Abstract
Background
Polycythaemia vera and essential thrombocythaemia are chronic Philadelphia‐negative myeloproliferative neoplasms that increase the risk of arterial and venous thrombosis, as well as bleeding. In addition to the different therapeutic strategies available, an antiplatelet drug is often used to reduce thrombotic risk.
Objectives
To quantify the benefit and harm of antiplatelet drugs for long‐term primary and secondary prophylaxis of arterial and venous thrombotic events in patients with polycythaemia vera or essential thrombocythaemia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library (Issue 1 2012), MEDLINE (1966 to 2012), and EMBASE (1980 to 2012), as well as online registers of ongoing trials and conference proceedings. The date of the last search was October 2012.
Selection criteria
We included all randomised controlled trials (RCTs) comparing long‐term (>6 months) use of an antiplatelet drug versus placebo or no treatment in participants with polycythaemia vera or essential thrombocythaemia, as diagnosed by established international criteria, with data for at least one of the selected outcomes.
Data collection and analysis
Using a pre‐defined extraction form, two review authors independently screened results, extracted data, and assessed quality. We planned to analyse the following outcomes: mortality from arterial and venous thrombotic events (primary efficacy outcome), mortality from bleeding episodes (primary safety outcome), fatal and non‐fatal arterial thrombotic events, fatal and non‐fatal venous thrombotic events, micro‐circulation events, transient neurological and ocular manifestations, major and minor bleeding episodes, and all‐cause mortality and any adverse events. We based quantitative analysis of outcome data on an intention‐to‐treat principle. We used the pooled odds ratio (OR) with 95% confidence interval (CI) with a fixed‐effect model (Mantel‐Haenszel) to estimate the overall treatment effect.
Main results
We identified no new studies from the updated searches. We included in this review two RCTs for a total of 630 participants. Both RCTs included participants with an established diagnosis of polycythaemia vera and with no clear indication or contraindication to aspirin therapy. We judged both studies to be of moderate quality. Published data from both studies were insufficient for a time‐to‐event data analysis and for some of the primary and secondary outcomes that we planned. The use of low‐dose aspirin, compared with placebo, was associated with a lower risk of fatal thrombotic events (although this benefit was not statistically significant (OR 0.20, 95% CI 0.03 to 1.14; P = 0.07). No data on mortality from bleeding episodes were available. A non‐significant benefit of aspirin was shown for all‐cause mortality (OR 0.46, 95% CI 0.21 to 1.01; P = 0.05). No increase in the risk of major bleeding was reported in participants taking aspirin compared with those given placebo (OR 0.99, 95% CI 0.23 to 4.36; P = 0.99), and a non‐significant increase with aspirin treatment was shown for minor bleeding (OR 1.85, 95% CI 0.90 to 3.79; P = 0.09). No published studies have reported findings in participants with essential thrombocythaemia or in the study of other antiplatelet drugs.
Authors' conclusions
For patients with polycythaemia vera who have no clear indication or contraindication to aspirin therapy, available evidence suggests that the use of low‐dose aspirin, when compared with no treatment, is associated with a statistically non‐significant reduction in the risk of fatal thrombotic events and all‐cause mortality, without an increased risk of major bleeding.
Keywords: Humans; Anticoagulants; Anticoagulants/administration & dosage; Anticoagulants/adverse effects; Aspirin; Aspirin/administration & dosage; Aspirin/adverse effects; Platelet Aggregation Inhibitors; Platelet Aggregation Inhibitors/administration & dosage; Platelet Aggregation Inhibitors/adverse effects; Polycythemia Vera; Polycythemia Vera/drug therapy; Polycythemia Vera/mortality; Randomized Controlled Trials as Topic; Thrombocythemia, Essential; Thrombocythemia, Essential/drug therapy; Thrombocythemia, Essential/mortality; Thrombosis; Thrombosis/prevention & control
Plain language summary
Antiplatelet drugs for preventing arterial and venous thrombotic events in patients with polycythaemia vera or essential thrombocythaemia
Low‐dose aspirin as an antiplatelet therapy is the drug of choice for preventing cardiovascular events, but the potential risk of bleeding has limited its use in myeloproliferative neoplasms in the past. Evidence from this review of 630 people in two trials suggests that, in patients with polycythaemia vera and with no clear indication or contraindication to aspirin therapy, low‐dose aspirin may reduce the risk of thrombotic and all‐cause mortality. No data were provided on mortality from bleeding episodes. No studies in participants with essential thrombocythaemia and with antiplatelet therapy other than aspirin have been published.
Summary of findings
for the main comparison.
| Antiplatelet drugs compared with placebo for polycythaemia vera | ||||||
|
Patient or population: patients with polycythaemia vera Settings: outpatient Intervention: aspirin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE)° | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Aspirin | |||||
|
Mortality for thrombotic events [mean follow‐up 31 months] |
Medium‐risk population | OR 0.20 [0.03, 1.14] | 630 (2) | ⊕⊕⊕⊝ moderate | ||
| 22 per 1000 | 3 per 1000 (‐18 to 22) | |||||
|
Mortality for bleedings [mean follow‐up 31 months] |
Medium‐risk population | NA | 630 (2) | ⊕⊕⊕⊝ moderate | ||
| NA | NA | |||||
|
All‐cause mortality [mean follow‐up 31 months] |
Medium‐risk population | OR 0.46 [0.21, 1.01] | 630 (2) | ⊕⊕⊕⊝ moderate | ||
| 63 per 1000 | 30 per 1000 (14 to 64) | |||||
|
Major bleeding [mean follow‐up 31 months] |
Medium‐risk population | OR 0.99 [0.23, 4.36] | 630 (2) | ⊕⊕⊕⊝ moderate | ||
| 9 per 1000 | 9 per 1000 (2 to 40) | |||||
|
Minor bleeding [mean follow‐up 31 months] |
Medium‐risk population | OR 1.85 [0.90, 3.79] | 630 (2) | ⊕⊕⊕⊝ moderate | ||
| 38 per 1000 | 68 per 1000 (34 to 130) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). °The quality of the trials was judged as moderate because only two small trials are available and the risk of bias in both trials is uncertain. CI: Confidence interval; OR: odds ratio; NA: not available. | ||||||
| The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Polycythaemia vera and essential thrombocythaemia are chronic Philadelphia‐negative myeloproliferative neoplasms, in which a multi‐potent haemopoietic stem cell autonomously replicates, independent of physiological stimuli (WHO 2002). Both diseases follow a chronic clinical course with increased risk of arterial and venous thrombosis and a 1% to 2% incidence per year of evolution to myelofibrosis with myeloid metaplasia or transformation to acute leukaemia (GISP 1995; Passamonti 2004; Finazzi 2005; Marchioli 2005). Also, the risk of bleeding is increased as the result of quantitative and qualitative platelet abnormalities.
The leading symptoms of polycythaemia vera are secondary to increased red cell production and red cell mass. The consequent blood hyperviscosity reduces blood flow velocity and increases the risk of both microvascular and thrombotic complications. The incidence of polycythaemia vera is 2.3 per 100,000 person‐years (Ania 1994). The overall mortality in study participants treated with various regimens was estimated to be 3.5 deaths per 100 persons per year — almost twice the risk of the general population (ECLAP, European Collaboration on Low‐dose Aspirin in Polycythaemia study) (Marchioli 2005). Thrombotic events, haematological transformation, and major bleeding are responsible for 41%, 13%, and 4% of deaths, respectively (Marchioli 2005). Participants are usually stratified into different thrombotic risk categories on the basis of the combination of age, a history of arterial or venous thrombosis, and the presence of other common cardiovascular risk factors (e.g. smoking, diabetes mellitus, congestive heart failure) (Campbell 2005; Marchioli 2005).
The incidence of essential thrombocythaemia is 2.5 per 100,000 person‐years (Mesa 1999). The hallmark of essential thrombocythaemia is an elevated peripheral platelet count together with excessive proliferation of the megakaryocytes. The occurrence of thrombotic complications is related to platelet count and function but is not dependent on the degree of platelet elevation. In about two thirds of all participants, the clinical course is characterised by the occurrence of minor and major thrombotic or bleeding complications, but in about one third, the disease is benign, and no complications are reported for many years (Mesa 1999; Passamonti 2004). Similar to polycythaemia vera, thrombotic risk categories can be identified on the basis of age, a history of arterial or venous thrombosis, and the presence of common cardiovascular risk factors (e.g. smoking, hypertension, hypercholesterolaemia) (Cortelazzo 1990; Cortelazzo 1995; Campbell 2005).
