Abstract
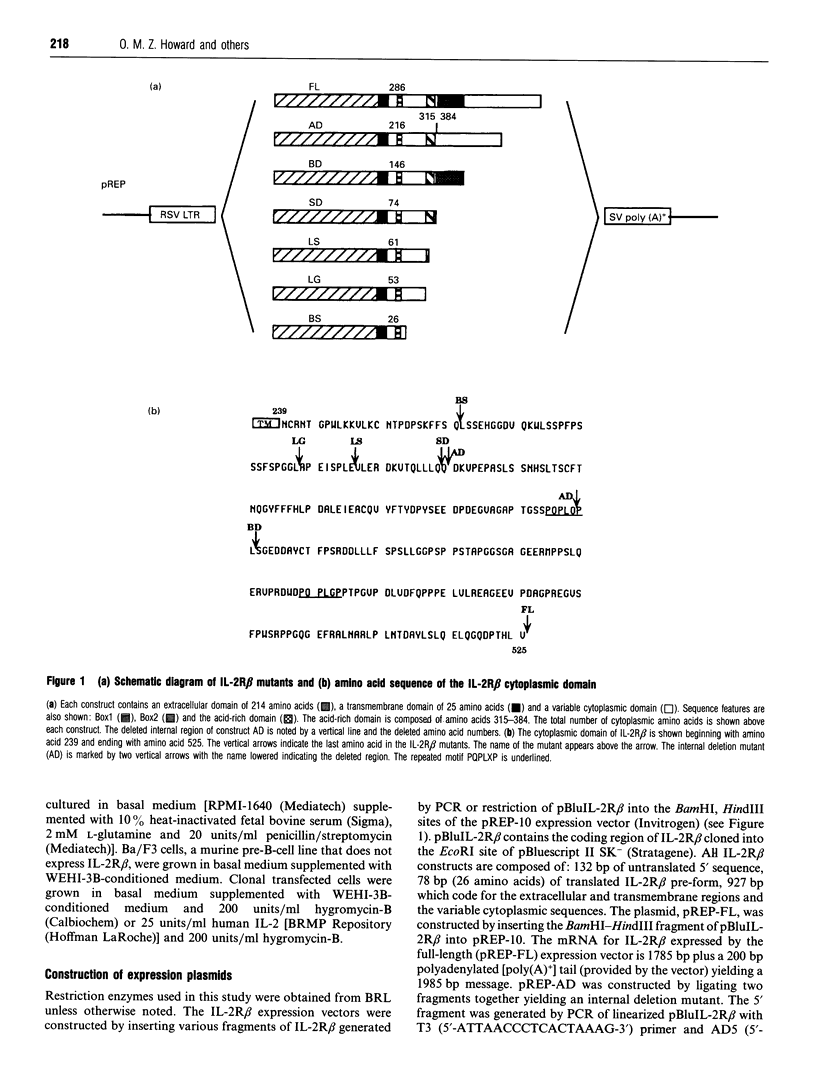
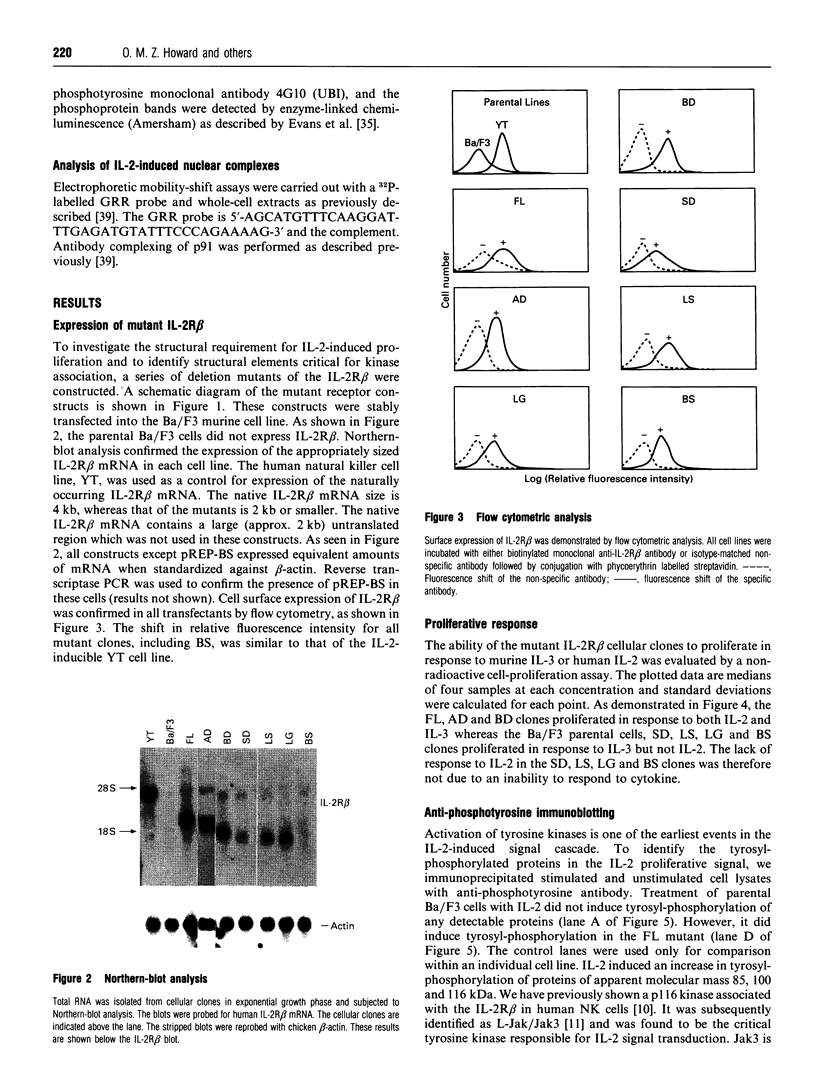
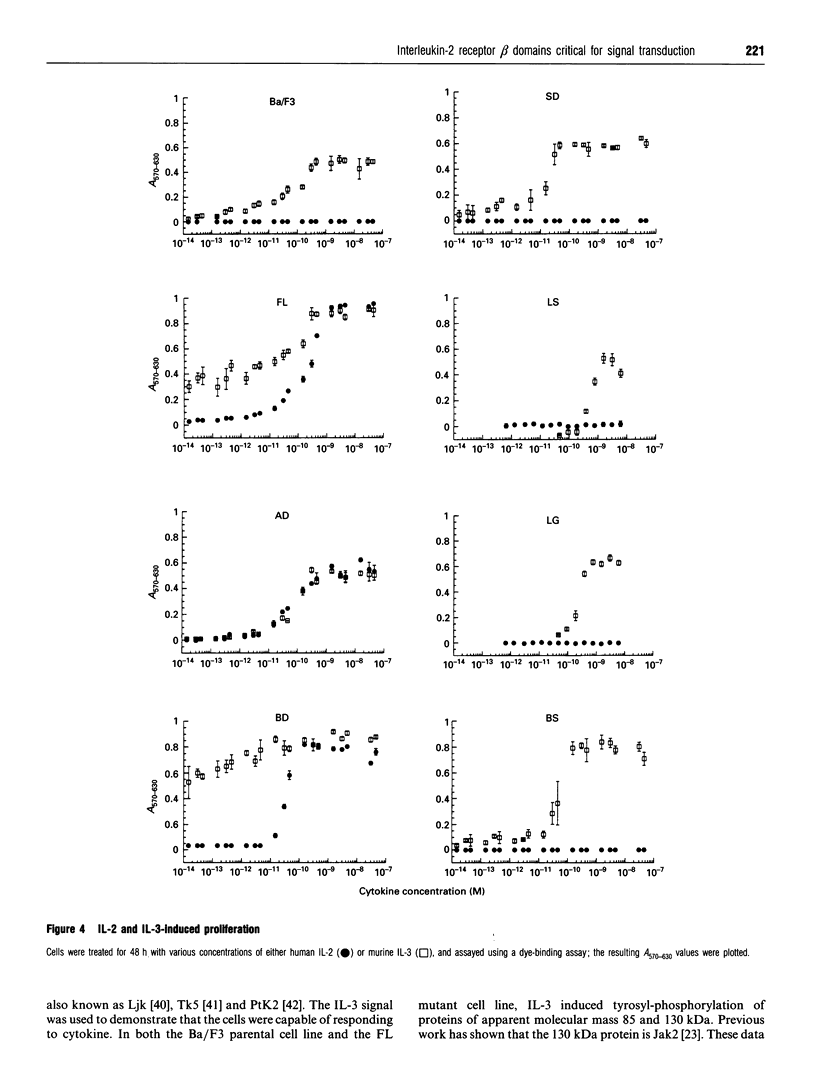
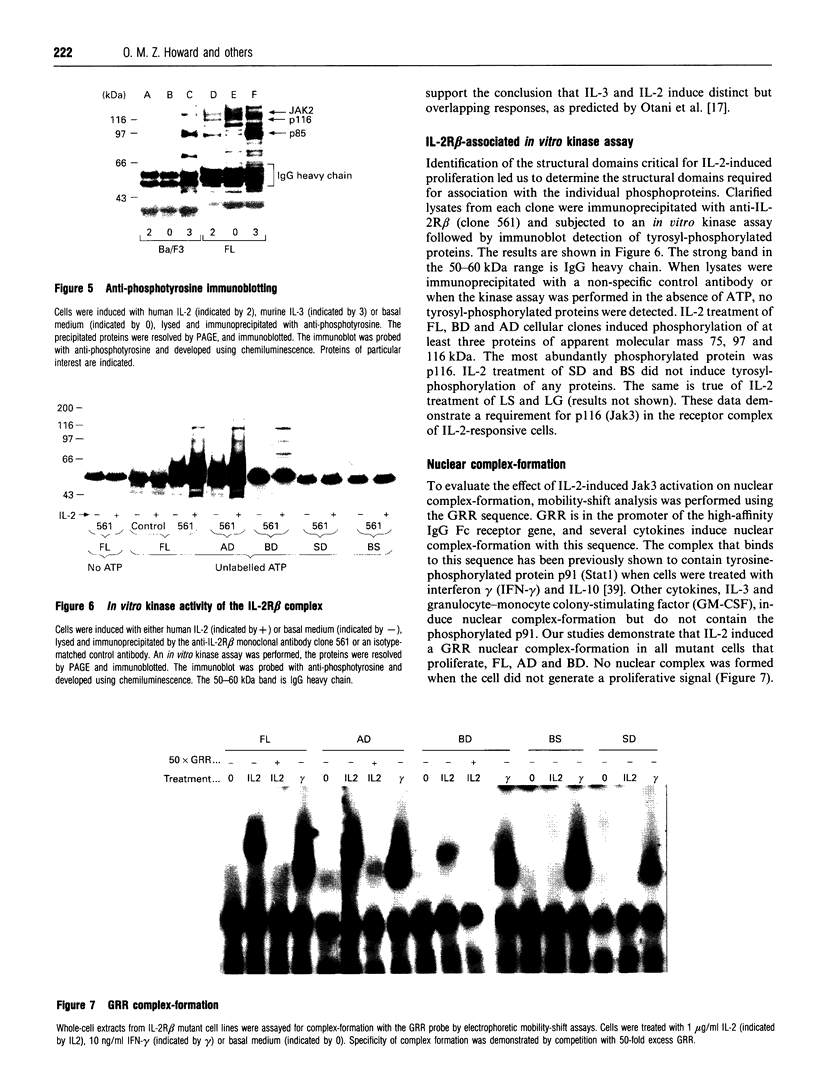
The structural domains of interleukin-2 receptor beta (IL-2R beta) were examined, characterizing the protein domains, associated phosphoproteins and nuclear complexes of IL-2-induced signal transduction. A series of IL-2R beta cytoplasmic deletion mutants were constructed and transfected into a murine pre-B-cell line, Ba/F3. The proliferative response of characterized clones was determined. A minimal linear cytoplasmic sequence required for proliferation and a sequence motif (PQPLXP) needed along with Box1-Box2 for IL-2-induced proliferation were identified. Anti-phosphotyrosine Western-blot analysis of a stimulated biologically active clone showed several IL-2-induced tyrosylphosphorylated proteins with molecular masses ranging from 45 to 116 kDa. In vitro kinase studies of biologically active clone-receptor complexes showed a 116 kDa protein (p116) to be the major tyrosine-phosphorylated component. The presence of the p116 kinase in the receptor complex correlates with IL-2-induced proliferation. An IL-2-inducible p116 kinase has recently been characterized as a Jak kinase family member and named Jak3. Nuclear complexes were formed with the GRR oligomer only when the IL-2R beta mutant supported proliferation. This led us to conclude that Box1-Box2 and PQPLXP motifs associate with Jak3 and that this association is an essential element in the IL-2 signal-transduction pathway culminating in the formation of a nuclear complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Cance W. G., Craven R. J., Weiner T. M., Liu E. T. Novel protein kinases expressed in human breast cancer. Int J Cancer. 1993 Jun 19;54(4):571–577. doi: 10.1002/ijc.2910540409. [DOI] [PubMed] [Google Scholar]
- Colosi P., Wong K., Leong S. R., Wood W. I. Mutational analysis of the intracellular domain of the human growth hormone receptor. J Biol Chem. 1993 Jun 15;268(17):12617–12623. [PubMed] [Google Scholar]
