Abstract
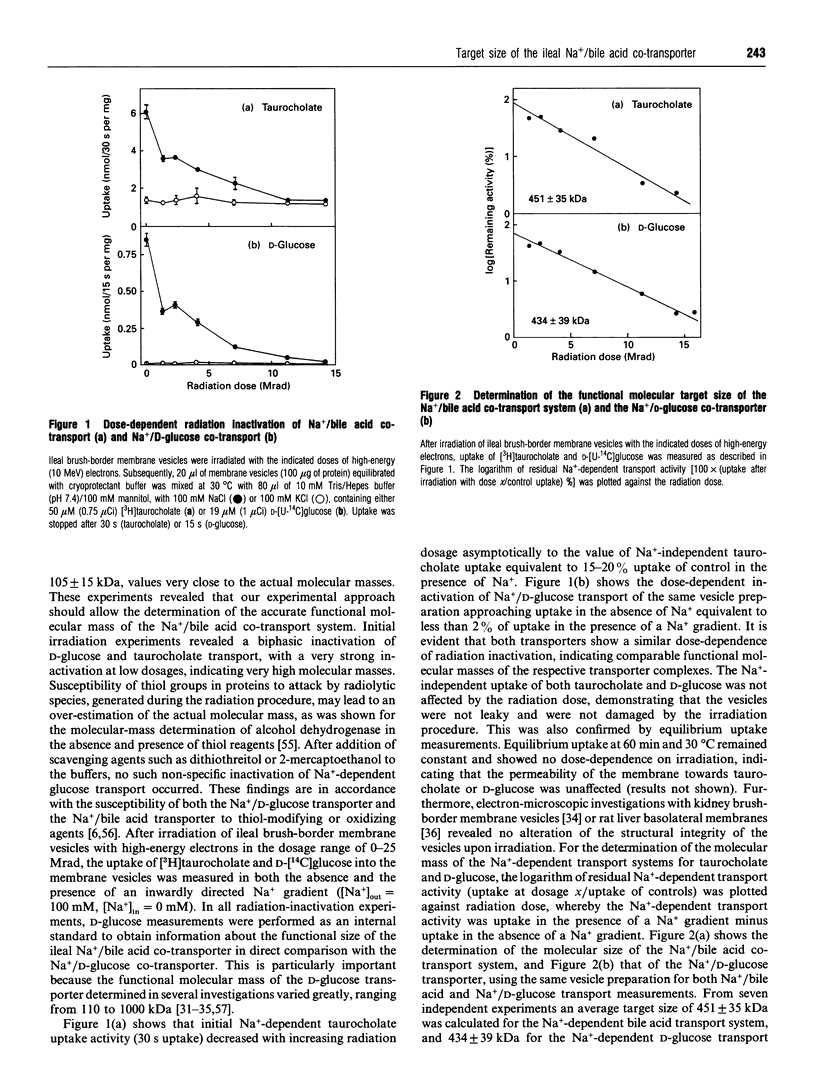
The functional-unit molecular size of the Na+/bile acid cotransport system and the apparent target size of the bile-acid-binding proteins in brush-border membrane vesicles from rabbit ileum were determined by radiation inactivation with high-energy electrons. The size of the functional transporting unit for Na(+)-dependent taurocholate uptake was determined to 451 +/- 35 kDa, whereas an apparent molecular mass of 434 +/- 39 kDa was measured for the Na(+)-dependent D-glucose transport system. Proteins of 93 kDa and 14 kDa were identified as putative protein components of the ileal Na+/bile acid cotransporter in the rabbit ileum, whereas a protein of 87 kDa may be involved in passive intestinal bile acid uptake. Photoaffinity labelling with 3- and 7-azi-derivatives of taurocholate revealed a target size of 229 +/- 10 kDa for the 93 kDa protein, and 132 +/- 23 kDa for the 14 kDa protein. These findings indicate that the ileal Na+/bile acid co-transport system is in its functional state a protein complex composed of several subunits. The functional molecular sizes for Na(+)-dependent transport activity and the bile-acid-binding proteins suggest that the Na+/bile acid co-transporter from rabbit ileum is a homotetramer (AB)4 composed of four AB subunits, where A represents the integral 93 kDa and B the peripheral 14 kDa brush-border membrane protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burckhardt G., Kramer W., Kurz G., Wilson F. A. Photoaffinity labeling studies of the rat renal sodium bile salt cotransport system. Biochem Biophys Res Commun. 1987 Mar 30;143(3):1018–1023. doi: 10.1016/0006-291x(87)90353-6. [DOI] [PubMed] [Google Scholar]
- Béliveau R., Demeule M., Ibnoul-Khatib H., Bergeron M., Beauregard G., Potier M. Radiation-inactivation studies on brush-border-membrane vesicles. General considerations, and application to the glucose and phosphate carriers. Biochem J. 1988 Jun 15;252(3):807–813. doi: 10.1042/bj2520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Elsner R. H., Ziegler K. Radiation inactivation of multispecific transport systems for bile acids and xenobiotics in basolateral rat liver plasma membrane vesicles. J Biol Chem. 1992 May 15;267(14):9788–9793. [PubMed] [Google Scholar]
- Elsner R., Ziegler K. Determination of the apparent functional molecular mass of the hepatocellular sodium-dependent taurocholate transporter by radiation inactivation. Biochim Biophys Acta. 1989 Jul 24;983(1):113–117. doi: 10.1016/0005-2736(89)90387-8. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Ingram J., Kenny A. J. Radiation inactivation analysis of kidney microvillar peptidases. FEBS Lett. 1986 Sep 15;205(2):323–327. doi: 10.1016/0014-5793(86)80921-8. [DOI] [PubMed] [Google Scholar]
- Gerardi-Laffin C., Delque-Bayer P., Sudaka P., Poiree J. C. Oligomeric structure of the sodium-dependent phlorizin binding protein from kidney brush-border membranes. Biochim Biophys Acta. 1993 Sep 5;1151(1):99–104. doi: 10.1016/0005-2736(93)90076-c. [DOI] [PubMed] [Google Scholar]