Description of the intervention
In keeping with the estimated individual risk of thrombotic and bleeding complications, different therapeutic strategies are used: phlebotomy for polycythaemia vera or platelet apheresis for essential thrombocythaemia, cytoreductive therapy (e.g. hydroxyurea, anagrelide, interferon‐α), and antiplatelet drugs to prevent platelet aggregation (Campbell 2005).
Several molecules that inhibit platelet aggregation are currently available in clinical practice, including aspirin, dipyridamole, and the old (ticlopidine, clopidogrel) and the new (prasugrel, ticagrelor) thienopyridines (ACCP 2012). Aspirin has an antiplatelet effect by inhibiting the production of thromboxane, thienopyridine by inhibiting adenosine diphosphate (ADP) receptor/P2Y12 inhibitors, and dipyridamole by inhibiting the production of thromboxane and by inhibiting the phosphodiesterase enzymes that normally break down cyclic adenosine monophosphate (cAMP). In addition to the increased risk of bleeding, major reported adverse events include the following: for aspirin, gastric ulcer and allergic reaction; for thienopyridine, neutropenia, thrombotic thrombocytopenic purpura, and dyspnoea (ticagrelor); and for dipyridamole, headache and dizziness (ACCP 2012).
How the intervention might work
Antiplatelet drugs are commonly used for primary and secondary prevention of arterial thrombosis in patients without myeloproliferative neoplasms. For example, among a wide range of high‐risk patients (with acute or previous vascular disease or some other predisposing condition), aspirin prevents 10 to 20 fatal and non‐fatal thrombotic events per 1000 patients per year (ATC 2002). There is no reason to suppose a lack of effect of antiplatelet drugs in patients with polycythaemia vera or essential thrombocythaemia. Aspirin has been preferentially used by clinicians and researchers in participants with polycythaemia vera and essential thrombocythaemia because, in these diseases, biosynthesis of thromboxane is increased, and one of the molecular effects of aspirin on platelet aggregation is the reduction of thromboxane biosynthesis (Landolfi 1992; Rocca 1995). However, other antiplatelet drugs are currently used in daily practice, in particular when aspirin is contraindicated (Alvarez‐Larran 2010).
Why it is important to do this review
It is important to assess the actual beneficial effects of antiplatelet drugs. As has been stated, different underlying pathophysiological mechanisms of arterial and venous thrombosis among patients with and without myeloproliferative neoplasms may result in different effects of antiplatelet drugs on thrombosis occurrence and survival. Moreover, in myeloproliferative neoplasms, bleeding may occur spontaneously or may be associated with antiplatelet drugs. Bleeding is typically mucocutaneous, with easy bruising and nose and gingival bleeding. Major haemorrhage requiring transfusion is less frequent and generally involves the gastrointestinal tract. Age, disease duration, use of an antiplatelet agent, and a history of bleeding were significantly associated with bleeding events during follow‐up (Marchioli 2005). Aspirin is associated with an approximately two‐fold increase in the risk of major upper gastrointestinal bleeding (one to two bleeding events per 1000 patients per year), with an absolute excess of haemorrhagic strokes of one to two per 10,000 patients with chronic Philadelphia‐negative myeloproliferative neoplasms (ECLAP 2004; Harrison 2005). Finally, a platelet count greater than 1,500,000/mm3 is a well‐recognised bleeding risk factor (Campbell 2005).
Therefore, the aim of this systematic review — an update of a previously published Cochrane review (Squizzato 2008) — is to assess the efficacy and safety of antiplatelet drugs in the primary and secondary prevention of thrombotic events in different risk subgroups of participants with polycythaemia vera or essential thrombocythaemia.
Objectives
To assess the effects of an antiplatelet drug in the long‐term primary and secondary prophylaxis of arterial and venous thrombotic events in patients with polycythaemia vera or essential thrombocythaemia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs)
Types of participants
Adult (age ≥18 years) participants with polycythaemia vera or essential thrombocythaemia, diagnosed by established international criteria (e.g. World Health Organization (WHO), Polycythaemia Vera Study Group (PVSG)).
Types of interventions
Antiplatelet drug (e.g. aspirin, ticlopidine, clopidogrel, dipyridamole, prasugrel, ticagrelor) versus placebo or no treatment for at least 6 months.
Types of outcome measures
Separate analyses for polycythaemia vera and for essential thrombocythaemia were planned. Primary and secondary end‐points were determined for both diseases.
Primary outcomes
Mortality from arterial and venous thrombotic events (fatal myocardial infarction (MI), fatal stroke, fatal acute visceral thromboembolism, fatal acute peripheral thromboembolism, fatal cerebral sinus and venous thrombosis, fatal visceral vein thrombosis, fatal pulmonary embolism, fatal venous thrombosis in unusual sites)
Mortality from bleeding episodes
Secondary outcomes
Each single fatal and non‐fatal arterial and venous thrombotic event
Arterial
Myocardial infarction (MI) (fatal and non‐fatal).
Unstable angina.
Ischaemic stroke (fatal and non‐fatal).
Transient ischaemic attack (TIA).
Acute peripheral and visceral thromboembolism (fatal and non‐fatal).
Venous
Deep venous thrombosis (legs and arms).
Pulmonary embolism (fatal and non‐fatal).
Fatal cerebral sinus and venous thrombosis.
Unusual sites of venous thrombosis (visceral vein thrombosis and cerebral sinus and venous thrombosis, fatal and non‐fatal).
Superficial venous thrombosis.
Each single micro‐circulation event
Erythromelalgia.
Other.
Each single transient neurological and ocular manifestation
Seizure.
Migraine.
Vertigo.
Tinnitus.
Scintillating scotoma.
Amaurosis fugax.
Other.
Major and minor bleeding episodes
Major bleeding (e.g. haemorrhagic stroke, gastric bleeding, any bleeding requiring blood transfusion, any bleeding causing a haemoglobin level drop of >2 mg/dL, or hospitalisation).
Minor bleeding.
All‐cause mortality
All adverse events
(e.g. renal failure, thrombotic thrombocytopaenic purpura (TTP), neutropenia, low platelets, gastric complaints, diarrhoea, skin rash).
We considered any RCTs with at least one of the above clinical outcomes for this review. We excluded RCTs with only laboratory outcomes.
We contacted Investigators to obtain unpublished data when necessary.
Search methods for identification of studies
We developed the search strategy for this review in accordance with the Cochrane Haematological Malignancies Group guidelines. We searched for RCTs using the following methods.
Electronic searches
We searched The Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library (Issue 1, 2012; see Appendix 1), MEDLINE (1946 to 2012; see Appendix 2), and EMBASE (1996 to 2012; see Appendix 3). The date of the last search was 12 October 2012.
No language restrictions were applied.
Searching other resources
We searched the Database of Abstracts of Reviews of Effects (DARE) in The Cochrane Library in October 2012.
We also searched websites for recent or ongoing trials (e.g. http://www.clinicaltrials.gov, http://www.controlled‐trials.com) in October 2012.
We searched the online conference proceedings of
American Society of Hematology (from 2004 to 2011) and
European Hematology Association (from 2002 to 2011)
as well as references from relevant review articles.
Data collection and analysis
Selection of studies
We selected studies on the basis of Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Two review authors (AS, ER) independently selected potentially eligible studies from the search. We rejected studies if one could determine from the title and/or abstract that the study was not suitable for inclusion in this review. We obtained the full text of the study when an article could not be excluded with certainty. We then compared excluded studies, and any disagreement was resolved through discussion between the authors. When necessary, we contacted the trial authors for additional information. To assess agreement between authors on study selection, we used the k statistic, which measures chance‐corrected agreement. A k value higher than 0.6 was considered to represent substantial agreement, and values higher than 0.8 almost perfect agreement (Maclure 1987).
We extracted the study characteristics using a pre‐defined form and included an assessment of quality. We used a consensus meeting to resolve any disagreement in the quality assessment of the trials. The third author (SM) checked assessments for the included studies.