- DaSilva L., Howard O. M., Rui H., Kirken R. A., Farrar W. L. Growth signaling and JAK2 association mediated by membrane-proximal cytoplasmic regions of prolactin receptors. J Biol Chem. 1994 Jul 15;269(28):18267–18270. [PubMed] [Google Scholar]
- Evans G. A., Howard O. M., Erwin R., Farrar W. L. Interleukin-2 induces tyrosine phosphorylation of the vav proto-oncogene product in human T cells: lack of requirement for the tyrosine kinase lck. Biochem J. 1993 Sep 1;294(Pt 2):339–342. doi: 10.1042/bj2940339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. L., Ferris D. K. Two-dimensional analysis of interleukin 2-regulated tyrosine kinase activation mediated by the p70-75 beta subunit of the interleukin 2 receptor. J Biol Chem. 1989 Jul 25;264(21):12562–12567. [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Nagata S. Growth and differentiation signals mediated by different regions in the cytoplasmic domain of granulocyte colony-stimulating factor receptor. Cell. 1993 Sep 24;74(6):1079–1087. doi: 10.1016/0092-8674(93)90729-a. [DOI] [PubMed] [Google Scholar]
- Fung M. R., Scearce R. M., Hoffman J. A., Peffer N. J., Hammes S. R., Hosking J. B., Schmandt R., Kuziel W. A., Haynes B. F., Mills G. B. A tyrosine kinase physically associates with the beta-subunit of the human IL-2 receptor. J Immunol. 1991 Aug 15;147(4):1253–1260. [PubMed] [Google Scholar]
- Garcia G. G., Evans G. A., Michiel D. F., Farrar W. L. Characterization of a tyrosine kinase activity associated with the high-affinity interleukin 2 receptor complex. Biochem J. 1992 Aug 1;285(Pt 3):851–856. doi: 10.1042/bj2850851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N., Yang G., Miyajima A., Howard M. Identification of an essential region for growth signal transduction in the cytoplasmic domain of the human interleukin-4 receptor. J Biol Chem. 1992 Nov 15;267(32):22752–22758. [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Izquierdo M., Cantrell D. A. Protein tyrosine kinases couple the interleukin-2 receptor to p21ras. Eur J Immunol. 1993 Jan;23(1):131–135. doi: 10.1002/eji.1830230121. