Abstract
Background
When treating elevated blood pressure (BP), doctors often want to know what blood pressure target they should try to achieve. The standard blood pressure target in clinical practice for some time has been less than 140 ‐ 160/90 ‐ 100 mmHg for the general population of people with elevated blood pressure. Several clinical guidelines published in recent years have recommended lower targets (less than 130/80 mmHg) for people with diabetes mellitus. It is not known whether attempting to achieve targets lower than the standard target reduces mortality and morbidity in those with elevated blood pressure and diabetes.
Objectives
To determine if 'lower' BP targets (any target less than 130/85 mmHg) are associated with reduction in mortality and morbidity compared with 'standard' BP targets (less than 140 ‐ 160/90 ‐ 100 mmHg) in people with diabetes.
Search methods
We searched the Database of Abstracts of Reviews of Effectiveness (DARE) and the Cochrane Database of Systematic Reviews for related reviews. We conducted electronic searches of the Hypertension Group Specialised Register (January 1946 ‐ October 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 9), MEDLINE (January 1946 ‐ October 2013), EMBASE (January 1974 ‐ October 2013) and ClinicalTrials.gov. The most recent search was performed on October 4, 2013.
Other search sources were the International Clinical Trials Registry Platform (WHO ICTRP), and reference lists of all papers and relevant reviews.
Selection criteria
Randomized controlled trials comparing people with diabetes randomized to lower or to standard BP targets as previously defined, and providing data on any of the primary outcomes below.
Data collection and analysis
Two review authors independently assessed and established the included trials and data entry. Primary outcomes were total mortality; total serious adverse events; myocardial infarction, stroke, congestive heart failure and end‐stage renal disease. Secondary outcomes were achieved mean systolic and diastolic BP, and withdrawals due to adverse effects.
Main results
We found five randomized trials, recruiting a total of 7314 participants and with a mean follow‐up of 4.5 years. Only one trial (ACCORD) compared outcomes associated with 'lower' (< 120 mmHg) or 'standard' (< 140 mmHg) systolic blood pressure targets in 4734 participants. Despite achieving a significantly lower BP (119.3/64.4 mmHg vs 133.5/70.5 mmHg, P < 0.0001), and using more antihypertensive medications, the only significant benefit in the group assigned to 'lower' systolic blood pressure (SBP) was a reduction in the incidence of stroke: risk ratio (RR) 0.58, 95% confidence interval (CI) 0.39 to 0.88, P = 0.009, absolute risk reduction 1.1%. The effect of SBP targets on mortality was compatible with both a reduction and increase in risk: RR 1.05 CI 0.84 to 1.30, low quality evidence. Trying to achieve the 'lower' SBP target was associated with a significant increase in the number of other serious adverse events: RR 2.58, 95% CI 1.70 to 3.91, P < 0.00001, absolute risk increase 2.0%.
Four trials (ABCD‐H, ABCD‐N, ABCD‐2V, and a subgroup of HOT) specifically compared clinical outcomes associated with 'lower' versus 'standard' targets for diastolic blood pressure (DBP) in people with diabetes. The total number of participants included in the DBP target analysis was 2580. Participants assigned to 'lower' DBP had a significantly lower achieved BP: 128/76 mmHg vs 135/83 mmHg, P < 0.0001. There was a trend towards reduction in total mortality in the group assigned to the 'lower' DBP target (RR 0.73, 95% CI 0.53 to 1.01), mainly due to a trend to lower non‐cardiovascular mortality. There was no difference in stroke (RR 0.67, 95% CI 0.42 to 1.05), in myocardial infarction (RR 0.95, 95% CI 0.64 to 1.40) or in congestive heart failure (RR 1.06, 95% CI 0.58 to 1.92), low quality evidence. End‐stage renal failure and total serious adverse events were not reported in any of the trials. A sensitivity analysis of trials comparing DBP targets < 80 mmHg (as suggested in clinical guidelines) versus < 90 mmHg showed similar results. There was a high risk of selection bias for every outcome analyzed in favor of the 'lower' target in the trials included for the analysis of DBP targets.
Authors' conclusions
At the present time, evidence from randomized trials does not support blood pressure targets lower than the standard targets in people with elevated blood pressure and diabetes. More randomized controlled trials are needed, with future trials reporting total mortality, total serious adverse events as well as cardiovascular and renal events.
Keywords: Adult, Humans, Blood Pressure, Diabetic Angiopathies, Diabetic Angiopathies/drug therapy, Diabetic Angiopathies/mortality, Diastole, Hypertension, Hypertension/drug therapy, Hypertension/mortality, Randomized Controlled Trials as Topic, Reference Values, Stroke, Stroke/prevention & control, Systole
Plain language summary
Blood pressure targets in people with diabetes
Review Question
We conducted this review to find and assess all trials designed to evaluate whether lower blood pressure targets are better than standard blood pressure targets for people with diabetes. We found and analyzed five studies.
Background
Cardiovascular disease is a frequent complication in people with diabetes. Hypertension (high blood pressure) is frequently found in people with diabetes. Recent clinical guidelines have recommended stricter control of blood pressure in people with diabetes compared with those without. For the general population of people with hypertension, the standard target has been to achieve a blood pressure of less than 140 to 160/90 to 100 mmHg, whereas for people with diabetes the guidelines have recommended lowering this target to less than 130/80 mmHg. This trend has been based on the assumption that achieving a lower blood pressure will produce a greater reduction in cardiovascular events.
Study Characteristics
The evidence is current to October 2013. We found and analyzed five randomized trials including 7134 adult participants with type 2 diabetes and high blood pressure, 40‐80 years old, who received treatment aimed to lower blood pressure to a standard compared to a lower blood pressure target and followed for 2 to 5 years to detect differences in mortality and adverse events. Four out of five studies were funded by the drug manufacturer, which had a potential of impacting the results. One study was sponsored by the National Heart, Lung, and Blood Institute (NHLBI) from the United States.
Key Results
The only significant benefit in the group assigned to 'lower' systolic blood pressure was a small reduction in the incidence of stroke, but with a significantly larger increase in the number of other serious adverse events. The effect of systolic blood pressure targets on mortality was compatible with both a reduction and increase in risk. There was no benefit associated with a 'lower' diastolic blood pressure target.
The evidence from randomized trials available at the present time is of low quality and does not support blood pressure targets lower than the standard in people with raised blood pressure and diabetes. Further research is likely to change these results and future studies should report all outcomes that are important to patients, such as mortality and adverse events.
Summary of findings
for the main comparison.
| Lower blood pressure (BP) targets compared with standard BP targets for mortality and morbidity | ||||||
|
Patient or population: Diabetes Mellitus with elevated blood pressure Settings: outpatients Intervention: BP < 130/85 mmHg Comparison: BP < 140 ‐ 160/90 ‐ 100 mmHg | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard BP targets | Lower BP targets | |||||
|
Total mortality Systolic targets |
RR 1.05 (0.84 to 1.30) | 4734 (1) | ⊕⊕⊝⊝ low4 | Only one RCT | ||
| Medium risk population | ||||||
| 60 per 10001 | 63 per 1000 (50 to 78) | |||||
|
Total mortality Diastolic targets |
Low risk population | RR 0.73 (0.53 to 1.01) | 2580 (4) | ⊕⊝⊝⊝ very low5 | High risk of bias | |
| 30 per 10002 | 22 per 1000 (16 to 30) | |||||
| Medium risk population | ||||||
| 60 per 10001 | 44 per 1000 (32 to 61) | |||||
| High risk population | ||||||
| 100 per 10003 | 73 per 1000 (53 to 101) | |||||
|
Total serious adverse events Systolic targets |
RR 1.01 (0.91 to 1.13) | 4734 (1) | ⊕⊕⊝⊝ low6 | Only one RCT | ||
| Medium risk population | ||||||
| 214 per 10001 | 216 per 1000 (197 to 244) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Based on the event rate in the standard BP target group. 2One‐half the medium event rate. 3Medium event rate times 1.7. 4Only one RCT and confidence intervals includes a 25% increase. 5Inadequate sequence generation, no blinding, subgroup analysis and early termination. 6Only one RCT and no blinding.
Background
Description of the condition
Epidemiological studies show a continuous direct relationship between adverse cardiovascular events and blood pressure (BP), with elevated blood pressure defined as one of the major risk factors for adverse cardiovascular events (MacMahon 1990; Stamler 1993; Kannel 1996; Prospective Studies 2002). The primary goal in the management of people with elevated blood pressure (hypertension) is to maximize the reduction in mortality and morbidity (Oparil 2003; ESH‐ESC 2007). The lower threshold at which this relationship no longer applies has not been definitively identified (Prospective Studies 2002).
Any numerical cut‐off value above which elevated blood pressure is defined is arbitrary. The standard for diagnosis of arterial hypertension is based on consensus recommendations, which attempt to predict the blood pressure above which it is expected that treatment will provide more benefit than harm.
Recent guidelines in Europe acknowledge the paucity of evidence related to the benefit from drugs in mild hypertension. The same limitation applies to BP targets to be reached on treatment: the benefit from drug treatment seems rather clear with treatments aiming at lowering BP levels at less than 160 for systolic or 100 mmHg for diastolic, whereas no data are available for promoting lower targets. This statement is based 1) on the targets adopted in trials that showed the benefit from drugs in primary prevention in hypertensive people without other conditions, and 2) on the analysis of the part of risk reduction due to treatment that could be attributed to BP lowering. This part has been estimated to be about 60% for stroke (Boissel 2005). Of note, the benefit from some BP‐lowering drugs has been established in other conditions with normal or even low BP levels, e.g. congestive heart failure, secondary prevention after stroke or myocardial infarction, high cardiovascular risk. In these situations, the benefit from these drugs has been established with fixed dosages, without any adjustment to the apparent BP level or response. The superiority of a strategy guided by a predefined BP target has not proved superior to a fixed‐dosage strategy.