Data extraction and management
Two authors independently extracted data (AS, ER) using a pre‐defined data extraction form. A consensus meeting was held to deal with differences in the extracted data.
We extracted data for any subgroup listed in the 'Subgroup analysis and investigation of heterogeneity' paragraph.
Extracted data consisted of the following:
General information: published/unpublished, title, authors, country, year of publication, duplicate publications.
Trial characteristics: design, duration, allocation concealment (and method), randomisation (and method), blinding (outcome assessors), checking of blinding, intention‐to‐treat analysis.
Intervention: loading dose, dosage, duration of treatment.
Participants: exclusion criteria, total number and numbers in comparison groups, age, gender, similarity of groups at baseline, withdrawals/losses to follow‐up, history of thrombotic events, JAK2 V617F mutation status, concomitant cardiovascular risk factors, levels of hematocrit (for polycythaemia vera) and of platelets (for essential thrombocythaemia), concomitant therapies.
Outcomes: listed above.
Assessment of risk of bias in included studies
We assessed the methodological quality of selected studies on the basis of Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We scored each of the following points as 'low,' 'high,' or 'unclear' (where 'low' indicates that the study is less open to bias) and report them in a risk of bias table (Characteristics of included studies; Figure 1):
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Method of randomisation (selection bias): Methods of randomisation using date of birth, date of admission, hospital numbers, or alternation are not appropriate because they do not allow each study participant to have the same chance of receiving each intervention.
Concealment of allocation (indication bias): Adequate measures to conceal allocations include central randomisation; serially numbered, opaque, sealed envelopes; and other descriptions with convincing concealment.
Blinding of investigators and participants (performance bias).
Blinding of outcome assessment (detection bias).
Adequate follow‐up (attrition bias): Attrition bias refers to systematic differences between the comparison groups in terms of loss of participants from the study. We will carefully check the reporting of withdrawals, dropouts, protocol deviations, and losses to follow‐up. It is adequate when more than 90% of participants have completed follow‐up, and when reasons for withdrawals, dropouts, protocol deviations, and losses to follow‐up are clearly reported.
Other possible bias.
To avoid selection bias, we did not reject any study because of methodological characteristics or any subjective quality criteria, except non‐randomised studies. However, we planned to examine differences in study methods in sensitivity analyses.
Measures of treatment effect
We analysed data of selected studies on the basis of Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
We used the Cochrane Review Manager software to analyse the data (RevMan 5). We based quantitative analysis of outcome on the intention‐to‐treat (ITT) principle. To measure the treatment effect for each study, we used the OR with 95% CI.
Unit of analysis issues
We planned to manage data with non‐standard designs according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Dealing with missing data
We contacted the investigators for additional information about missing data, but no data were provided. As only two RCTs were finally meta‐analysed, we decided to analyse only available outcomes for both studies, as imputing the missing data with replacement values would have provided misleading information.
Assessment of heterogeneity
As trials may have been carried out according to different protocols, we planned to assess statistical heterogeneity of trial data by using the Mantel‐Haenszel Chi2 test of heterogeneity and the I² statistic of heterogeneity (Deeks 2011). For the first method, trial data were considered to be heterogeneous if P < 0.10. As significant heterogeneity may have occurred, we planned to attempt to explain the differences as they relate to types of participants and study design. The I² method is expressed as a percentage of total variation across studies with an uncertainty interval (Higgins 2003). I² < 30% indicates mild heterogeneity, 30% to 50% moderate heterogeneity, and > 50% severe heterogeneity.
Assessment of reporting biases
We planned to assess publication bias by using funnel plots, if a sufficient number of RCTs were included (at least 10 studies) (Lau 2006, Egger 1997, Sterne 2011). We also planned to apply a rank correlation test if necessary (Lau 2006). However, we did not assess reporting bias because only two trials were included.
Data synthesis
We planned to extract the hazard ratio (HR) and associated variances for time‐to‐event data (i.e. mortality) directly from the trial publications or, if not reported, to extract them indirectly using the methods described by Parmar involving either other available summary statistics or data extracted from published Kaplan‐Meier curves (also with the help of Lesley Stewart's Microsoft Excel sheet, which is available at the Editorial Base of the Cochrane Haematological Malignancies Group) (Parmar 1998).
We planned to obtain a pooled HR from the derived observed (O) less expected (E) number of events and the variance for each trial, using the fixed‐effect model.
We planned to report ratios of treatment effects for time‐to‐event outcomes so that HRs less than 1.0 favour antiplatelet drugs and values greater than 1.0 favour placebo or no treatment.
For binary endpoints, measure of effect was the OR with 95% CI. We estimated the overall treatment effect by the pooled OR with 95% CI using a fixed‐effect model (Mantel‐Haenszel). Each test for significance was two‐sided.
Given that published data from both studies were insufficient for a time‐to‐event data analysis or for any of the primary and secondary outcomes that we planned, and the corresponding authors of studies did not provide them, we meta‐analysed outcomes as simple binary endpoints.
The main value of this review is derived from the examination of whether long‐term administration of an antiplatelet drug has consistent effects in primary and secondary thrombotic prevention and in pre‐defined thrombotic risk subgroups.
For this reason, we planned subgroup analyses. However, we performed no subgroup analysis because available data were insufficient.
Subgroup analysis and investigation of heterogeneity
For each myeloproliferative disease —polycythaemia vera and essential thrombocythaemia — we planned the following subgroup analyses:
Antiplatelet drug dosage (low dose vs high dose).
Antiplatelet drug for primary prophylaxis (to prevent first thrombotic events).
Antiplatelet drug for secondary prophylaxis (after an arterial or a venous thrombotic event).
JAK2 V617F mutation status.
Different ages.
Concomitant cardiovascular risk factors (e.g. hypertension, dyslipidaemia, diabetes mellitus, smoking).
Levels of hematocrit (for polycythaemia vera) and of platelets (for essential thrombocythaemia).
Concomitant therapies.
Sensitivity analysis
We re‐analysed data using a random‐effects model instead of a fixed‐effect model.
Moreover, to test the robustness of the review results, we planned to re‐analyse data by including/excluding studies on the basis of differences in quality, sample size and study methods, and imputed values for missing data. However, as reported in the Dealing with missing data section, only two RCTs were finally meta‐analysed, limiting any sensitivity analysis.
Results
Description of studies
Results of the search
In the previous search of March 2007, we found 536 references with 74 duplicates; on the basis of title and/or abstract, we excluded 441 publications. In the updated search in October 2012, we identified 348 references with 24 duplicates (Figure 2); on the basis of title and/or abstract, we excluded 324 publications. Overall, we found 786 references with 98 duplicates; on the basis of title and/or abstract, we excluded 765 publications. We retrieved full copies of 21 articles. We excluded nine because they were review articles without original data, seven because they were not randomised trials, and one because it was a protocol. From four potentially eligible RCTs, two studies were further excluded because they did not meet inclusion criteria (Characteristics of excluded studies). Only two RCTs were finally included in this systematic review (GISP 1997; ECLAP 2004). Both studies included only participants with polycythaemia vera, and in both trials, the administered antiplatelet drug was aspirin given at low dose (Characteristics of included studies). We did not find and therefore did not include any new studies in our update search. An ongoing study, which was potentially eligible, was identified from a search of the online trial registry (Characteristics of ongoing studies).
2.
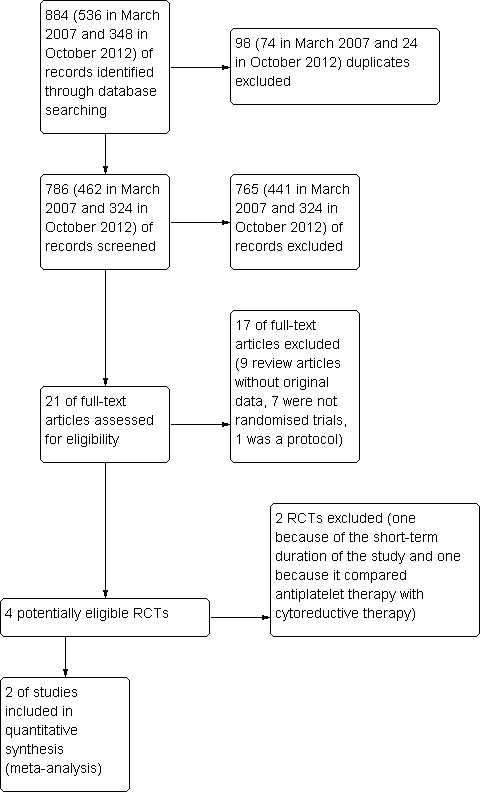
Study flow diagram (PRISMA).