- Gong Y. Z., Zwarych P. P., Jr, Lin M. C., Wilson F. A. Effect of antiserum to a 99 kDa polypeptide on the uptake of taurocholic acid by rat ileal brush border membrane vesicles. Biochem Biophys Res Commun. 1991 Aug 30;179(1):204–209. doi: 10.1016/0006-291x(91)91355-g. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B., Lübbert H., Stieger B., Meier P. J. Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J Biol Chem. 1990 Apr 5;265(10):5357–5360. [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Kempner E. S., Fleischer S. Radiation inactivation of membrane components and molecular mass determination by target analysis. Methods Enzymol. 1989;172:410–439. doi: 10.1016/s0076-6879(89)72027-9. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Haigler H. T. The influence of low temperature on the radiation sensitivity of enzymes. J Biol Chem. 1982 Nov 25;257(22):13297–13299. [PubMed] [Google Scholar]
- Kempner E. S., Miller J. H. Radiation inactivation of glutamate dehydrogenase hexamer: lack of energy transfer between subunits. Science. 1983 Nov 11;222(4624):586–589. doi: 10.1126/science.6635656. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., George S. G., Aparicio S. G. The localization of peptidases in microvilli from the brush border of the proximal renal tubule of the rabbit. Biochem J. 1969 Nov;115(3):18P–18P. doi: 10.1042/bj1150018pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev. 1982 Jan;62(1):91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kramer W., Bickel U., Buscher H. P., Gerok W., Kurz G. Bile-salt-binding polypeptides in plasma membranes of hepatocytes revealed by photoaffinity labelling. Eur J Biochem. 1982 Dec;129(1):13–24. doi: 10.1111/j.1432-1033.1982.tb07015.x. [DOI] [PubMed] [Google Scholar]
- Kramer W., Burckhardt G., Wilson F. A., Kurz G. Bile salt-binding polypeptides in brush-border membrane vesicles from rat small intestine revealed by photoaffinity labeling. J Biol Chem. 1983 Mar 25;258(6):3623–3627. [PubMed] [Google Scholar]
- Kramer W., Buscher H. P., Gerok W., Kurz G. Bile salt binding to serum components. Taurocholate incorporation into high-density lipoprotein revealed by photoaffinity labelling. Eur J Biochem. 1979 Dec;102(1):1–9. doi: 10.1111/j.1432-1033.1979.tb06257.x. [DOI] [PubMed] [Google Scholar]
- Kramer W., Dechent C., Girbig F., Gutjahr U., Neubauer H. Intestinal uptake of dipeptides and beta-lactam antibiotics. I. The intestinal uptake system for dipeptides and beta-lactam antibiotics is not part of a brush border membrane peptidase. Biochim Biophys Acta. 1990 Nov 30;1030(1):41–49. doi: 10.1016/0005-2736(90)90236-h. [DOI] [PubMed] [Google Scholar]
- Kramer W., Dürckheimer W., Girbig F., Gutjahr U., Leipe I., Oekonomopulos R. Influence of amino acid side-chain modification on the uptake system for beta-lactam antibiotics and dipeptides from rabbit small intestine. Biochim Biophys Acta. 1990 Oct 5;1028(2):174–182. doi: 10.1016/0005-2736(90)90152-e. [DOI] [PubMed] [Google Scholar]