Diastolic blood pressure was privileged for the inclusion criteria or the treatment target in the first trials conducted with antihypertensive drugs, during the last three decades of the previous century. Systolic BP has been used more recently, initially to explore whether isolated systolic hypertension frequently observed after 60 years of age was associated with a benefit from these drugs, and subsequently for two reasons: its prognostic value appeared greater than that of diastolic BP, and it is observable in all age ranges, whereas the value of diastolic BP was observable in young people and disappeared or even reverted in older people.
Diabetes is also identified as one of the major risk factors for adverse cardiovascular (CV) events, and CV complications are the most frequent reason for mortality in people with diabetes (Stamler 1993b). Furthermore, arterial hypertension is frequently detected in people with diabetes mellitus, possibly hastening the development and progression of complications (Adler 2000). At the present time the threshold above which antihypertensive treatment benefits outweigh harm in people with diabetes remains unclear.
Description of the intervention
The target blood pressure is used in clinical practice as the goal of antihypertensive therapy. It guides the clinical practitioner when making treatment decisions related to the intensity of the antihypertensive regimen used for each patient.
How the intervention might work
Blood pressure targets lower than standard have become more prevalent in recent guidelines and thus in clinical practice. This trend toward 'the lower the pressure the better' was expressed in an editorial accompanying the publication of the 2004 British Hypertension Society guidelines (Laurent 2004), and assumes that treatment to lower blood pressure targets with antihypertensive drugs will achieve the predicted reduction in cardiovascular morbidity and mortality seen in epidemiological observational studies. This trend has been especially strong for people with diabetes mellitus and those with chronic renal disease, for whom even lower targets have been promoted based on the assumption that they have a higher cardiovascular risk.
Why it is important to do this review
The importance of this review is emphasized by the blood pressure targets recommended for people with diabetes mellitus in several clinical guidelines published in recent years (JNC 7 2003; WHO/ISH 2003; BHS 2004; AHA 2007; ESH‐ESC 2007; LA 2009; CHEP 2011; ADA 2012). These guidelines recommend a BP target lower than 130/80 mmHg in people with diabetes, mainly based on observational data and on retrospective analyses of outcome trials. Most of these guidelines also recommend initiating antihypertensive drug treatment in people with diabetes with systolic blood pressure (SBP) higher than 130 mmHg or diastolic blood pressure (DBP) higher than 80 to 85 mmHg.
However, elevated blood pressure can be considered as a marker of vascular disease, and an aggressive reduction in blood pressure does not necessarily mean that the pathological and functional vascular abnormalities already established will be reversed.
Attempting to achieve lower blood pressure targets has several consequences. The most obvious is the need for large doses or an increased number of antihypertensive drugs. This has costs to patients in terms of inconvenience and economic burdens. More drugs and higher doses will also increase adverse drug effects, which if serious could cancel any potential benefit associated with any lower blood pressures achieved. In addition, there is the potential that lowering blood pressure too much with drugs can cause adverse cardiovascular events, such as the so‐called 'J‐curve phenomenon', mentioned for many years in medical articles and revisited in recent years, especially in the elderly or in people with established coronary disease (Farnett 1991; Vokó 1999; Zanchetti 2003; Messerli 2006; Sleight 2009; Bangalore 2010; Dorresteijn 2012).
The only way to prove that a lower BP target is beneficial is through clinical trials where participants are randomized to different treatment targets. In that respect, in a previous Cochrane systematic review and meta‐analysis of randomized controlled clinical trials (Arguedas 2009), we showed that, in the general population of people with hypertension, treating to blood pressure targets lower than 130/85 mmHg by pharmacological means did not result in lower mortality or cardiovascular morbidity compared with standard targets (lower than 140 ‐ 160 mmHg systolic and lower than 90 ‐ 100 mmHg diastolic). In the same Cochrane review, a sensitivity analysis in people with diabetes did not provide sufficient evidence for recommending lower blood pressure targets in that patient population. Therefore, the assumption that treating to lower targets would provide a greater reduction in cardiovascular risk, as suggested by epidemiological studies, was not proven, and 'the lower the better' strategy in hypertension was challenged (Arguedas 2010; Filippone 2011; Grossman 2011). A recent observational analysis did not show any difference in cardiovascular outcomes between SBP less than 130 mmHg and less than 140 mmHg in people with diabetes and coronary disease (Cooper‐DeHoff 2010).
A more recent clinical guideline, published by the National Institute for Health and Clinical Excellence (NICE) and the British Hypertension Society (NICE 2011) states “aim for a target clinic blood pressure below 140/90 mmHg in people aged under 80 years with treated hypertension” without providing any different criteria for people with diabetes. The latest American Diabetes Association clinical guideline recommends that people with diabetes and hypertension should be treated to a systolic blood pressure goal of less than 140 mmHg, and to a diastolic blood pressure goal of less than 80 mmHg (ADA 2013). Finally, the most recently published European guideline (ESH/ESC2013) recommends a target lower than 140/85 mmHg for people with diabetes.
Given that many clinical guidelines are still recommending lower blood pressure targets for people with diabetes, our goal was to identify all randomized controlled trials where people with diabetes were randomized to lower targets (less than 130/85 mmHg) compared with the standard targets. Standard targets were defined as a systolic blood pressure target less than or equal to 140 ‐ 160 mmHg, and a diastolic blood pressure target less than or equal to 90 ‐ 100 mmHg. We have chosen a range for both standard target categories to be inclusive and to make sure that the two treatment groups are mutually exclusive. Treatment targets higher than those previously mentioned were not eligible because they were considered to be inappropriately high.
Objectives
Primary objective
To determine if 'lower' BP targets (any target less than 130/85 mmHg) are associated with reduction in mortality and morbidity compared with 'standard' BP targets (less than 140 ‐ 160/90 ‐ 100 mmHg) in people with diabetes.
Secondary objectives
To determine if there is a change in mean achieved systolic and diastolic blood pressure associated with 'lower targets' compared with 'standard targets' in people with diabetes and elevated blood pressure.
To determine if there is a change in withdrawals due to adverse effects with 'lower targets' compared with 'standard targets', in people with diabetes and elevated blood pressure.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomized controlled clinical trials. Trials cannot be blinded to blood pressure targets because the treating physicians must know the target to which each participant has been assigned in order to make the proper adjustment in the therapy to achieve the blood pressure goal.
All trials that reported any of the outcomes were included. Trials were not limited by any concomitant disease, other factor or baseline cardiovascular risk. There was no language restriction.
Types of participants
Participants were adults with diabetes mellitus and elevated blood pressure, documented in a standard way on at least two occasions, or already receiving treatment for elevated blood pressure.
Since any numerical definition of elevated blood pressure is arbitrary, we included trials if people with diabetes were randomized to one of the two targets described below, irrespective of their baseline blood pressure.
Types of interventions
We included trials if individuals were randomized to a 'lower' compared with a 'standard' target blood pressure as defined above.
Types of outcome measures
Primary outcomes
All‐cause mortality plus cardiovascular and non‐cardiovascular mortality separately.
Total serious adverse events (total serious morbidity and mortality).
Cardiovascular serious adverse events, including myocardial infarction, stroke, congestive heart failure, end‐stage renal failure.
All other serious adverse events.
Secondary outcomes
Systolic blood pressure achieved.
Diastolic blood pressure achieved.
Withdrawals due to adverse effects.
Number of antihypertensive drugs needed per participant.
Search methods for identification of studies
Electronic searches
We searched the Database of Abstracts of Reviews of Effectiveness (DARE) and the Cochrane Database of Systematic Reviews for related reviews.
We searched the following electronic databases for primary studies: the Hypertension Group Specialised Register (January 1946 ‐ October 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 9), MEDLINE (January 1946 ‐ October 2013), EMBASE (January 1974 ‐ October 2013) and ClinicalTrials.gov.
We searched the electronic databases using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) with selected MeSH terms and free‐text terms relating to diabetes and hypertension. We applied no language restrictions. The MEDLINE search strategy (Appendix 1) was translated into EMBASE (Appendix 2), CENTRAL (Appendix 3), The Hypertension Group Specialised Register (Appendix 4), and ClinicalTrials.gov (Appendix 5) using the appropriate controlled vocabulary as applicable. The latest search date for all databases was October 2013.
Searching other resources
Other sources: a) International Clinical Trials Registry Platform (WHO ICTRP); b) Reference lists of all papers and relevant reviews identified; c) We tried to contact authors of relevant papers regarding any further published or unpublished work; d) We tried to contact authors of trials reporting incomplete information to provide the missing information; e) We searched ISI Web of Science for papers which cite studies included in the review.
Data collection and analysis
Selection of studies
We prespecified the outcomes to be compared and the trial eligibility criteria before the result of any contributing trial was known. Two independent review authors assessed the eligibility of the trials, resolving discrepancies by discussion or by recourse to a third individual if necessary.
Data extraction and management
Two review authors independently extracted data from the included trials. For the synthesis and analysis of the data, we used Cochrane Review Manager software (RevMan 2012). Quantitative analyses of outcomes were based on an intention‐to‐treat principle.
Assessment of risk of bias in included studies
Two review authors independently performed the assessment of risk of bias for each study, using the six domains of the 'Risk of bias' Tool according to the method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We used the risk ratio (RR) and a fixed‐effect model to combine outcomes across trials.