Included studies
In the pilot study of the Gruppo Italiano Studio Policitemia (GISP), 112 participants with a PVSG diagnosis of polycythaemia vera were randomly assigned to low‐dose aspirin (40 mg per day) or to placebo (GISP 1997). In keeping with the 'uncertainty principle,' people were eligible if physicians were uncertain whether aspirin was indicated, and were ineligible if physicians were reasonably certain that aspirin was indicated, was not indicated, or was contraindicated. Exclusion criteria included stage III chronic renal failure, active gastrointestinal disease, and a history of a major haemorrhagic episode during the preceding four months. The primary objective of the study was to evaluate safety and tolerability by assessing major haemorrhagic events, compliance with the assigned treatment, and gastric intolerance. Secondary endpoints consisted of the occurrence of acute MI, TIAs, stroke, venous thromboembolism, and acute arterial thrombosis. Aspirin administration was not associated with any bleeding complications. The mean duration of follow‐up was 16 months.
In the ECLAP phase III study, 518 participants with a PVSG diagnosis of polycythaemia vera were randomly assigned to low‐dose aspirin (100 mg per day) or to placebo (ECLAP 2004). Participants were eligible if they had no clear indication and no clear contraindication for aspirin treatment, and had no clinically significant co‐existing conditions. No age limits were applied. Two pre‐defined combined primary efficacy endpoints were composed as follows: first, non‐fatal MI, non‐fatal stroke, or death from cardiovascular causes; second, non‐fatal MI, non‐fatal stroke, pulmonary embolism, major venous thrombosis, or death from cardiovascular causes. The secondary endpoints involved the following single events: fatal or non‐fatal cerebrovascular events, fatal or non‐fatal cardiac events, minor thrombotic complications (including atypical cerebral or visual symptoms of ischaemia, erythromelalgia, and thrombophlebitis), and major and minor thrombotic complications as previously defined. The safety of low‐dose aspirin was assessed by examining rates of fatal and non‐fatal major haemorrhage (any haemorrhage requiring transfusion, hospitalisation, or both), minor haemorrhage, and any adverse events leading to discontinuation of treatment. Aspirin significantly reduced only the second of the combined endpoints. All‐cause mortality and cardiovascular mortality were not reduced significantly. The incidence of major bleeding episodes was not significantly increased in the aspirin group. The study was planned to have a follow‐up duration of five years, but as the result of a slow recruitment rate after two years, the study was prematurely stopped. Total follow‐up consisted of 1478 person‐years, with a minimum of 12 months per participant.
Excluded studies
The reasons for exclusion are specified in the Characteristics of excluded studies table. We excluded one study because of the short‐term duration of the study (60 days) (Finelli 1991) and another one because it compared antiplatelet therapy with cytoreductive therapy (Tartaglia 1986).
Risk of bias in included studies
The ECLAP 2004 and GISP 1997 studies were double‐blind, placebo‐controlled, randomised trials (Characteristics of included studies, Figure 1). We judged the methodology of the GISP study to have a moderate quality. This judgement was based on the fact that selective loss to follow‐up or study withdrawal (8% of participants) could not be excluded because data in the published article were insufficient. The authors provided no further data. The study protocol of the ECLAP study was published in advance, before the study had been completed (Landolfi 1997). We also considered this study to have a moderate quality, because we could not determine whether there was selective loss to follow‐up or study withdrawal (6%). The authors provided no further data.
Allocation
Both trials had a co‐ordinating centre that randomly allocated participants.
Blinding
Randomisation was double blind, centrally co‐ordinated, and stratified by centre for both trials.
Incomplete outcome data
In both studies, loss to follow‐up or study withdrawal (more than 5% in both trials) could not be excluded because data in the published article were insufficient.
Selective reporting
It is unclear in both studies whether selective reporting occurred, because the authors did not provide further data.
Effects of interventions
See: Table 1
Data from two trials assessing the efficacy of low‐dose aspirin in a total of 630 people with polycythaemia vera were available. The primary efficacy outcome in both trials was the sum of fatal arterial and venous thrombotic events.
Published data from both studies were insufficient for a time‐to‐event data analysis and for some of the planned primary and secondary outcomes. We contacted the corresponding authors of the GISP and ECLAP studies (the same for both studies) for additional data, but they did not provide them. Consequently, we meta‐analysed four main outcomes as simple binary endpoints, measuring the OR with 95% CI.
Low‐dose aspirin treatment showed a marked benefit in terms of mortality from thrombotic events, although this finding was not statistically significant (OR 0.20, 95% CI 0.03 to 1.14, P = 0.07, fixed‐effect model; see Figure 3). In absolute terms, 3 of 1000 participants treated with aspirin had a fatal thrombotic event, compared with 22 participants treated with placebo (risk difference 1.9%, 95% CI 0% to 4%).
3.
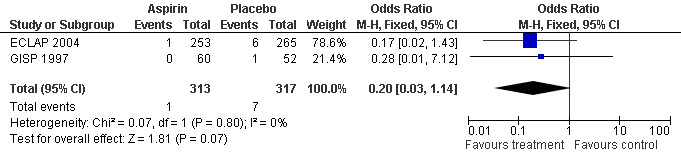
Forest plot of comparison: 1 Polycythaemia vera, outcome: 1.1 Mortality for thrombotic events.
A non‐significant benefit of aspirin was also shown for all‐cause mortality (OR 0.46, 95% CI 0.21 to 1.01; P = 0.05; fixed‐effect model; see Figure 4).
4.
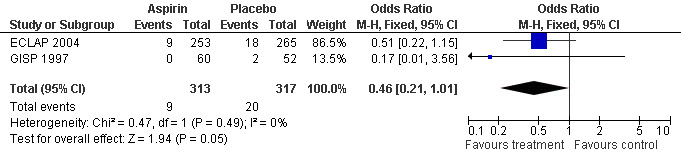
Forest plot of comparison: 1 Polycythaemia vera, outcome: 1.2 All cause mortality.
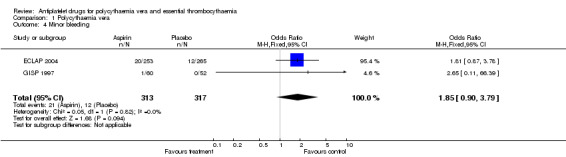
The primary safety outcome in our review was mortality from bleeding, but no data were available. A non‐significant increase with aspirin treatment was shown only for minor bleeding (OR 1.85, 95% CI 0.90 to 3.79; P = 0.09; fixed‐effect model; see Figure 5). Major bleeding occurred at a similar incidence in both treatment groups (OR 0.99, 95% CI 0.23 to 4.36; P = 0.99; fixed‐effect model; see Figure 6).
5.
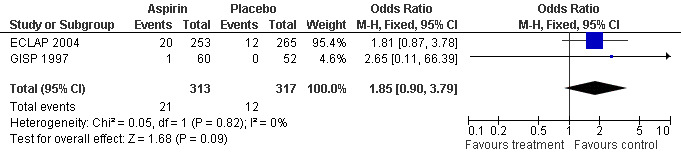
Forest plot of comparison: 1 Polycythaemia vera, outcome: 1.4 Minor bleeding.
6.
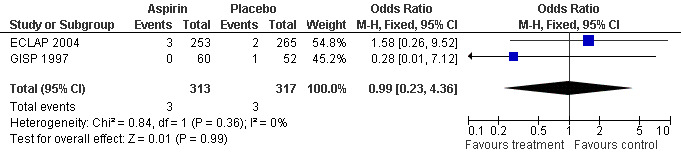
Forest plot of comparison: 1 Polycythaemia vera, outcome: 1.3 Major bleeding.
Using a random‐effects model for each outcome, we obtained very similar results.