- Kramer W., Girbig F., Gutjahr U., Kowalewski S., Adam F., Schiebler W. Intestinal absorption of beta-lactam antibiotics and oligopeptides. Functional and stereospecific reconstitution of the oligopeptide transport system from rabbit small intestine. Eur J Biochem. 1992 Mar 1;204(2):923–930. doi: 10.1111/j.1432-1033.1992.tb16713.x. [DOI] [PubMed] [Google Scholar]
- Kramer W., Girbig F., Gutjahr U., Kowalewski S., Jouvenal K., Müller G., Tripier D., Wess G. Intestinal bile acid absorption. Na(+)-dependent bile acid transport activity in rabbit small intestine correlates with the coexpression of an integral 93-kDa and a peripheral 14-kDa bile acid-binding membrane protein along the duodenum-ileum axis. J Biol Chem. 1993 Aug 25;268(24):18035–18046. [PubMed] [Google Scholar]
- Kramer W., Girbig F., Gutjahr U., Leipe I. Application of high-performance liquid chromatography to the purification of the putative intestinal peptide transporter. J Chromatogr. 1990 Nov 23;521(2):199–210. doi: 10.1016/0021-9673(90)85044-v. [DOI] [PubMed] [Google Scholar]
- Kramer W., Girbig F., Leipe I., Petzoldt E. Direct photoaffinity labelling of binding proteins for beta-lactam antibiotics in rabbit intestinal brush border membranes with [3H]benzylpenicillin. Biochem Pharmacol. 1988 Jun 15;37(12):2427–2435. doi: 10.1016/0006-2952(88)90370-x. [DOI] [PubMed] [Google Scholar]
- Kramer W. Identification of identical binding polypeptides for cephalosporins and dipeptides in intestinal brush-border membrane vesicles by photoaffinity labeling. Biochim Biophys Acta. 1987 Nov 27;905(1):65–74. doi: 10.1016/0005-2736(87)90009-5. [DOI] [PubMed] [Google Scholar]
- Kramer W., Kurz G. Photolabile derivatives of bile salts. Synthesis and suitability for photoaffinity labeling. J Lipid Res. 1983 Jul;24(7):910–923. [PubMed] [Google Scholar]
- Kramer W., Nicol S. B., Girbig F., Gutjahr U., Kowalewski S., Fasold H. Characterization and chemical modification of the Na(+)-dependent bile-acid transport system in brush-border membrane vesicles from rabbit ileum. Biochim Biophys Acta. 1992 Oct 19;1111(1):93–102. doi: 10.1016/0005-2736(92)90278-t. [DOI] [PubMed] [Google Scholar]
- Kramer W., Schneider S. 3-Diazirine-derivatives of bile salts for photoaffinity labeling. J Lipid Res. 1989 Aug;30(8):1281–1288. [PubMed] [Google Scholar]
- Kwon H. M. Radiation target sizes of the Na,K-ATPase and p-aminohippurate transport system in the basolateral membrane of renal proximal tubule. Biochim Biophys Acta. 1990 Sep 7;1027(3):253–256. doi: 10.1016/0005-2736(90)90315-f. [DOI] [PubMed] [Google Scholar]
- Lack L. Properties and biological significance of the ileal bile salt transport system. Environ Health Perspect. 1979 Dec;33:79–89. doi: 10.1289/ehp.793379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. C., Kramer W., Wilson F. A. Identification of cytosolic and microsomal bile acid-binding proteins in rat ileal enterocytes. J Biol Chem. 1990 Sep 5;265(25):14986–14995. [PubMed] [Google Scholar]
- Lin M. C., Weinberg S. L., Kramer W., Burckhardt G., Wilson F. A. Identification and comparison of bile acid-binding polypeptides in ileal basolateral membrane. J Membr Biol. 1988 Nov;106(1):1–11. doi: 10.1007/BF01871762. [DOI] [PubMed] [Google Scholar]