Dealing with missing data
We tried to contact authors in case of missing information in the retrieved articles.
Assessment of heterogeneity
We used the Chi² and I² statistics to test for heterogeneity of treatment effect between the trials (Higgins 2003). A Chi² value less than 0.05 or an I² value greater than 50% was considered indicative of significant heterogeneity. We planned to use a random‐effects model to test for statistical significance if significant heterogeneity existed.
Results
Description of studies
Results of the search
The search identified 597 records. There remained 235 publications after partial screen and removal of duplicates by Trials Research Coordinator. Most of those publications were rejected after reading the abstract or the complete report. This left 11 references that seemed appropriate for this systematic review (Figure 1). The detailed analysis of those 11 publications revealed:
1.
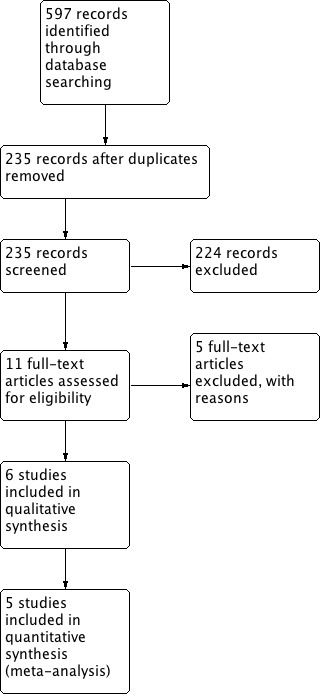
Results of the search.
‐ 5 randomized controlled trials from 6 publications that met the inclusion criteria
‐ 5 randomized controlled trials that did not meet the inclusion criteria
Included studies
One trial (ACCORD BP 2010) compared clinical outcomes associated with different systolic blood pressure (SBP) targets within our definitions for ‘lower’ and ‘standard’ targets. Four trials (ABCD‐H 1998, ABCD‐N 2002, ABCD‐2V 2006, and the subgroup of people with diabetes in HOT 1998) compared clinical outcomes associated with different diastolic blood pressure (DBP) targets meeting our definitions for ‘lower’ and ‘standard’ targets
a. Methods:
The 5 included trials were randomized and open label. In each trial an independent end point committee, which was blinded to the study intervention arms, reviewed all cardiovascular events.
In ACCORD BP 2010 the participants were also randomly assigned (in a 2‐by‐2 factorial design) to either intensive or standard glycemic control (the ACCORD glycemia trial). In HOT 1998 the participants were also randomly assigned in a factorial design to receive aspirin or placebo.
The follow‐up period varied from 1.9 to 5 years.
ACCORD BP 2010 was conducted in the United States and Canada. HOT 1998 included participants from 26 countries from Europe, Asia, North and South America.
b. Participants:
The inclusion criteria varied among the trials (see table of characteristics of included studies). ACCORD BP 2010, ABCD‐H 1998, ABCD‐N 2002, and ABCD‐2V 2006 only included people with type 2 diabetes. The type of diabetes was not specified in HOT 1998. ACCORD BP 2010 also required a high cardiovascular risk, defined by already established cardiovascular disease, evidence of subclinical cardiovascular disease, or at least two additional risk factors for cardiovascular disease.
The baseline blood pressure required for inclusion also varied. ACCORD BP 2010 required a systolic blood pressure between 130 and 180 mmHg when taking three or fewer antihypertensive medications to be included. Participants in ABCD‐H 1998 had a baseline diastolic blood pressure equal to or higher than 90 mm Hg. Participants in ABCD‐N 2002 had a baseline diastolic blood pressure between 80 and 89 mmHg and were not receiving antihypertensive medications at the randomization visit, but some of them had elevated systolic blood pressure. ABCD‐2V 2006 included people with a systolic BP lower than 140 mmHg, and a diastolic BP between 80 and 90 mmHg. In HOT 1998 the baseline diastolic blood pressure was between 100 mmHg and 115 mmHg.
The trials included people between the ages of 40 and 81 years. The number of participants included in each study was: ABCD‐2V 2006: 129 participants; ABCD‐H 1998: 470 participants; ABCD‐N 2002: 480 participants; ACCORD BP 2010: 4733 participants; HOT 1998: 1501 participants.
c. Interventions:
Participants in ACCORD BP 2010 were randomly assigned to intensive therapy that targeted systolic blood pressure of less than 120 mmHg, or standard therapy that targeted systolic blood pressure of less than 140 mmHg.
Participants in ABCD‐H 1998 and ABCD‐2V 2006 were randomized into two treatment arms consisting of ‘intensive’ treatment with a diastolic blood pressure goal of 75 mmHg, and ‘moderate’ treatment with a diastolic blood pressure goal of 80‐89 mmHg.
Participants in ABCD‐N 2002 were randomized into two treatment arms consisting of "intensive" or "moderate" treatment. The goal in the "intensive" treatment group was to achieve a decrease of 10 mmHg below baseline in diastolic blood pressure (i.e. 70 to 79 mmHg), whereas the goal in the "moderate" treatment group was to maintain a diastolic blood pressure between 80 and 89 mmHg.
Participants in HOT 1998 were randomly assigned to one of three diastolic blood pressure target groups: less than or equal to 90 mmHg, less than or equal to 85 mmHg, or less than or equal to 80 mmHg.
In ABCD‐H 1998 and ABCD‐N 2002 the initial antihypertensive agent was either nisoldipine or enalapril, randomly assigned in a factorial way. Valsartan was the initial antihypertensive medication in ABCD‐2V 2006, following a established drug procedure afterwards. In HOT 1998 felodipine was given as baseline therapy with the addition of other agents according to a five‐step regimen. No specific drug procedure was decribed in ACCORD BP 2010.
In ABCD‐N 2002 and ABCD‐2V 2006 participants assigned to the ‘standard’ therapy groups were given placebo initially.
d. Outcomes:
Several types of major cardiovascular events were the primary outcomes in ACCORD BP 2010 and HOT 1998. Surrogate markers of renal function were the primary outcome in the ABCD trials (ABCD‐2V 2006, ABCD‐H 1998, ABCD‐N 2002) and some types of cardiovascular events were reported as secondary outcomes.
e. Additional notes:
The ACCORD BP 2010 trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI) from the United States. The remaining trials were supported by pharmaceutical companies.
ACCORD BP 2010 was conducted between January 2001 and October 2005. HOT was conducted between October 1992 and August 1997. The dates for the other trials were not specified.
Excluded studies
United Kingdom Prospective Diabetes Study (UKPDS 38 1998)
This study was excluded because the target for systolic blood pressure in the 'tight control' group was higher than stated in our protocol. In addition, and more importantly, the targets for both systolic and diastolic blood pressure in the 'less tight control' group were much higher than specified in the protocol for this systematic review.
Hypertension in Diabetes Study IV (HDS 1996)
This trial was excluded from the review for the same reasons as the UKPDS 38 trial. Furthermore, it is likely that participants in this trial represent a subgroup of participants included in UKPDS 38, because the study design is similar and the authors are the same.
SANDS (SANDS 2008)
This trial was not included because the dual intervention would not allow us to address the events specifically associated with a lower blood pressure target. Besides, both systolic blood pressure targets in this trial were within the values considered as 'lower targets' in our systematic review.
Lewis et al (Lewis 1999)
It was excluded because it did not provide data on any of the outcomes defined for this systematic review.
Steno‐2 study (Steno‐2 2003)
This trial was not included because the multifactorial intervention prevented any inference as to whether any difference in clinical outcomes could be attributed to a lower blood pressure target or to any of the other combined interventions.
Risk of bias in included studies
The summary of the risk of bias assessment of each trial is shown in Figure 2.
2.
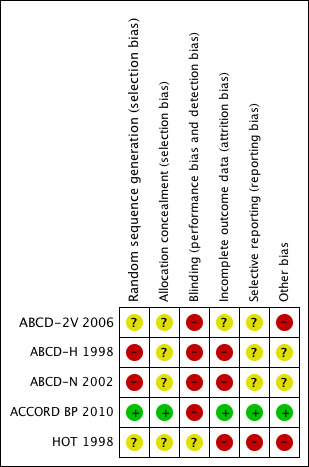
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In HOT 1998 and ACCORD BP 2010 randomization was performed centrally and computer‐generated. The method of randomization was not described in the other trials.
Blinding
None of the trials was blinded to blood pressure goal because of the need to titrate treatment to achieve the specific target. Clinical events were evaluated in every trial by an independent committee masked to the group allocation.
Incomplete outcome data
In the entire HOT 1998 trial 2.6% of the participants were lost to follow‐up, and they were equally distributed between the target arms; such information is not available for the subgroup of diabetic participants. In ACCORD BP 2010 4.9% were lost to follow‐up, and their distribution is not known. No specific information about drop‐outs was provided in the remaining trial reports.
Selective reporting
Some of the outcomes were not evaluated or reported in the trials. The clearest example of potential selective reporting bias is total serious adverse events, which were reported in only one trial.
Other potential sources of bias
As explained in detail below, baseline characteristics in the ABCD trials differed between the 'lower' and the 'standard' target groups.
Effects of interventions
See: Table 1
1. Systolic blood pressure target
Only one randomized trial (ACCORD BP 2010) was identified comparing targets for systolic blood pressure within the definitions established for this review. The data were obtained from the main report of the trial and the published appendices.
1.1 Total mortality
There was no difference in total mortality between the two blood pressure target groups: risk ratio (RR) 1.05, 95% confidence interval (CI) 0.84 to 1.30, P = 0.69 (Analysis 1.1).