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Kawamura M., McVicar D. W., Johnston J. A., Blake T. B., Chen Y. Q., Lal B. K., Lloyd A. R., Kelvin D. J., Staples J. E., Ortaldo J. R. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6374–6378. doi: 10.1073/pnas.91.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirken R. A., Rui H., Evans G. A., Farrar W. L. Characterization of an interleukin-2 (IL-2)-induced tyrosine phosphorylated 116-kDa protein associated with the IL-2 receptor beta-subunit. J Biol Chem. 1993 Oct 25;268(30):22765–22770. [PubMed] [Google Scholar]
- Kirken R. A., Rui H., Malabarba M. G., Farrar W. L. Identification of interleukin-2 receptor-associated tyrosine kinase p116 as novel leukocyte-specific Janus kinase. J Biol Chem. 1994 Jul 22;269(29):19136–19141. [PubMed] [Google Scholar]
- Kobayashi N., Kono T., Hatakeyama M., Minami Y., Miyazaki T., Perlmutter R. M., Taniguchi T. Functional coupling of the src-family protein tyrosine kinases p59fyn and p53/56lyn with the interleukin 2 receptor: implications for redundancy and pleiotropism in cytokine signal transduction. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4201–4205. doi: 10.1073/pnas.90.9.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koettnitz K., Kalthoff F. S. Human interleukin-4 receptor signaling requires sequences contained within two cytoplasmic regions. Eur J Immunol. 1993 Apr;23(4):988–991. doi: 10.1002/eji.1830230437. [DOI] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993 Dec 17;262(5141):1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Lütticken C., Wegenka U. M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Yasukawa K., Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994 Jan 7;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- Maslinski W., Remillard B., Tsudo M., Strom T. B. Interleukin-2 (IL-2) induces tyrosine kinase-dependent translocation of active raf-1 from the IL-2 receptor into the cytosol. J Biol Chem. 1992 Aug 5;267(22):15281–15284. [PubMed] [Google Scholar]