Insufficient data prevented meta‐analysis of the following secondary outcomes: MI, unstable angina, ischaemic stroke, TIA, acute peripheral and visceral thromboembolism, deep venous thrombosis, pulmonary embolism, unusual sites of venous thrombosis (visceral vein thrombosis and cerebral sinus and venous thrombosis), superficial venous thrombosis, erythromelalgia, seizures, migraine, vertigo, tinnitus, scintillating scotomas, amaurosis fugax, and all adverse events (Secondary outcomes).
Overall, even though no outcome was statistically significant, we would expect 19 fatal thrombotic events (95% CI 0 to 40) to be prevented for every 1000 participants treated with aspirin, without an excess of major bleeding.
Discussion
Summary of main results
This systematic review of RCTs on the effects of antiplatelet drugs for long‐term primary and secondary prophylaxis of arterial and venous thrombotic events in participants with polycythaemia vera or essential thrombocythaemia included two original RCTs. These were designed and performed by the same network of haematologists and included only participants with polycythaemia vera. The experimental antiplatelet agent was low‐dose aspirin in both RCTs. Published data from both studies were insufficient for a time‐to‐event data analysis and for some of the primary and secondary outcomes that we had planned.
Overall, available evidence showed a marked, but not statistically significant, beneficial effect of low‐dose aspirin treatment compared with placebo in terms of mortality from thrombotic events and all‐cause mortality, with no increase in major bleeding, in participants with polycythaemia vera with no clear indication or contraindication to aspirin.
Overall completeness and applicability of evidence
For correct interpretation of these data, some comments are necessary. We had planned to extract the HRs and associated variances for a time‐to‐event data meta‐analysis. However, the necessary data (such as several single secondary outcomes) were not available in the published articles and could not be obtained from the trial investigators. This means that the overall treatment effect may be estimated with bias. For this reason, we decided to report data as simple binary outcomes and only data from four available efficacy and safety outcomes (mortality from thrombotic events, all‐cause mortality, and major and minor bleeding).
We could not perform our planned subgroup analysis. Consequently, results of these meta‐analyses are not directly extensible to all thrombotic risk groups of polycythaemic participants. Both RCTs included only participants with no clear indication for aspirin treatment and no clear contraindication to it, and who had no clinically significant co‐existing conditions.
Moreover, no studies in participants with essential thrombocythaemia have been published. Given the different natural course of the two diseases, data pertaining to participants with polycythaemia vera are only indirectly applicable to thrombocythaemic participants. Further studies are necessary to clarify the role of antiplatelet therapy in the treatment of patients with these conditions. Unfortunately, no ongoing RCTs that potentially meet our inclusion/exclusion criteria were identified for essential thrombocythaemia.
Aspirin was the only antiplatelet drug that was investigated. Other antiplatelet drugs are currently used daily (Alvarez‐Larran 2010), but no information for clinical practice can be extrapolated from our systematic review. The only ongoing trial on this topic is a phase II study on the safety and efficacy of clopidogrel and aspirin for the treatment of polycythaemia vera (ISCLAP). Lack of research in this area may have two possible explanations: complexity in conducting a trial in rare chronic diseases; and competing trials on new drugs, the Janus kinase 2 inhibitors, for the management of chronic Philadelphia‐negative myeloproliferative neoplasms (Pardanani 2011).
Quality of the evidence
Another limitation is represented by the methodological quality of the included RCTs (Characteristics of included studies). Both studies were well designed (ECLAP 2004, GISP 1997). However, they were judged to have a moderate quality because insufficient data were available to exclude an attrition bias.
Potential biases in the review process
An important limit of our meta‐analysis is the relevant weight (almost 80%) of the ECLAP 2004 study. However, we decided to meta‐analyse data as the GISP 1997 study had a very similar protocol, and because meta‐analysis is particularly useful in rare diseases, such as polycythaemia vera, in which only a limited number of participants can be enrolled.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this systematic review is the first ever published on this topic.
Authors' conclusions
Implications for practice.
In patients with polycythaemia vera who have no clear indication or contraindication to aspirin therapy, the use of low‐dose aspirin, when compared with no treatment, is associated with a statistically non‐significant reduction in the risk of fatal thrombotic events and all‐cause mortality, without increased risk of major bleeding. Given the available evidence and until new data are published. low‐dose aspirin would therefore be the antiplatelet drug of choice.
Implications for research.
Even though polycythaemia vera and essential thrombocythaemia are rare diseases, further RCTs are necessary to clarify definitively which subgroup of patients may benefit from antiplatelet drugs. Aspirin should be the drug of choice to be tested in future trials. However, other antiplatelet drugs should also be tested to determine whether they are as beneficial and safe as aspirin, as some patients with polycythaemia vera and essential thrombocythaemia may have an absolute contraindication to aspirin (e.g. allergy).
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2012 | New citation required but conclusions have not changed | Search has been re‐run to 12 October 2012. Only an ongoing study was included in this update |
| 12 October 2012 | New search has been performed | New search |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 13 January 2011 | Amended | typo correction |
| 15 August 2008 | Amended | Converted to new review format. |
Acknowledgements
None
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (October 2012)
#1 MeSH descriptor: [Myeloproliferative Disorders] explode all trees
#2 MeSH descriptor: [Polycythemia Vera] explode all trees
#3 polycyth*em*
#4 p vera*
#5 (erythrocytos* or erythrocytot*)
#6 erythr*em*
#7 Osler Vaquez*
#8 MeSH descriptor: [Thrombocytosis] explode all trees
#9 thrombocyth*em*
#10 (thrombocytos* or thrombocytot*)
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 MeSH descriptor: [Platelet Activation] explode all trees
#13 MeSH descriptor: [Blood Platelets] explode all trees
#14 MeSH descriptor: [Platelet Aggregation Inhibitors] explode all trees
#15 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*)
#16 (platelet* near/5 inhibit*)
#17 (thrombocyt* near/5 inhibit*)
#18 (platelet* near/5 antagon*)
#19 MeSH descriptor: [Aspirin] explode all trees
#20 (aspirin* or acetyl* salicyl* acid* or acetyl*salicyl* acid*)
#21 MeSH descriptor: [Ticlopidine] explode all trees
#22 (Ticlopidin* or Ticl*d* or Tikl*d* or Desitic* or ticlodon* or ticlodin* or anagregal* or panaldin*)
#23 (clopidogrel* or Plavix* or Iscover* or Aggrenox)
#24 MeSH descriptor: [Dipyridamole] explode all trees
#25 (dipyridamol* or dipyramidol*)
#26 (permol* or apotex* or antistenocardin* or persantin*)
#27 (curantyl* or kurantyl* or curantil* or kurantil*)
#28 prasugrel*
#29 (brilinta* or briliqu* or possia*)
#30 (effient* or efient*)
#31 (cs‐747 or cs747)
#32 (ly640315 or 150322‐43‐3)
#33 ticagrelor*
#34 (brilinta* or briliqu* or possia*)
#35 (azd6140 or azd‐6140)
#36 MeSH descriptor: [Thienopyridines] explode all trees
#37 thienopyridine*
#38 ((adenosine* diphoshate* or adp*) near/5 receptor* near/5 antagonist*)
#39 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38
#40 #11 and #39
Appendix 2. MEDLINE search strategy
MEDLINE (supplier OVID) from 1946 to October Week 3 2012
1 exp MYELOPROLIFERATIVE DISORDERS/
2 exp POLYCYTHEMIA VERA/
3 polycyth?em$.tw,kf,ot.
4 p vera.tw,kf,ot.
5 (erythrocytos$ or erythrocytot$).tw,kf,ot.
6 erythr?em$.tw,kf,ot.
7 Osler Vaquez.tw,kf,ot.
8 exp THROMBOCYTOSIS/
9 thrombocyth?em$.tw,kf,ot.
10 (thrombocytos$ or thrombocytot$).tw,kf,ot.
11 or/1‐10
12 exp PLATELET ACTIVATION/
13 BLOOD PLATELETS/
14 Platelet aggregation inhibitors/
15 (antiplatelet$ or anti‐platelet$ or antiaggreg$ or anti‐aggreg$ or (platelet$ adj5 inhibit$) or (thrombocyt$ adj5 inhibit$) or (platelet$ adj5 antagon$)).tw,kf,ot.