1.1. Analysis.
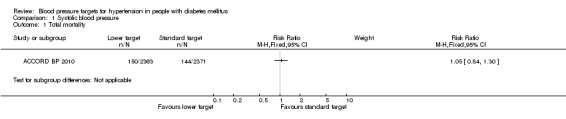
Comparison 1 Systolic blood pressure, Outcome 1 Total mortality.
1.2 Cardiovascular (CV) mortality
There was no difference in cardiovascular mortality between the two blood pressure target groups: RR 1.04, 95% CI 0.73 to 1.48, P = 0.84 (Analysis 1.2).
1.2. Analysis.
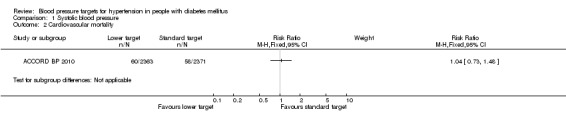
Comparison 1 Systolic blood pressure, Outcome 2 Cardiovascular mortality.
1.3 Non‐CV mortality
There was no difference in non‐cardiovascular mortality between the two blood pressure target groups: RR 1.05, 95% CI 0.79 to 1.40, P = 0.74 (Analysis 1.3).
1.3. Analysis.
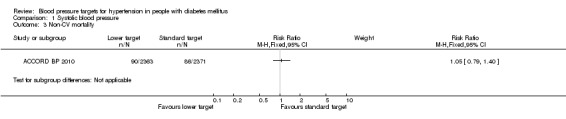
Comparison 1 Systolic blood pressure, Outcome 3 Non‐CV mortality.
1.4 Total serious adverse events
The total number of serious adverse events was not reported in the ACCORD BP 2010 trial. We calculated the sum of total mortality, non‐fatal myocardial infarction, non‐fatal stroke, non‐fatal heart failure, end‐stage renal disease or need for dialysis, and other serious adverse events attributed to blood pressure medications. There was no difference in the calculated total serious adverse events between the two blood pressure target groups: RR 1.01, 95% CI 0.91 to 1.13, P = 0.81 (Analysis 1.4).
1.4. Analysis.
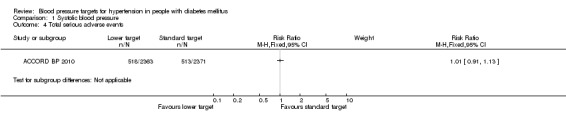
Comparison 1 Systolic blood pressure, Outcome 4 Total serious adverse events.
1.5 Myocardial infarction
There were 133 myocardial infarctions (126 non‐fatal) in the intensive therapy group and 151 (146 non‐fatal) in the standard therapy group, with no significant difference between the groups: RR 0.88, 95% CI 0.71 to 1.11, P = 0.28 (Analysis 1.5).
1.5. Analysis.
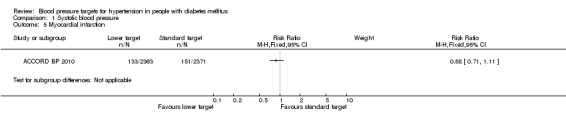
Comparison 1 Systolic blood pressure, Outcome 5 Myocardial infarction.
1.6 Stroke
There were 36 strokes (34 non‐fatal) in the intensive therapy group and 62 (55 non‐fatal) in the standard therapy group. The difference between the groups was statistically significant: RR 0.58, 95% CI 0.39 to 0.88, P = 0.009, absolute risk reduction 1.1% (Analysis 1.6).
1.6. Analysis.

Comparison 1 Systolic blood pressure, Outcome 6 Stroke.
1.7 Congestive heart failure
There was no difference in fatal or non‐fatal heart failure between the two blood pressure target groups: RR 0.93, 95% CI 0.69 to 1.24, P = 0.60 (Analysis 1.7).
1.7. Analysis.
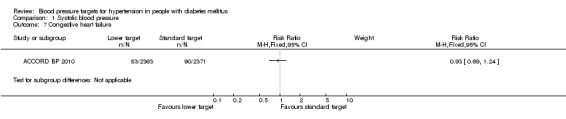
Comparison 1 Systolic blood pressure, Outcome 7 Congestive heart failure.
1.8 End‐stage renal failure
There was no difference in end‐stage renal failure or need for dialysis between the two blood pressure target groups: RR 1.02, 95% CI 0.71 to 1.46, P = 0.84 (Analysis 1.8).
1.8. Analysis.
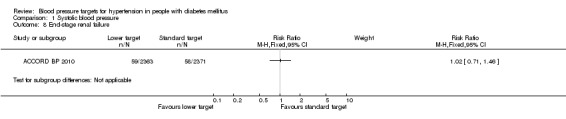
Comparison 1 Systolic blood pressure, Outcome 8 End‐stage renal failure.
1.9 All other serious adverse events
The ACCORD BP 2010 investigators reported separately other serious adverse events attributed to blood pressure medications, including hypotension, syncope, bradycardia or arrhythmia, hyperkalemia, angioedema, and renal failure. These serious adverse events were significantly more prevalent in the 'intensive therapy' group: RR 2.58, 95% CI 1.70 to 3.91, P < 0.00001, absolute risk increase 2.0% (Analysis 1.9).
1.9. Analysis.
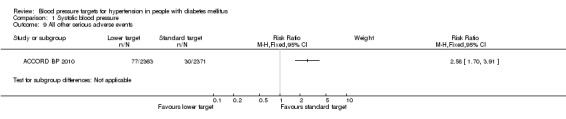
Comparison 1 Systolic blood pressure, Outcome 9 All other serious adverse events.
1.10 Systolic blood pressure achieved
After the first year of therapy, the average systolic blood pressure achieved was significantly lower in the 'intensive therapy' group, mean difference 14.2 mmHg, P < 0.00001 (119.3 mmHg vs 133.5 mmHg in the 'intensive' vs the 'standard' groups respectively; Analysis 1.10).
1.10. Analysis.
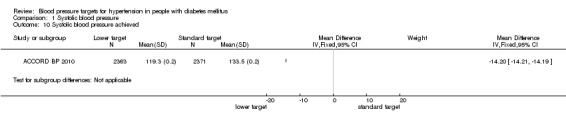
Comparison 1 Systolic blood pressure, Outcome 10 Systolic blood pressure achieved.
1.11 Diastolic blood pressure achieved
After the first year of therapy, the average diastolic blood pressure achieved was significantly lower in the 'intensive therapy' group, mean difference 6.1 mmHg, P < 0.00001 (64.4 mmHg vs 70.5 mmHg in the 'intensive' vs the 'standard' groups respectively; Analysis 1.11).
1.11. Analysis.
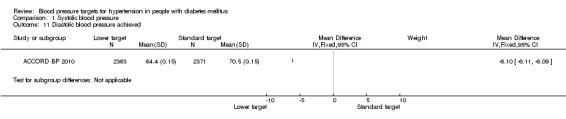
Comparison 1 Systolic blood pressure, Outcome 11 Diastolic blood pressure achieved.
1.12 Withdrawals due to adverse events
There is no information available.
1.13 Number of antihypertensive drugs used
The mean number of antihypertensive drugs used after the first year was significantly greater in the 'intensive therapy' group (3.4 vs 2.1), P < 0.00001; Analysis 1.13.
1.13. Analysis.
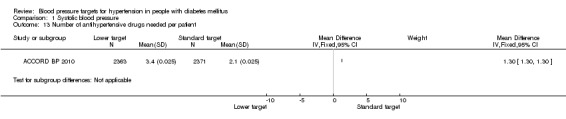
Comparison 1 Systolic blood pressure, Outcome 13 Number of antihypertensive drugs needed per patient.
2. Diastolic blood pressure (DBP) target
Three trials compared clinical outcomes associated with different diastolic blood pressure targets specifically in people with diabetes (ABCD‐H 1998; ABCD‐N 2002; ABCD‐2V 2006). Although it enrolled non‐diabetic participants, the HOT 1998 trial could be included in this analysis because it reported outcomes in the subgroup of participants with diabetes separately. Some outcomes, not provided in the published reports of the trials, were obtained from the Blood Pressure Lowering Treatment Trialists` Collaboration (BPLTTC 2003).
Several issues regarding the design and the characteristics of these trials must be mentioned before analyzing the results:
a. Regarding baseline characteristics, if the three ABCD trials are combined, a significantly greater proportion of participants randomized to 'standard target' had established cardiovascular or cerebrovascular disease compared with the 'lower target' group (41.0% vs 33.5%, P = 0.007). This difference in baseline characteristics is important and would be expected to have an impact on the clinical outcomes against the 'standard target' group, because having a greater proportion of participants with established vascular disease increases the risk of having future events. The magnitude of this potential bias is increased by the fact that the ABCD trials account for 42% of the total number of participants included in the analysis for diastolic BP targets.
In the HOT 1998 trial, randomization was blocked for several factors, including diabetes mellitus, and therefore it is possible that baseline characteristics of participants with diabetes were similar between the target blood pressure groups, but the details are not available and therefore the potential for bias also exists for this trial.
b. In terms of inclusion criteria, the ABCD‐N 2002 and ABCD‐2V 2006 trials included only normotensive diabetic participants, defined as having a diastolic blood pressure between 80 and 89 mmHg. Twenty‐six participants (5.4%) with isolated systolic hypertension (systolic blood pressure greater than 160 mmHg and diastolic blood pressure between 80 and 89 mmHg) were enrolled in ABCD‐N 2002 during the first year of recruitment, but none thereafter. On the other hand, ABCD‐H 1998 and HOT 1998 only included participants with elevated blood pressure, but the criteria for inclusion were different. In ABCD‐H 1998 participants had a baseline diastolic blood pressure equal to or higher than 90 mmHg, whereas an inclusion criterion in HOT 1998 was a baseline diastolic blood pressure between 100 and 115 mmHg.
c. When the HOT 1998, ABCD‐H 1998 and ABCD‐N 2002 trials were conducted, the diagnostic criteria for diabetes mellitus were different from those currently used. At that time, diabetes mellitus was defined as two fasting plasma glucose levels, measured on different days, higher than 7.7 mmol/L (140 mg/dL), instead of 7.0 mmol/L (126 mg/dL), as currently defined.