- Mills G. B., Arima N., May C., Hill M., Schmandt R., Li J., Miyamoto N. G., Greene W. C. Neither the LCK nor the FYN kinases are obligatory for IL-2-mediated signal transduction in HTLV-I-infected human T cells. Int Immunol. 1992 Nov;4(11):1233–1243. doi: 10.1093/intimm/4.11.1233. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Zhang N., Schmandt R., Fung M., Greene W., Mellors A., Hogg D. Transmembrane signalling by interleukin 2. Biochem Soc Trans. 1991 Apr;19(2):277–287. doi: 10.1042/bst0190277. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Yamada K., Kobayashi N., Kawahara A., Perlmutter R. M., Taniguchi T. Association of p56lck with IL-2 receptor beta chain is critical for the IL-2-induced activation of p56lck. EMBO J. 1993 Feb;12(2):759–768. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., Cleveland J. L., Ihle J. N. Inactivation of erythropoietin receptor function by point mutations in a region having homology with other cytokine receptors. Mol Cell Biol. 1993 Mar;13(3):1788–1795. doi: 10.1128/mcb.13.3.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993 Nov 11;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Nakamura Y., Russell S. M., Ziegler S. F., Tsang M., Cao X., Leonard W. J. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993 Dec 17;262(5141):1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- O'Neal K. D., Yu-Lee L. Y. The proline-rich motif (PRM): a novel feature of the cytokine/hematopoietin receptor superfamily. Lymphokine Cytokine Res. 1993 Oct;12(5):309–312. [PubMed] [Google Scholar]
- Otani H., Siegel J. P., Erdos M., Gnarra J. R., Toledano M. B., Sharon M., Mostowski H., Feinberg M. B., Pierce J. H., Leonard W. J. Interleukin (IL)-2 and IL-3 induce distinct but overlapping responses in murine IL-3-dependent 32D cells transduced with human IL-2 receptor beta chain: involvement of tyrosine kinase(s) other than p56lck. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2789–2793. doi: 10.1073/pnas.89.7.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui H., Kirken R. A., Farrar W. L. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994 Feb 18;269(7):5364–5368. [PubMed] [Google Scholar]