16 ASPIRIN/
17 (aspirin$ or acetyl$ salicyl$ acid$ or acetyl?salicyl$ acid$).tw,kf,ot.
18 TICLOPIDINE/
19 (Ticlopidin$ or Ticl?d$ or Tikl?d$ or Desitic$ or ticlodon$ or ticlodin$ or anagregal$ or panaldin$).tw,kf,ot.
20 (clopidogrel$ or Plavix$ or Iscover$ or Aggrenox).tw,kf,ot.
21 DIPYRIDAMOLE/
22 (dipyridamol$ or dipyramidol$).tw,kf,ot.
23 (permol$ or apotex$ or antistenocardin$ or persantin$).tw,kf,ot.
24 (curant?l$ or kurant?l$).tw,kf,ot.
25 prasugrel$.tw,kf,ot.
26 (brilinta$ or briliqu$ or possia$).tw,kf,ot.
27 (effient$ or efient$).tw,kf,ot.
28 (cs‐747 or cs747).tw,kf,ot.
29 (ly640315 or 150322‐43‐3).tw,kf,ot.
30 ticagrelor$.tw,kf,ot.
31 azd6140.tw.
32 exp Thienopyridines/
33 thienopyridine$.tw,kf,ot.
34 ((adenosine$ diphoshate$ or adp$) adj5 receptor$ adj5 antagonist$).tw,kf,ot.
35 or/12‐34
36 11 and 35
37 randomized controlled trial.pt.
38 controlled clinical trial.pt.
39 randomi?ed.ab.
40 placebo.ab.
41 drug therapy.fs.
42 randomly.ab.
43 trial.ab.
44 groups.ab.
45 or/37‐44
46 humans.sh.
47 45 and 46
48 36 and 47
Appendix 3. EMBASE search strategy
EMBASE (supplier OVID) from 1996 to 2012 Week 40
1 exp MYELOPROLIFERATIVE DISORDER/
2 ERYTHROCYTOSIS/
3 polycyth?em$.tw,hw,ot.
4 p vera.tw,hw,ot.
5 (erythrocytos$ or erythrocytot$).tw,hw,ot.
6 erythr?em$.tw,hw,ot.
7 Osler Vaquez.tw,hw,ot.
8 THROMBOCYTOSIS/
9 thrombocyth?em$.tw,hw,ot.
10 (thrombocytos$ or thrombocytot$).tw,hw,ot.
11 or/1‐10
12 THROMBOCYTE ACTIVATION/
13 THROMBOCYTE AGGREGATION/
14 THROMBOCYTE AGGREGATION INHIBITION/
15 ANTITHROMBOCYTIC AGENT/
16 (antiplatelet$ or anti‐platelet$ or antiaggreg$ or anti‐aggreg$ or (platelet$ adj5 inhibit$) or (thrombocyt$ adj5 inhibit$) or (platelet$ adj5 antagon$)).tw,hw,ot.
17 ACETYLSALICYLIC ACID/
18 (aspirin$ or acetyl$ salicyl$ acid$ or acetyl?salicyl$ acid$).tw,hw,ot.
19 TICLOPIDINE/
20 (Ticlopidin$ or Ticl?d$ or Tiklid$ or Desitic$ or ticlodon$ or ticlodin$ or anagregal$ or panaldin$).tw,hw,ot.
21 DIPYRIDAMOLE/
22 (dipyridamol$ or dipyramidol$ or Persantin$).tw,hw,ot.
23 (permol$ or apotex$ or antistenocardin$ or persantin$).tw,hw,ot.
24 (curantyl$ or kurantyl$ or curantil$ or kurantil$).tw,hw,ot.
25 prasugrel$.tw,hw,ot.
26 (brilinta$ or briliqu$ or possia$).tw,hw,ot.
27 (effient$ or efient$).tw,hw,ot.
28 (cs‐747 or cs747).tw,hw,ot.
29 (ly640315 or 150322‐43‐3).tw,hw,ot.
30 ticagrelor$.tw,hw,ot.
31 azd6140.tw,hw,ot.
32 THIENOPYRIDINE DERIVATIVE/
33 Thienopyridine.tw,hw,ot.
34 ((adenosine$ diphoshate$ or adp$) adj5 receptor$ adj5 antagonist$).tw,hw,ot.
35 CLOPIDOGREL/
36 (clopidogrel$ or Plavix$ or Iscover$).tw,hw,ot.
37 ACETYLSALICYLIC ACID PLUS DIPYRIDAMOLE/
38 Aggrenox.tw,hw,ot.
39 or/12‐38
40 11 and 39
41 (random$ or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab.
42 RETRACTED ARTICLE/
43 or/41‐42
44 (animal$ not human$).sh,hw.
45 (book or conference paper or editorial or letter or review).pt. not exp randomized controlled trial/
46 (random sampl$ or random digit$ or random effect$ or random survey or random regression).ti,ab. not exp randomized controlled trial/
47 43 not (44 or 45 or 46)
48 40 and 47
Data and analyses
Comparison 1. Polycythaemia vera.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality for thrombotic events | 2 | 630 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.03, 1.14] |
| 2 All cause mortality | 2 | 630 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.21, 1.01] |
| 3 Major bleeding | 2 | 630 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.23, 4.36] |
| 4 Minor bleeding | 2 | 630 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.90, 3.79] |
1.1. Analysis.
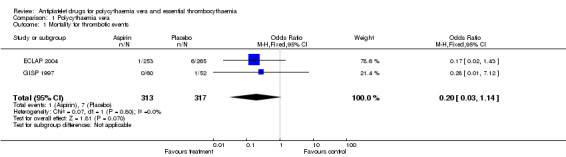
Comparison 1 Polycythaemia vera, Outcome 1 Mortality for thrombotic events.
1.2. Analysis.
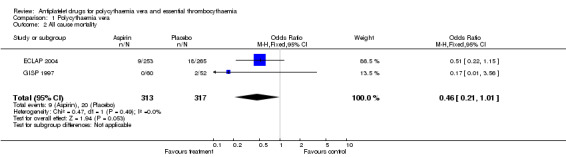
Comparison 1 Polycythaemia vera, Outcome 2 All cause mortality.
1.3. Analysis.
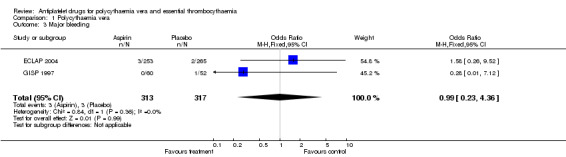
Comparison 1 Polycythaemia vera, Outcome 3 Major bleeding.
1.4. Analysis.
Comparison 1 Polycythaemia vera, Outcome 4 Minor bleeding.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ECLAP 2004.