2.1 Total mortality
The data on mortality from ABCD‐H 1998 deserve an explanation. The first publication of the trial (ABCD‐H 1998) mentioned 30 death in total, but it did not provide details on the distribution according to blood pressure targets. Later publications stated that “patients randomized to intensive therapy had a lower incidence of all‐cause mortality when compared to moderate therapy, 5.5% vs 10.7%, p= 0.037” (ABCD‐H 2000; ABCD 2007), without providing absolute numbers. Given that 237 participants were assigned to the intensive treatment group, and 233 participants to the moderate treatment group, the absolute number of deaths calculated from the reported percentages would be 13 and 25 respectively, for a total of 38 deaths, which differs from the total mortality mentioned in the first report. Finally, the Blood Pressure Lowering Treatment Trialists`Collaboration reported 32 deaths in the same trial, 10 in the intensive treatment group and 22 in the moderate treatment group (BPLTTC 2003). Due to the lack of concordance, the information from the BPLLTTC was used for this analysis, because it was the only one providing absolute numbers and because they were closer to the figures mentioned in the original report.
There was a statistically non‐significant trend toward reduced risk of total mortality in the 'lower target' group: RR 0.73, 95% CI 0.53 to 1.01, P = 0.05 (Analysis 2.1).
2.1. Analysis.
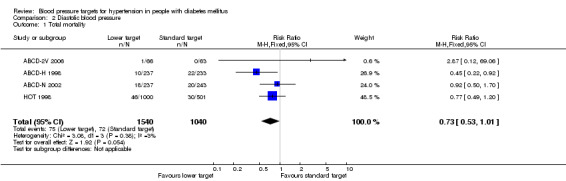
Comparison 2 Diastolic blood pressure, Outcome 1 Total mortality.
2.2 Cardiovascular mortality
There was no difference in cardiovascular mortality between the '''lower target' and the 'standard target' groups: RR 0.79, 95% CI 0.52 to 1.19, P = 0.26 (Analysis 2.2).
2.2. Analysis.
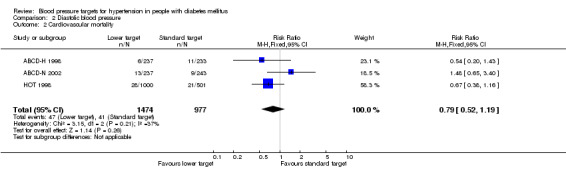
Comparison 2 Diastolic blood pressure, Outcome 2 Cardiovascular mortality.
2.3 Non‐CV mortality
There was no difference in non‐cardiovascular mortality in the 'lower target' compared with the 'standard target' group: RR 0.62, 95% CI 0.36 to 1.06, P = 0.08 (Analysis 2.3).
2.3. Analysis.
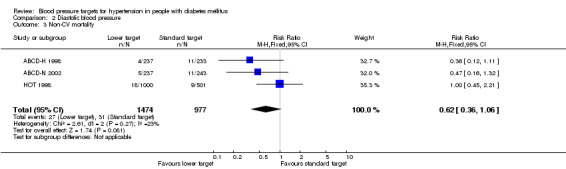
Comparison 2 Diastolic blood pressure, Outcome 3 Non‐CV mortality.
2.4 Total serious adverse events
Total serious adverse events were not reported in any of the trials.
2.5 Myocardial infarction
There was no difference in the incidence of myocardial infarction between the 'lower target' and the 'standard target' groups: RR 0.95, 95% CI 0.64 to 1.40, P = 0.79 (Analysis 2.5).
2.5. Analysis.
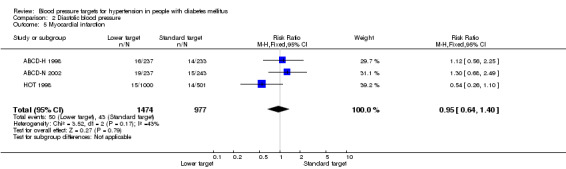
Comparison 2 Diastolic blood pressure, Outcome 5 Myocardial infarction.
2.6 Stroke
There was no difference in the incidence of stroke in the 'lower target' compared with the 'standard target' group, RR 0.67, 95% CI 0.42 to 1.05), P = 0.08 (Analysis 2.6).
2.6. Analysis.
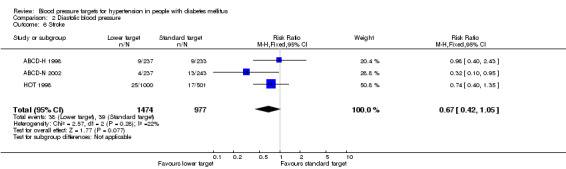
Comparison 2 Diastolic blood pressure, Outcome 6 Stroke.
2.7 Congestive heart failure
Data on this outcome from the subgroup of participants with diabetes included in HOT 1998 and the ABCD‐2V 2006 trial are not available. Data from ABCD‐H 1998 were provided by BPLTTC. There was no difference in the incidence between the two treatment target groups: RR 1.06, 95% CI 0.58 to 1.92, P = 0.86 (Analysis 2.7).
2.7. Analysis.
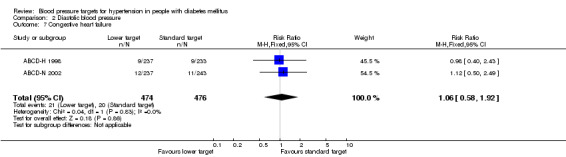
Comparison 2 Diastolic blood pressure, Outcome 7 Congestive heart failure.
2.8 End‐stage renal failure
End‐stage renal failure was not reported in any of the trials.
2.9 All other serious adverse events
All other serious adverse events were not reported in any of the trials.
2.10 Systolic blood pressure achieved
No information is available from the subgroup of participants with diabetes in HOT 1998. The average systolic blood pressure achieved in the ABCD trials was significantly lower in the 'lower target' group, mean difference 7.31 mmHg, P < 0.00001 (mean SBP = 128 mmHg vs 135 mmHg in the 'lower target' vs the 'standard target' groups respectively; Analysis 2.10).
2.10. Analysis.
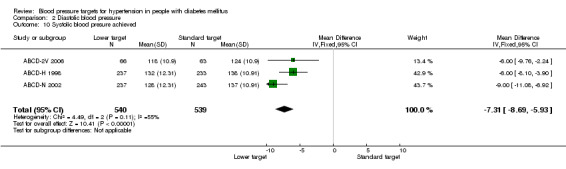
Comparison 2 Diastolic blood pressure, Outcome 10 Systolic blood presure achieved.
2.11 Diastolic blood pressure achieved
No information is available from the subgroup of participants with diabetes in HOT 1998. The average diastolic blood pressure achieved in the ABCD trials was significantly lower in the 'lower target' group, mean difference 6.85 mmHg, P < 0.00001 (mean DBP= 76 mmHg vs 83 mmHg in the 'lower target' vs the 'standard target' groups respectively; Analysis 2.11).
2.11. Analysis.
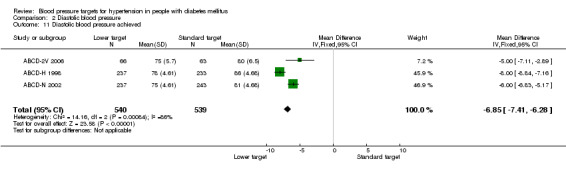
Comparison 2 Diastolic blood pressure, Outcome 11 Diastolic blood pressure achieved.
2.12 Withdrawals due to adverse effects
Withdrawals due to adverse events were not reported in any of the trials.
2.13 Number of antihypertensive drugs used
The number of antihypertensive drugs used was not reported in any of the trials.
Sensitivity analysis for diastolic blood pressure < 80 mm Hg vs < 90 mm Hg
Because clinical guidelines recommend a diastolic blood pressure target lower than 80 mmHg, we decided to perform a sensitivity analysis in trials comparing a target of lower than 80 mmHg versus the standard target of lower than 90 mmHg. This sensitivity analysis only excluded the treatment arm lower than 85 mmHg from the HOT 1998 trial. It therefore has the same limitations described for the entire analysis on DBP, except that in this case the unbalance in baseline characteristics against the standard target from the ABCD trials has a more pronounced effect because they represent more than 50% of the total population.
The results from this sensitivity analysis are shown in the following table. They are very similar to the entire analysis for DBP mentioned above. In this case the reduction in total mortality associated with the lower target achieved statistical significance. The contributions from both cardiovascular and non‐CV mortality were numerically similar.
| Outcome | Studies | Participants | Statistical method | Effect estimate | P |
| Total mortality | 4 | 2079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64, 95% CI 0.45 to 0.92 | 0.02 |
| Cardiovascular mortality | 3 | 1950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64, 95% CI 0.39 to 1.03 | 0.07 |
| Non‐cardiovascular mortality | 3 | 1950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62, 95% CI 0.35 to 1.08 | 0.09 |
| Total serious adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable | |
| Myocardial infarction | 4 | 2079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98, 95% CI 0.65 to 1.48 | 0.93 |
| Stroke | 4 | 2079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64, 95% CI 0.39to 1.06 | 0.08 |
| Congestive heart failure | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06, 95% CI 0.58 to 1.92 | 0.86 |
Discussion
This systematic review and meta‐analysis summarizes the available evidence from randomized trials that have evaluated clinical outcomes associated with 'lower' versus 'standard' blood pressure (BP) targets, as previously defined, in participants with diabetes mellitus. Even though a review of people with diabetes and hypertension can be considered as a subgroup analysis of the hypertensive population, the approach is justified because most of the trials were conducted specifically in people with diabetes, for whom different BP targets have been proposed.