- Sato N., Sakamaki K., Terada N., Arai K., Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993 Nov;12(11):4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmandt R., Fung M., Arima N., Zhang N., Leung B., May C., Gibson S., Hill M., Green W., Mills G. B. T-lymphocyte proliferation: tyrosine kinases in interleukin 2 signal transduction. Baillieres Clin Haematol. 1992 Jul;5(3):551–573. doi: 10.1016/s0950-3536(11)80007-7. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Morrow T. A., Desiderio S. V. itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11194–11198. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvennoinen O., Ihle J. N., Schlessinger J., Levy D. E. Interferon-induced nuclear signalling by Jak protein tyrosine kinases. Nature. 1993 Dec 9;366(6455):583–585. doi: 10.1038/366583a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N., Boulton T. G., Farruggella T., Ip N. Y., Davis S., Witthuhn B. A., Quelle F. W., Silvennoinen O., Barbieri G., Pellegrini S. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994 Jan 7;263(5143):92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Sánchez M. P., Tapley P., Saini S. S., He B., Pulido D., Barbacid M. Multiple tyrosine protein kinases in rat hippocampal neurons: isolation of Ptk-3, a receptor expressed in proliferative zones of the developing brain. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1819–1823. doi: 10.1073/pnas.91.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Torigoe T., Saragovi H. U., Reed J. C. Interleukin 2 regulates the activity of the lyn protein-tyrosine kinase in a B-cell line. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2674–2678. doi: 10.1073/pnas.89.7.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. C., Tonks N. K., Rapp U. R., Reed J. C. Interleukin 2 regulates Raf-1 kinase activity through a tyrosine phosphorylation-dependent mechanism in a T-cell line. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5544–5548. doi: 10.1073/pnas.90.12.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994 Jul 14;370(6485):153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]