| Methods | Randomised controlled trial. | |
| Participants | 518 people with polycythaemia vera (210 females, 308 males; mean age 61 years: a total of 26 percent of participants were 70 years of age or older). Participants were eligible if they had no clear indication for aspirin treatment and no clear contraindication to it, were able to provide written informed consent, and had no clinically significant co‐existing conditions. | |
| Interventions | Low‐dose aspirin, 100 mg per day (N = 253) versus placebo (N = 265). All participants who were recruited received other recommended treatments: Phlebotomy, cytoreductive drugs, and standard cardiovascular drugs were given as required. | |
| Outcomes | The two pre‐defined primary composed efficacy endpoints were: non‐fatal myocardial infarction, non‐fatal stroke, or death from cardiovascular causes; and non‐fatal myocardial infarction, non‐fatal stroke, pulmonary embolism, major venous thrombosis, or death from cardiovascular causes. The secondary endpoints consisted of fatal or non‐fatal cerebrovascular events, fatal or non‐fatal cardiac events, minor thrombotic complications (including atypical cerebral or visual symptoms of ischaemia, erythromelalgia, and thrombophlebitis), and major and minor thrombotic complications as previously defined. Safety was assessed by examining rates of fatal and non‐fatal major haemorrhage (any haemorrhage requiring transfusion, hospitalisation, or both), minor haemorrhage, and any adverse events leading to discontinuation of treatment. |
|
| Notes | Cytoreductive therapy was used in the following percentages of participants: ‐ Phlebotomy: 175 participants (69.2%) in the aspirin group and 197 participants (74.3%) in the placebo group. ‐ Any cytoreductive drug (radioactive phosphorus, hydroxyurea, busulfan, chlorambucil, pipobroman, interferon alpha): 149 participants (58.9%) in the aspirin group and 145 participants (54.7%) in the placebo group. After a planned interim safety analysis (in December 2000), the steering committee was informed that fewer centres than expected were recruiting effectively; that after the planned two years of recruitment, the rate of randomisation was reduced to nearly zero; that an impractically long follow‐up period would be required to accumulate the number of events needed to reach the pre‐defined rate of end points; and that no additional support for the trial could be obtained. For these reasons, the study was stopped, and follow‐up of participants who had undergone randomisation was completed during the next 12 months. These decisions were made with the advice and consent of the data and safety monitoring board and were communicated to the investigators, who were monitored to ensure that they conducted a final follow‐up visit. We obtained updated follow‐up information after September 1, 2001, for 92% of the participants who had undergone randomisation, for a total duration of follow‐up of 1478 person‐years. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '... A double‐blind, placebo‐controlled design was used. A total of 253 participants were randomly assigned to receive aspirin (100 mg daily), and 265 were randomly assigned to receive placebo ... Participants were assigned to treatments with the use of a program based on the biased‐coin algorithm, which allowed for stratification according to centre ...' |
| Allocation concealment (selection bias) | Low risk | '... randomisation was centralised and was performed over the telephone ...' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inability to judge whether there was selective loss to follow‐up or study withdrawal (6%). No further data were provided by the authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | '... A double‐blind, placebo‐controlled design was used. A total of 253 participants were randomly assigned to receive aspirin (100 mg daily), and 265 were randomly assigned to receive placebo ... Participants were assigned to treatments with the use of a program based on the biased‐coin algorithm, which allowed for stratification according to centre ...' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | '... The validation of the clinical events included in the primary endpoints was ensured by an ad hoc committee of expert clinicians who were unaware of the treatment‐group assignments. Each event was validated independently by two evaluators, and disagreement between evaluators was assessed by the chairman of the study ...' |
GISP 1997.
| Methods | Randomised controlled trial. | |
| Participants | 112 participants with polycythaemia vera (42 females, 70 males; aged 17 to 80 years), in whom physicians were uncertain whether aspirin was indicated. Participants were ineligible if physicians were reasonably certain that aspirin was indicated, was not indicated, or was contraindicated. Moreover, exclusion criteria included stage III chronic renal failure, active gastrointestinal disease, and a history of a major haemorrhagic episodes during the preceding four months. | |
| Interventions | Low‐dose aspirin, 40 mg per day (N = 60), versus placebo (N = 52) (in addition to the standard management policies adopted by the participating centres). | |
| Outcomes | Primary endpoints: major haemorrhagic events, compliance with the assigned treatment, gastric intolerance. Secondary endpoints: acute myocardial infarction, transient ischaemic attacks, stroke, venous thromboembolism, and acute arterial thrombosis. Follow‐up duration: 16 ± 6 months. |
|
| Notes | No data on concomitant cytoreductive therapy are provided in the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '... Participants were randomly allocated ... Randomisation was double blind, centrally co‐ordinated, and stratified by centre and according to thrombotic risk ...' |
| Allocation concealment (selection bias) | Low risk | '... Randomisation was double blind, centrally co‐ordinated, and stratified by centre and according to thrombotic risk ...' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This judgement was based on the fact that selective loss to follow‐up or study withdrawal (8% of participants) could not be excluded because data in the published article were insufficient. The authors provided no further data. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | '... Participants were randomly allocated to receive active treatment, ..., or placebo ... Randomisation was double blind, centrally co‐ordinated, and stratified by centre and according to thrombotic risk ...' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | '... Randomisation was double blind, centrally co‐ordinated, and stratified by centre and according to thrombotic risk ... All randomised participants were seen by their haematologists every 3 months and underwent a clinical and laboratory evaluation of their status ... ' |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Finelli 1991 | Participants with polycythaemia vera and an additional cardiovascular risk factor were randomly assigned to ticlopidine (250 mg twice a day) or to placebo for 60 days. As the main objective of our systematic review is to examine the benefit and harm of long‐term antiplatelet drug administration in preventing thrombotic events compared with no treatment, study drug administration less than 6 months is an exclusion criterion. |
| Tartaglia 1986 | Participants with a diagnosis of polycythaemia vera were randomly assigned to phlebotomy plus aspirin (300 mg three times a day) and dipyridamole (75 mg three times a day), or to phlebotomy plus radioactive phosphorus. As the main objective of our systematic review is to examine the benefit and harm of antiplatelet drugs in preventing thrombotic events compared with no treatment, direct comparison with an active cytoreductive drug is an exclusion criterion. |
Characteristics of ongoing studies [ordered by study ID]
ISCLAP.
| Trial name or title | Clopidogrel and Aspirin for the Treatment of Polycythemia Vera (ISCLAP). |
| Methods | Randomised controlled trial, phase II. |
| Participants | Inclusion criteria:
Participants are included in the study if all of the following criteria are met: A documented diagnosis of polycythaemia vera established within 5 years of registration. There must be documentation that the participant has met the revised WHO criteria for the diagnosis of polycythaemia vera. Participants must meet the 2 major criteria and 1 of the minor criteria. To verify that the criteria have been met, appropriate laboratory or pathology reports must be submitted demonstrating that the participant has documentation of these diagnostic criteria. Major criteria: Hemoglobin >18.5 g/dL in men, >16.5 g/dL in women, or other evidence of increased red cell volume. Presence of JAK 2V617F or other functionally similar mutation such as JAK2 exon 12 mutation. Minor criteria: Bone marrow biopsy showing hypercellularity for age with trilineage growth (panmyelosis) with prominent erythroid, granulocyte, and megakaryocytic proliferation. Serum erythropoietin level below the reference range for normal. Endogenous erythroid colony formation in vitro. High cardiovascular risk due to a prior vascular event such as ischaemic stroke, MI, or venous thromboembolism. Objective documentation of these events must be accurately reviewed and registered. Stroke and pulmonary embolism must be documented by an imaging study, deep vein thrombosis by ultrasound or other objective methods, and MI by typical electrocardiogram (ECG) changes and/or an increase in serum troponin. Minor thrombotic events such as TIAs, superficial thrombophlebitis, or atypical microcirculatory disturbances alone or in combination are considered to be qualifying events. No contraindication to aspirin use such as allergy, a history of a previous hemorrhagic stroke, or a major gastrointestinal bleed in the previous three months. Use of hydroxyurea as a cytoreductive agent. Signed informed consent: Participants must have signed consents for both the ISCLAP protocol and for the mandatory correlative biomarker MPD‐RC 107 protocol to be eligible. Serum bilirubin levels less than and/or equal to two times the upper limit of the normal range for the laboratory (ULN). Serum glutamic‐pyruvic transaminase (SGPT) alanine aminotransferase (ALT) levels and serum aspartate aminotransferase (AST) less than and/or equal to 2 x ULN. Serum creatinine levels less than and/or equal to 1.5 x ULN. Women of childbearing potential must have a negative serum or urine pregnancy test before receiving clopidogrel treatment and should be advised to avoid becoming pregnant. Women of childbearing potential must practice effective methods of contraception (those generally accepted as standard‐of‐care measures). Women of childbearing potential are those who have not been menopausal for 12 months or who have not undergone previous surgical sterilization. If the participant is a woman of childbearing potential, she must use a medically acceptable form of contraception during the study period and for 30 days thereafter. Age greater than or equal to 18 years to 81 years of age. Exclusion criteria: Participants are excluded from this study if one or more of the following criteria are met: Therapy with clopidogrel within the last 12 months. Any history of prior treatment with aspirin that has resulted in a significant clinical adverse event requiring discontinuation of aspirin therapy (e.g. bleeding, GI intolerance, etc., or intolerance to aspirin). Participants requiring anticoagulation treatment with warfarin, heparin, or low‐molecular‐weight heparin for any medical condition. Nursing and pregnant female participants. Should a woman become pregnant or suspect that she is pregnant while participating in this study, she should inform her physician immediately. History of a major bleeding event (requiring blood transfusion or hospitalisation, bleeding at a critical site, or life‐threatening bleeding). Clinical indication for the use of clopidogrel and/or a different antithrombotic regimen. History of active substance or alcoholic abuse within the last year. Known hypersensitivity or contraindication to study treatments. Chronic viral hepatitis or chronic liver disease from any other cause associated with a Model for End‐Stage Liver Disease (MELD) score equal to or higher than 8. Presence of any disease (e.g. cancer) that is likely to significantly shorten life expectancy. >81 years of age. New York Heart Association (NYHA) Grade II or greater congestive heart failure. A history of gastrointestinal bleeding in the last 12 months. Major surgical procedure, open biopsy, or significant traumatic injury within 28 days, or anticipation of the need for a major surgical procedure during the course of the study. Biopsy or other minor surgical procedure, excluding placement of a vascular access device or bone marrow biopsy, within 7 days before study enrolment. Ongoing serious, non‐healing wound, ulcer, or bone fracture. Treatment with a CYP3A4 inhibitor, including azole antifungals (topicals are permitted); protease inhibitors; nefazodone; cyclosporine; erythromycin; clarithromycin; and troleandomycin. Serum AST greater than or equal to 2 x ULN. Serum ALT greater than or equal 2 x ULN. Total bilirubin greater than or equal to 2 x ULN, Serum creatinine greater than or equal 1.5 x ULN. Participants with a diagnosis of polycythaemia vera >5 years from the time of registration. Participants who do not have high‐risk polycythaemia vera as defined by experiencing a thrombotic event occurring since the initial diagnosis of PV. |
| Interventions | Clopidogrel (oral, 75 mg per day) versus placebo, in addition to low‐dose aspirin and hydroxyurea. |
| Outcomes | Primary outcome measures: to determine the safety of clopidogrel plus aspirin in participants with polycythaemia at 2 years of follow‐up. |
| Starting date | June 2009. |
| Contact information | Raffaele Landolfi (rlandolfi@rm.unicatt.it) on behalf of Myeloproliferative Disorders‐Research Consortium. |
| Notes | http://clinicaltrials.gov, last updated on June 24, 2011: 'the study has been terminated (could not get drug).' No data have been published yet. |
Contributions of authors
Squizzato A. Drafting of protocol/review, searching and selection of studies, data extraction, data analysis, data interpretation. Final approval of the version to be published.