We found five randomized trials, with a total of 7314 participants, and a mean follow‐up period of 4.5 years.
Even though pharmacological treatment usually decreases both systolic and diastolic blood pressure, we decided to analyze each target separately, because each one of them really constitutes an individual target in clinical practice. For example, current medical practice is to start pharmacological antihypertensive therapy when either systolic blood pressure (SBP) or diastolic blood pressure (DBP) remains elevated despite non‐pharmacologic strategies. If both SBP and DBP are elevated, the recommendation is to adjust drug regimens until both targets are achieved. Besides, systolic and diastolic pressures are determined by different pathophysiological mechanisms and can be associated with different clinical implications (systolic BP is a constant risk factor at all ages, whereas diastolic BP is mainly a risk factor in younger people). Finally, combining trials evaluating SBP and DBP targets can be misleading because both pressures may not decrease to the same extent, and therefore the treatment strategy may differ. For example, a pharmacological strategy to achieve a 'standard' target in SBP could produce a very intense decrease in DBP within the range defined for the 'intensive' target. In fact, that situation occurred in the ACCORD BP 2010 trial, where participants assigned to the 'standard' systolic target achieved a mean DBP well below our definition for the 'lower' target.
Summary of main results
Systolic blood pressure (SBP) target:
No randomized trial has ever evaluated directly clinical outcomes associated with a SBP less than 130 mmHg as recommended in most clinical guidelines for people with diabetes. ACCORD BP 2010 is the only trial that has evaluated outcomes associated with systolic blood pressure targets within the range established as 'lower' or 'standard' and it clearly stated that trying to achieve a SBP lower than 120 mmHg instead of lower than 140 mmHg was associated with an isolated benefit in the risk of stroke. Even though it was statistically significant, its magnitude was small, with an absolute risk reduction of 1.1%, which means that 91 people must be treated intensively during 4.7 years to prevent one additional stroke. Moreover, the benefit in stroke was counterbalanced by a significant absolute risk increase of 2% in other serious adverse events attributed to blood pressure medications, which means that one excessive serious adverse event occurred for every 50 people treated intensively.
Three main issues have arisen from the ACCORD BP 2010 results:
1. Maybe the systolic target of lower than 120 mmHg was too low, and could have produced a J‐curve phenomenon. Whether a target lower than 130 mmHg instead of lower than 120 mmHg would avoid that potential inconvenience is unknown. 2. Based on the previous argument, it has been suggested that tight BP control could be beneficial if implemented early (Zanchetti 2009; Parati 2011). For example, young people with diabetes, who are in the early stages of the vascular atherosclerotic process, could benefit from a lower target without the potential risk of a J‐curve phenomenon. However, this interesting argument mentioned in one of the most recent clinical guidelines (ADA 2013) is not supported by evidence, and it must be properly evaluated and proved before being implemented in clinical practice. 3. It has also been proposed that the risk/benefit ratio associated with the lower target could be acceptable for people at high risk of stroke, such as people with diabetes with a history of cerebrovascular disease (Mancia 2011). Once again, this idea must be properly evaluated.
Diastolic blood pressure (DBP) target:
The four remaining trials evaluated DBP targets according to our definitions. There was a trend of borderline statistical significance toward a decrease in total mortality (RR 0.73, 95% CI 0.53, 1.01, P = 0.05) associated with the 'lower target'. However, a more detailed analysis leads one not to place much weight on this finding:
1. The trend toward a decrease in total mortality was mainly driven by the non‐cardiovascular causes of death. It is hard to explain how a more intensive antihypertensive therapy can decrease the mortality due to non‐cardiovascular causes. 2. There was no difference in other clinical outcomes. 3. As previously mentioned, there is a high risk of bias in these trials. The main potential sources of bias are the subgroup analysis in the HOT 1998 trial, and a greater proportion of participants with established cardiovascular disease at baseline assigned to the 'standard' BP target in the ABCD trials, which might have biased the results against the 'standard' target group. 4. The trials were performed when the diagnostic criteria for diabetes were less strict than the currently applied criteria. Besides, other preventive cardiovascular pharmacological resources frequently used in current medical practice were seldom used when the trials were performed. Therefore, participants included in those trials may differ in several aspects from many people with diabetes in current medical practice. 5. The lack of information on total serious adverse events and on withdrawals due to adverse effects precludes a global assessment of the benefits and harms ratio. 6. Most of the outcomes had wide confidence intervals because the total number of trial participants was small.
Overall completeness and applicability of evidence
Hypertension and diabetes are highly prevalent conditions, frequently coexisting in the same patient. Treating hypertension is therefore an important part of the medical care of people with diabetes. The intensity of the antihypertensive treatment is greatly determined by the blood pressure target. Despite those important facts, the available evidence from randomized trials to define the optimal blood pressure targets is scanty.
For SBP, the ACCORD BP 2010 trial provides very useful information. However, as mentioned before, there are many important unanswered questions.
The situation is more critical for DBP. The evidence is provided by an insufficient number of participants, under conditions that differ in several aspects from current medical practice, and is associated with a high risk of bias.
Quality of the evidence
We downgraded the quality of evidence to low or very low for the outcomes of mortality and serious adverse events (Table 1). This was primarily due to imprecision of effect estimates from the systolic target trial, and due to imprecision and very serious risk of bias in the diastolic target trials.
Potential biases in the review process
The main potential bias is due to the fact that studies were not blinded. Lack of blinding is necessary because adjustments in the treatment must be performed when trying to achieve a specific blood pressure target. However, lack of blinding may lead to ascertainment bias when investigators are adjudicating outcomes.
The trials at the highest risk for other types of biases were the HOT 1998 trial (because it is a subgroup analysis) and the ABCD trials, because of important differences in baseline characteristics suggesting that adequate sequence generation or allocation concealment or both were not achieved.
Agreements and disagreements with other studies or reviews
The main disagreement relates to clinical guidelines, which based their recommendations mainly on observational data or on post hoc analyses of achieved blood pressures in outcome trials. It must be remembered that observational studies are very susceptible to selection bias and to other confounding factors. The interpretation of outcome trials based on achieved blood pressure, rather than on the allocated treatment target, is also misleading, because it neglects the basic principles of randomization and intention‐to‐treat analysis. Looking at the cohort of participants with lower achieved blood pressure probably selects for those who had the lowest baseline blood pressure, for those in whom the blood pressure was most easily reduced with low doses of antihypertensive drugs, and for those who are more compliant with drug and non‐drug therapies. All of these factors are associated with a lower risk of having a cardiovascular event.
To the best of our knowledge, three meta‐analyses have evaluated similar questions. A meta‐analysis of randomized controlled trials in people with diabetes or impaired fasting glucose analyzed outcomes according to achieved, rather than targeted, blood pressures, in trials designed to test different hypotheses (Bangalore 2011). It must be noted that 10 of the 13 trials included in that meta‐analysis were not designed to compare outcomes specifically associated with different BP targets, and therefore other factors could potentially influence the results. Despite those important methodological differences, and a somewhat different conclusion, the results were very similar to ours. In fact, the author of the editorial accompanying that meta‐analysis states that the data “do not support an across‐the‐board strategy of lowering SBP to ≤ 135 mmHg because their findings did not reveal significant benefit of intensive BP‐lowering strategy over the standard BP control strategy on macrovascular and microvascular events” (Deedwania 2011). With greater SBP reduction (lower than 130 mmHg), the only additional benefit found was an isolated decrease in the risk of stroke, but with an increased risk of serious adverse events. They conducted no analysis regarding DBP.
Another meta‐analysis compared clinical outcomes associated with different BP intervention strategies in people with diabetes (Reboldi 2011). One of the comparisons performed was “more tight” versus “less tight” BP control, without defining specific values for those categories. As a result, they combined trials evaluating SBP and DBP targets, and also included the UKPDS 38 1998 trial which was excluded from our analysis. As mentioned before, the reasons for excluding UKPDS 38 1998 from our review were two‐fold: firstly, the target for the 'low target' group in UKPDS 38 was 150/85 mmHg. This target is in the range for the 'standard target' for systolic blood pressure in our review; secondly, the blood pressure target in the less intensive treatment group was less than 200/105 mmHg, and after five years it was reduced to less than 180/105 mmHg. These high targets are very similar to the cutoffs for the no‐treatment group in trials comparing treatment with no treatment, and were abandoned in clinical practice many years ago. The inclusion of UKPDS 38 1998 is therefore misleading and inappropriate when comparing 'lower' and 'standard' blood pressure targets in current medical practice.
Finally, another meta‐analysis combined randomized trials comparing both systolic and diastolic blood pressure targets together (McBrien 2012). This meta‐analysis compared an intensive target defined as less than 130/80 mmHg with a standard target defined as less than 140 ‐ 160/85 ‐ 100 mmHg. There was statistical between‐studies heterogeneity. It showed that the intensive target was associated with a small reduction in stroke, without changing the risk for mortality or myocardial infarction. As mentioned above, we think that combining targets for SBP and DBP has a number of limitations.
