalbumin. Biochemistry. 1991 Feb 12;30(6):1723–1730. doi: 10.1021/bi00220a039. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Gibson H. L., Hallewell R. A., Barr P. J., Travis J. Recombinant DNA-derived forms of human alpha 1-proteinase inhibitor. Studies on the alanine 358 and cysteine 358 substituted mutants. J Biol Chem. 1986 Aug 5;261(22):10404–10409. [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Tsuda M., Kusumi T., Takada S., Shimamura T., Katsunuma T. Enhancement by alpha-1-antichymotrypsin of antibody response in vivo. Biochem Biophys Res Commun. 1981 May 15;100(1):478–482. doi: 10.1016/s0006-291x(81)80121-0. [DOI] [PubMed] [Google Scholar]
- Mistry R., Snashall P. D., Totty N., Guz A., Tetley T. D. Isolation and characterization of sheep alpha 1-proteinase inhibitor. Biochem J. 1991 Feb 1;273(Pt 3):685–690. doi: 10.1042/bj2730685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Harada M., Iwata T. Purification of human plasma alpha 1-proteinase inhibitor and its inactivation by Pseudomonas aeruginosa elastase. J Biochem. 1984 Mar;95(3):795–804. doi: 10.1093/oxfordjournals.jbchem.a134671. [DOI] [PubMed] [Google Scholar]
- Nelson R. B., Siman R. Clipsin, a chymotrypsin-like protease in rat brain which is irreversibly inhibited by alpha-1-antichymotrypsin. J Biol Chem. 1990 Mar 5;265(7):3836–3843. [PubMed] [Google Scholar]
- Ohkubo K., Ogata S., Misumi Y., Takami N., Ikehara Y. Molecular cloning and characterization of rat contrapsin-like protease inhibitor and related proteins. J Biochem. 1991 Feb;109(2):243–250. [PubMed] [Google Scholar]
- Ohkubo K., Ogata S., Misumi Y., Takami N., Sinohara H., Ikehara Y. Cloning, structure and expression of cDNA for mouse contrapsin and a related protein. Biochem J. 1991 Jun 1;276(Pt 2):337–342. doi: 10.1042/bj2760337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages G., Rouayrenc J. F., Le Cam G., Mariller M., Le Cam A. Molecular characterization of three rat liver serine-protease inhibitors affected by inflammation and hypophysectomy. Protein and mRNA analysis and cDNA cloning. Eur J Biochem. 1990 Jun 20;190(2):385–391. doi: 10.1111/j.1432-1033.1990.tb15587.x. [DOI] [PubMed] [Google Scholar]
- Pannell R., Johnson D., Travis J. Isolation and properties of human plasma alpha-1-proteinase inhibitor. Biochemistry. 1974 Dec 17;13(26):5439–5445. doi: 10.1021/bi00723a031. [DOI] [PubMed] [Google Scholar]
- Patterson S. D. Mammalian alpha 1-antitrypsins: comparative biochemistry and genetics of the major plasma serpin. Comp Biochem Physiol B. 1991;100(3):439–454. doi: 10.1016/0305-0491(91)90202-o. [DOI] [PubMed] [Google Scholar]
- Potempa J., Watorek W., Travis J. The inactivation of human plasma alpha 1-proteinase inhibitor by proteinases from Staphylococcus aureus. J Biol Chem. 1986 Oct 25;261(30):14330–14334. [PubMed] [Google Scholar]
- Salahuddin P. Isolation and characterization of alpha-1-proteinase inhibitor from goat plasma. Biochem Int. 1991 May;24(2):321–338. [PubMed] [Google Scholar]
- Schechter N. M., Sprows J. L., Schoenberger O. L., Lazarus G. S., Cooperman B. S., Rubin H. Reaction of human skin chymotrypsin-like proteinase chymase with plasma proteinase inhibitors. J Biol Chem. 1989 Dec 15;264(35):21308–21315. [PubMed] [Google Scholar]
- Sinha U., Sinha S., Janoff A. Characterization of sheep alpha-1-proteinase inhibitor. Important differences from the human protein. Am Rev Respir Dis. 1988 Mar;137(3):558–563. doi: 10.1164/ajrccm/137.3.558. [DOI] [PubMed] [Google Scholar]
- Steele F. R., Chader G. J., Johnson L. V., Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Yoshida K., Honda E., Sinohara H. Molecular cloning and sequence analysis of cDNAs coding for guinea pig alpha 1-antiproteinases S and F and contrapsin. J Biol Chem. 1991 Jan 15;266(2):928–932. [PubMed] [Google Scholar]
- Takahara H., Sinohara H. Mouse plasma trypsin inhibitors. Isolation and characterization of alpha-1-antitrypsin and contrapsin, a novel trypsin inhibitor. J Biol Chem. 1982 Mar 10;257(5):2438–2446. [PubMed] [Google Scholar]
- Travis J., Owen M., George P., Carrell R., Rosenberg S., Hallewell R. A., Barr P. J. Isolation and properties of recombinant DNA produced variants of human alpha 1-proteinase inhibitor. J Biol Chem. 1985 Apr 10;260(7):4384–4389. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Vankan D. M., Bell K. Caprine plasma proteinase inhibitors--I. Partial characterization. Comp Biochem Physiol B. 1993 Jan;104(1):101–108. doi: 10.1016/0305-0491(93)90344-5. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]