- Schwarz L. R., Burr R., Schwenk M., Pfaff E., Greim H. Uptake of taurocholic acid into isolated rat-liver cells. Eur J Biochem. 1975 Jul 15;55(3):617–623. doi: 10.1111/j.1432-1033.1975.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Jeppesen L., Staun M., Svensson B., Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. Eur J Biochem. 1978 Aug 1;88(2):503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Fernandez A., Hirayama B., Wright E. M., Kempner E. S. Intestinal brush border membrane Na+/glucose cotransporter functions in situ as a homotetramer. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1456–1460. doi: 10.1073/pnas.87.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B. R., Kempner E. S., Wright E. M. Radiation inactivation probe of membrane-bound enzymes: gamma-glutamyltranspeptidase, aminopeptidase N, and sucrase. Anal Biochem. 1986 Nov 1;158(2):278–282. doi: 10.1016/0003-2697(86)90550-6. [DOI] [PubMed] [Google Scholar]
- Suarez M. D., Ferguson-Miller S. Yeast and horse liver alcohol dehydrogenases: potential problems in target size analysis and evidence for a monomer active unit. Biochemistry. 1987 Jun 16;26(12):3340–3347. doi: 10.1021/bi00386a014. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Malathi P., Preiser H., Jung C. Y. Radiation inactivation studies on the rabbit kidney sodium-dependent glucose transporter. J Biol Chem. 1985 Sep 5;260(19):10551–10556. [PubMed] [Google Scholar]
- Takahashi M., Malathi P., Preiser H., Jung C. Y. Radiation inactivation studies on the rabbit kidney sodium-dependent glucose transporter. J Biol Chem. 1985 Sep 5;260(19):10551–10556. [PubMed] [Google Scholar]
- Turner R. J., Kempner E. S. Radiation inactivation studies of the renal brush-border membrane phlorizin-binding protein. J Biol Chem. 1982 Sep 25;257(18):10794–10797. [PubMed] [Google Scholar]
- Vodenlich A. D., Jr, Gong Y. Z., Geoghegan K. F., Lin M. C., Lanzetti A. J., Wilson F. A. Identification of the 14 kDa bile acid transport protein of rat ileal cytosol as gastrotropin. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1147–1154. doi: 10.1016/0006-291x(91)90659-u. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wieland T., Nassal M., Kramer W., Fricker G., Bickel U., Kurz G. Identity of hepatic membrane transport systems for bile salts, phalloidin, and antamanide by photoaffinity labeling. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5232–5236. doi: 10.1073/pnas.81.16.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. H., Oelkers P., Craddock A. L., Dawson P. A. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994 Jan 14;269(2):1340–1347. [PubMed] [Google Scholar]
- Ziegler K., Elsner R. H. Functional molecular mass of the 14C-azidobenzamidotaurocholic acid binding proteins in hepatocellular bile acid transport systems. Biochim Biophys Acta. 1992 Jan 31;1103(2):229–232. doi: 10.1016/0005-2736(92)90091-y. [DOI] [PubMed] [Google Scholar]
- von Dippe P., Levy D. Characterization of the bile acid transport system in normal and transformed hepatocytes. Photoaffinity labeling of the taurocholate carrier protein. J Biol Chem. 1983 Jul 25;258(14):8896–8901. [PubMed] [Google Scholar]