Authors' conclusions
Implications for practice.
At the present time the best available evidence from randomized controlled trials (RCTs) does not support blood pressure (BP) targets lower than 140/90 mmHg in people with elevated blood pressure and diabetes. This review analyzed lower systolic and diastolic blood pressure (SBP, DBP) targets separately, with similar findings for both targets. The isolated small reduction in stroke associated with a lower SBP target must be weighed against a larger increase in serious adverse events.
Therefore, the lower target for blood pressure recommended for people with diabetes in many clinical guidelines is not supported by evidence from randomized controlled trials.
Implications for research.
This review provides strong support for the need for independently‐conducted RCTs evaluating lower BP targets in the population of people with diabetes mellitus. The absence of information in people with type 1 diabetes is particularly regrettable. Future trials should report total mortality and total serious adverse events as well as individual cardiovascular, cerebrovascular and renal events. Other outcomes such as health‐related quality of life should also be investigated.
Finally, future research must evaluate whether trying to achieve a BP target is better than alternative therapeutic strategies, such as a fixed‐dose strategy.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2013 | Amended | Detailed search strategies added as appendices and included in Search methods section of the review. Corrected date of search. |
Acknowledgements
The authors would like to acknowledge the Cochrane Hypertension Group's advice and assistance.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 4 October 2013 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp diabetes mellitus/ 2 diabet$.tw. 3 or/1‐2 4 exp hypertension/ 5 hypertens$.tw. 6 exp blood pressure/ 7 (blood pressure or bloodpressure).tw. 8 or/4‐7 9 ((strict$ or target$ or tight$ or intens$ or below) adj3 (blood pressure or systolic or diastolic or bp or level$)).tw. 10 ((bp or blood pressure) adj2 lowering).tw. 11 or/9‐10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 clinical trials as topic/ 17 randomly.ab. 18 trial.ti. 19 or/12‐18 20 animals/ not (humans/ and animals/) 21 19 not 20 22 3 and 8 and 11 and 21
Appendix 2. EMBASE search strategy
Database: Embase <1974 to 2013 Week 39> Search Date: 4 October 2013 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp diabetes mellitus/ 2 diabet$.tw. 3 or/1‐2 4 exp hypertension/ 5 hypertens$.tw. 6 blood pressure.mp. 7 or/4‐6 8 ((strict$ or target$ or tight$ or intens$ or below) adj3 (blood pressure or systolic or diastolic or bp or level$)).tw. 9 ((bp or blood pressure) adj2 lowering).tw. 10 or/8‐9 11 randomized controlled trial/ 12 crossover procedure/ 13 double‐blind procedure/ 14 randomi?ed.tw. 15 (crossover$ or cross‐over$).tw. 16 randomly.ab. 17 placebo$.tw. 18 (doubl$ adj blind$).tw. 19 allocat$.ab. 20 comparison.ti. 21 or/11‐20 22 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 23 21 not 22 24 3 and 7 and 10 and 23 25 24 and (2012$ or 2013$).em.
Appendix 3. CENTRAL search strategy
Database: (Wiley) Cochrane Central Register of Controlled Trials <Issue 9 2013> Search Date: 4 October 2013 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ ID Search #1 MeSH descriptor: [Diabetes Mellitus] explode all trees #2 diabet*:ti,ab #3 #1 or #2 #4 MeSH descriptor: [Hypertension] explode all trees #5 hypertens*:ti,ab #6 MeSH descriptor: [Blood Pressure] explode all trees #7 (blood pressure or bloodpressure):ti,ab #8 #4 or #5 or #6 or #7 #9 (strict* or target* or tight* or intens* or below) near/5 (blood pressure or systolic or diastolic or bp or level*):ti,ab #10 (bp or blood pressure) near/5 (lower or lowered or lowering):ti,ab #11 #9 or #10 #12 #3 and #8 and #11
Appendix 4. Hypertension Group Specialised Register search strategy
Database: Hypertension Group Specialised Register Search Date: 4 October 2013 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 strict* AND (bp) AND (diabet*) #2 strict* AND (hypertens*) AND (diabet*) #3 target* AND (blood pressure) AND (diabet*) #4 target* AND (bp) AND (diabet*) #5 target* AND (hypertens*) AND (diabet*) #6 tight* AND (blood pressure) AND (diabet*) #7 tight* AND (bp) AND (diabet*) #8 tight* AND (hypertens*) AND (diabet*) #9 below AND (blood pressure) AND (hypertens*) AND (diabet*) #10 below AND (bp) AND (hypertens*) AND (diabet*) #11 (intens* blood pressure) AND (hypertens*) AND (diabet*) #12 (intens* bp) AND (hypertens*) AND (diabet*) #13 (intens* lower*) AND (hypertens*) AND (diabet*) #14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 AND INREGISTER
Appendix 5. ClinicalTrials.gov search strategy
Database: ClinicalTrials.gov (via Cochrane Register of Studies) Search Date: 4 October 2013 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Study type: Interventional Studies Conditions: hypertension Outcome Measures: blood pressure Search terms: diabetes randomized
Data and analyses
Comparison 1. Systolic blood pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Cardiovascular mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Non‐CV mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Total serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Myocardial infarction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Stroke | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Congestive heart failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8 End‐stage renal failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 All other serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Systolic blood pressure achieved | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 Diastolic blood pressure achieved | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12 Withdrawals due to adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Number of antihypertensive drugs needed per patient | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 2. Diastolic blood pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 4 | 2580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.01] |
| 2 Cardiovascular mortality | 3 | 2451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.19] |
| 3 Non‐CV mortality | 3 | 2451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.06] |
| 4 Total serious adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Myocardial infarction | 3 | 2451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.40] |
| 6 Stroke | 3 | 2451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.42, 1.05] |
| 7 Congestive heart failure | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.58, 1.92] |
| 8 End‐stage renal failure | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 All other serious adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Systolic blood presure achieved | 3 | 1079 | Mean Difference (IV, Fixed, 95% CI) | ‐7.31 [‐8.69, ‐5.93] |
| 11 Diastolic blood pressure achieved | 3 | 1079 | Mean Difference (IV, Fixed, 95% CI) | ‐6.85 [‐7.41, ‐6.28] |
| 12 Withdrawals due to adverse events | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Number of antihypertensive drugs used per patients | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ABCD‐2V 2006.
| Methods | Single‐center, open‐label, randomized trial. An independent Endpoint Committee, which was blinded to the study intervention arms, reviewed all cardiovascular events. The mean follow‐up period was 1.9 years. |
|
| Participants | 129 type‐2 diabetic participants, 40 to 81 years of age, with a systolic BP < 140 mmHg, a diastolic BP between 80 and 90 mmHg, and without evidence of overt albuminuria (< 200µg/min). Exclusion criteria included pregnant or lactating women, need for any antihypertensive medications, documented myocardial infarction or cerebrovascular accident within the past 6 months, severe peripheral vascular disease, history of bilateral renal artery stenosis or stenosis in a solitary kidney, evidence of severe liver disease, hyperkalemia, or history of active cancer. | |
| Interventions | Participants were randomized to either intensive BP control aiming for a diastolic BP goal of 75 mmHg or to moderate BP control aiming to maintain DBP between 80 and 90 mmHg. Participants randomized to intensive BP control were started on valsartan 80 mg per day with a target diastolic BP of 75 mmHg. Antihypertensive medications were increased in a step‐wise manner in order to achieve the BP target: valsartan 80 mg, then valsartan 160 mg/day, hydrochlorothiazide 12.5 ‐ 25 mg per day, metoprolol 50 ‐ 100 mg twice a day, additional medications at the discretion of the medical director. Participants randomized to moderate BP control were placed on placebo to maintain their diastolic BP between 80 and 90 mmHg. During the study period, if the systolic BP reached > 140 mmHg and/or the diastolic BP reached > 90 mmHg, antihypertensive medications were initiated in the moderate BP control group following the same procedure mentioned for the intensive BP control group. |
|
| Outcomes | The primary outcome was a combination of surrogate markers of renal function. Deaths and cardiovascular events were also recorded. | |
| Notes | The trial was stopped early because of funding constraints. The trial was sponsored by the industry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participant and investigator not possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trial terminated early |
| Selective reporting (reporting bias) | Unclear risk | Individual outcomes not reported |
| Other bias | High risk | Trial was terminated early; Industry funded |
ABCD‐H 1998.
| Methods | Randomized, open‐label clinical trial. An independent end point committee, which was blinded to the study intervention arms, reviewed all cardiovascular events. The follow‐up period was 5 years. |
|
| Participants | 472 participants, between the ages of 40 and 74 years, with type 2 diabetes mellitus and a diastolic blood pressure equal to or higher than 90 mm Hg were included. Exclusion criteria included myocardial infarction or a cerebrovascular accident within the previous 6 months, coronary artery bypass surgery within the previous 3 months, unstable angina pectoris within the previous 6 months, congestive heart failure NYHA class III or IV, a demonstrated absolute need for ACE inhibitors or CCB, and a serum creatinine level > 3 mg/dL. |
|
| Interventions | Patients were randomized into two treatment arms consisting of "intensive" treatment with a diastolic blood pressure goal of 75 mmHg, and "moderate" treatment with a diastolic blood pressure goal of 80‐89 mmHg. They were also allocated to either nisoldipine or enalapril as the initial antihypertensive medication. If the target blood pressure was not achieved with increasing doses, then open‐label antihypertensive medications were added in a step‐wise fashion, initially with metoprolol, then hydrochlorothiazide or additional drugs, but neither a calcium channel blocker nor an ACE inhibitor. Blood pressure recordings were obtained at the time when peak drug levels were expected and were an average of three seated readings obtained at each visit. |
|
| Outcomes | The primary end point was the change in 24‐hour creatinine clearance. Secondary end points included cardiovascular events, retinopathy, clinical neuropathy, and urinary albumin excretion. | |
| Notes | Patients were also randomized to either nisoldipine or enalapril as the initial antihypertensive medication. A test for interaction between study‐drug assignment and blood‐pressure‐control strategy showed that no interaction was present. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants assigned to "moderate" treatment had a greater prevalence of established vascular disease, which became significant when combined with ABCD‐N. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participant and investigator not possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on losses to follow‐up was not reported |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes reported |
| Other bias | Unclear risk | Funding not reported |
ABCD‐N 2002.