Romualdi E. Co‐drafting of protocol/review, searching and selection of studies, data extraction, data interpretation. Final approval of the version to be published.
Passamonti F. Analysis and interpretation of data, providing a clinical prospective. Revising the review critically for important intellectual concept. Final approval of the version to be published.
Middeldorp S. Conceiving, designing, coordinating, and general advice on the review. Co‐drafting protocol/review. Providing methodological, statistical, and clinical perspectives. Final approval of the version to be published.
Sources of support
Internal sources
University of Insubria, Italy.
External sources
CHMG Editorial Base is funded under the auspices of the German Federal Ministry of Education and Research (BMBF), application no: 01GH0501, Germany.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ECLAP 2004 {published data only}
- Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. Efficacy and safety of low‐dose aspirin in polycythaemia vera. New England Journal of Medicine 2004;350:114‐24. [DOI] [PubMed] [Google Scholar]
GISP 1997 {published data only}
- Gruppo Italiano Studio Policitemia Vera (GISP). Low‐dose aspirin in polycythaemia vera: a pilot study. British Journal of Haematology 1997;97:453‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Finelli 1991 {published data only}
- Finelli C, Palareti G, Poggi M, Torricelli P, Vianelli N, Fiacchini M, et al. Ticlopidine lowers plasma fibrinogen in patients with polycythaemia rubra vera and additional thrombotic risk factors. Acta Haematologica 1991;85:113‐8. [DOI] [PubMed] [Google Scholar]
Tartaglia 1986 {published data only}
- Tartaglia AP, Goldberg JD, Berck PD, Wasserman LR. Adverse effects of antiaggregant platelet therapy in the treatment of polycythaemia vera. Seminars in Hematology 1986;23:172‐6. [PubMed] [Google Scholar]
References to ongoing studies
ISCLAP {published data only}
- Clopidogrel and Aspirin for the Treatment of Polycythemia Vera (ISCLAP). NCT00940784. http://clinicaltrials.gov last search: 26 February 2012.
Additional references
ACCP 2012
- Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2012;2 Suppl:e89S‐e119S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez‐Larran 2010
- Alvarez‐Larran A, Cervantes F, Pereira A, Arellano‐Rodrigo E, Perez‐Andreu V, Hernandez‐Boluda JC, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low‐risk essential thrombocythemia. Blood 2010;116:1205‐10. [DOI] [PubMed] [Google Scholar]
Ania 1994
- Ania BJ, Suman VJ, Sobell JL, et al. Trends in the incidence of polycythemia vera among Olmsted County, Minnesota residents, 1935‐1989. American Journal of Hematology 1994;47:89‐93. [DOI] [PubMed] [Google Scholar]
ATC 2002
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. British Medical Journal 2002;324:71‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2005
- Campbell PJ, Green AR. Management of polycythaemia vera and essential thrombocythaemia. Hematology/Education Program of the American Society of Hematology 2005;1:201‐8. [DOI] [PubMed] [Google Scholar]
Cortelazzo 1990
- Cortelazzo S, Viero P, Finazzi G, D'Emilio A, Rodeghiero F, Barbui T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythaemia. Journal of Clinical Oncology 1990;8:556‐62. [DOI] [PubMed] [Google Scholar]
Cortelazzo 1995
- Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, et al. Hydroxyurea for patients with essential thrombocythaemia and high risk of thrombosis. New England Journal of Medicine 1995;332:1132‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finazzi 2005
- Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, et al. Acute leukaemia in polycythaemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105:2664‐70. [DOI] [PubMed] [Google Scholar]
GISP 1995
- Gruppo Italiano Studio Policitemia. Polycythemia vera: the natural history of 1213 patients followed for 20 years. Annals of Internal Medicine 1995;123:656‐64. [DOI] [PubMed] [Google Scholar]
Harrison 2005
- Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. Hydroxyurea compared with anagrelide in high‐risk essential thrombocythemia. New England Journal of Medicine 2005;353(1):33‐45. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 www.cochrane‐handbook.org.
Landolfi 1992
- Landolfi R, Ciabattoni G, Pugliese F, et al. Increased thromboxane biosynthesis in patients with polycythaemia vera: evidence for aspirin‐suppressible platelet activation in vivo. Blood 1992;80:1965‐71. [PubMed] [Google Scholar]
Landolfi 1997
- Landolfi R, Marchioli R. European Collaboration on Low‐dose Aspirin in Polycythemia Vera (ECLAP): a randomised trial. Seminars in Thrombosis and Haemostasis 1997;23:473‐8. [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. British Medical Journal 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maclure 1987
- Maclure M, Willett WC. Misinterpretation and misuse of the kappa statistic. American Journal of Epidemiology 1987;126:161‐9. [DOI] [PubMed] [Google Scholar]
Marchioli 2005
- Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythaemia vera. Journal of Clinical Oncology 2005;23:2224‐32. [DOI] [PubMed] [Google Scholar]
Mesa 1999
- Mesa RA, Silverstein MN, Jacobsen SJ, et al. Population‐based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. American Journal of Hematology 1999;61:10‐15. [DOI] [PubMed] [Google Scholar]
Pardanani 2011
- Pardanani A, Tefferi A. Targeting myeloproliferative neoplasms with JAK inhibitors. Curr Opin Hematol 2011;18:105‐110. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Passamonti 2004
- Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythaemia vera and essential thrombocythaemia. American Journal of Medicine 2004;117:755‐61. [DOI] [PubMed] [Google Scholar]
PRISMA
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement.. J Clin Epidemiol 2009;62:1006‐12. [DOI] [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagan: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rocca 1995
- Rocca B, Ciabattoni G, Tartaglione R, et al. Increased thromboxane biosynthesis in essential thrombocythaemia. Thrombosis and Haemostasis 1995;74:1225‐30. [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].. The Cochrane Collaboration, 2011 www.cochrane‐handbook.org.
WHO 2002
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002;100:2292‐302. [DOI] [PubMed] [Google Scholar]