| Methods | Randomized, open label controlled clinical trial. An independent end point committee, which was blinded to the study intervention arms, reviewed all cardiovascular events. The follow‐up period was 5 years. | |
| Participants | 480 participants, aged 40 ‐ 74 years, with type 2 diabetes mellitus were included. All of them had a baseline diastolic blood pressure between 80 and 89 mmHg and were not receiving antihypertensive medications at the randomization visit. The main exclusion criteria were: myocardial infarction or cerebrovascular accident within the previous 6 months, coronary artery bypass surgery within the previous 3 months, unstable angina pectoris within the previous 6 months, congestive heart failure NYHA class III or IV, a demonstrated absolute need for ACE inhibitors or CCB, and a serum creatinine level > 3 mg/dl. |
|
| Interventions | Participants were randomized into 2 treatment arms consisting of 'intensive' or 'moderate' treatment. The goal in the 'intensive' treatment group was to achieve a decrease of 10 mmHg below baseline in diastolic blood pressure (i.e. 70 ‐ 79 mmHg), whereas the goal in the 'moderate' treatment group was to maintain a diastolic blood pressure between 80 and 89 mmHg. Participants in the 'moderate' therapy group were given placebo, whereas those randomized to 'intensive' therapy received either nisoldipine or enalapril in a blinded manner as the initial antihypertensive medication. If the target blood pressure was not achieved with increasing doses, then open‐label antihypertensive medications were added in a step‐wise fashion, initially with metoprolol, then hydrochlorothiazide or additional drugs, but not a calcium channel blocker nor ACE inhibitor. Blood pressure recordings were obtained at the time when peak drug levels were expected and were an average of 3 seated readings obtained at each visit. |
|
| Outcomes | The primary end point was the change in 24‐hour creatinine clearance. Secondary end points included cardiovascular events, retinopathy, clinical neuropathy, and urinary albumin excretion. | |
| Notes | Participants randomized to intensive therapy received either nisoldipine or enalapril in a blinded manner as the initial antihypertensive medication. Participants in the moderate group were given placebo. However, by the end of the study 117 participants (48%) initially randomized to moderate therapy required treatment (systolic blood pressure > 159 and/or diastolic blood pressure > 89 mmHg on 2 consecutive visits). These individuals were started on either nisoldipine or enalapril according to randomization at entry into the study with the goal of maintaining the systolic blood pressure < 160 mmHg and diastolic blood pressure < 90 mmHg. A test for interaction between study drug assignment and blood‐pressure control strategy showed that no interaction was present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants assigned to 'moderate' treatment had a greater prevalence of established vascular disease, which became significant when combined with ABCD‐N. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participant and investigator not possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on losses to follow‐up was not reported |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes reported |
| Other bias | Unclear risk | Funding not reported |
ACCORD BP 2010.
| Methods | Randomized multicenter trial performed in the United States and Canada. An independent end point committee, which was blinded to the study intervention arms, reviewed all cardiovascular events. The mean follow‐up was 4.7 years |
|
| Participants | 4733 participants were included in the ACCORD BP trial. Participants were eligible if they had type 2 diabetes mellitus and a glycated hemoglobin level of 7.5% or more, and were 40 years of age or older with cardiovascular disease or 55 years of age or older with anatomical evidence of a substantial amount of atherosclerosis, albuminuria, left ventricular hypertrophy, or at least 2 additional risk factors for cardiovascular disease (dyslipidemia, hypertension, smoking, or obesity). Participants with a systolic blood pressure between 130 and 180 mmHg who were taking 3 or fewer antihypertensive medications and who had the equivalent of a 24‐hour protein excretion rate of less than 1.0 g were also eligible for the blood pressure trial. Exclusion criteria included a body mass index of more than 45, a serum creatinine level of more than 1.5 mg per deciliter, and other serious illness. |
|
| Interventions | Intensive therapy was defined by a target systolic blood pressure < 120 mmHg, whereas standard therapy targeted a systolic blood pressure< 140 mmHg. There was no specific drug regimen to achieve the target blood pressure. However, all the antihypertensive regimens were to include drug classes that had been shown to result in a reduction in cardiovascular events among participants with diabetes. |
|
| Outcomes | The primary outcome was the first occurrence of a major cardiovascular event, which was defined as the composite of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. Prespecified secondary outcomes included the combination of the primary outcome plus revascularization or hospitalization for congestive heart failure, the combination of a fatal coronary event, nonfatal myocardial infarction, or unstable angina; nonfatal myocardial infarction; fatal or nonfatal stroke, nonfatal stroke, death from any cause, death from cardiovascular causes, and hospitalization or death due to heart failure. | |
| Notes | The entire ACCORD trial enrolled 10,251 participants with type 2 diabetes mellitus considered to be at high risk. All participants were randomly assigned to either intensive or standard glycemic control. In addition, 4733 participants were also randomly assigned (in a 2‐by‐2 factorial design) to either intensive or standard blood pressure control (the ACCORD blood pressure trial). The trial was conducted between January 2001 and October 2005 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participant and investigator not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | The trial was sponsored by the National Heart, Lung, and Blood Institute from the United States. No other funding reported. |
HOT 1998.
| Methods | Randomized, open‐label, 3‐by‐2 factorial design controlled trial, with blinded endpoint evaluation (PROBE) design. An Independent Clinical Event Committee, masked to the group allocation, evaluated all clinical events. The trial was conducted in 26 countries from Europe, Asia, North and South America. The average follow‐up was 3.8 years. | |
| Participants | The entire study population was composed of 18,790 patients with elevated blood pressure, aged 50 ‐ 80 years. Of these, 1501 participants had diabetes at baseline and constitute the population included in this analysis. Baseline diastolic blood pressure between 100 mmHg and 115 mmHg on 2 occasions, at least 1 week apart, was an inclusion criterion. The main exclusion criteria were malignant hypertension, secondary hypertension, diastolic blood pressure > 115 mmHg, stroke or myocardial infarction within 12 months prior to randomization, decompensated congestive heart failure, other serious concomitant diseases which could affect survival during the next 2 ‐ 3 years, participants who required a beta‐blocker, ACE inhibitor or diuretic for reasons other than hypertension, participants who required antiplatelet or anticoagulant therapy, and insulin‐treated diabetics. |
|
| Interventions | Participants were randomly assigned to one of 3 diastolic blood pressure target groups: ≤ to 90 mmHg, ≤ 85 mmHg, or ≤ 80 mmHg and to low dose acetylsalicylic acid 75 mg or placebo. Blood pressure was measured 3 times, by an oscillometric semiautomatic device, with the participant in the sitting position after 5 minutes of rest. The time of day when blood pressure was measured was not specified. Block randomization was performed taking into consideration the following baseline variables: age, sex, previous antihypertensive therapy, smoking, previous myocardial infarction, previous coronary heart disease, previous stroke and diabetes mellitus. All participants were treated with the same drugs in the same order. The following were the required steps allowed to attempt to achieve the target blood pressure: Step 1‐ felodipine 5 mg once a day; Step 2‐ a starting dose of an angiotensin converting enzyme (ACE) inhibitor or beta‐blocker was added; Step 3‐ the dose of felodipine was increased to 10 mg once a day; Step 4‐ the dose of the ACE inhibitor or the beta‐blocker was doubled; Step 5‐ a diuretic was added. |
|
| Outcomes | The outcomes measured were: total and cardiovascular mortality, all (fatal and non‐fatal) myocardial infarctions including silent infarctions, all (fatal and non‐fatal) strokes, and major cardiovascular events (all myocardial infarctions plus all strokes plus other cardiovascular deaths). | |
| Notes | In the entire trial 24% of all investigators' reported events were rejected by the Clinical Event Committee. The corresponding number for the subgroup of participants with diabetes was not specified. The trial was conducted between October 1992 and August 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subgroup analysis |
| Allocation concealment (selection bias) | Unclear risk | Subgroup analysis |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of participant and investigator not possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on losses to follow‐up was not reported |
| Selective reporting (reporting bias) | High risk | Data on participants with diabetes represent a subgroup analysis of the entire HOT trial. The baseline characteristics in the subgroup of participants with diabetes are unknown, and therefore an unbalance at baseline cannot be ruled out. |
| Other bias | High risk | Industry funded |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| HDS 1996 | This study was excluded from the meta‐analysis because the target for systolic blood pressure in the 'tight control' group was higher than that stated in our protocol. In addition and more importantly the targets for both systolic and diastolic blood pressure in the 'less tight control group' were much higher than specified in the protocol for this systematic review. |
| Lewis 1999 | It was excluded because it did not provide data on any of the outcomes defined for this systematic review. |
| SANDS 2008 | This trial was not included because the dual intervention would not allow to address the events specifically associated with a lower blood pressure target. Besides, both systolic blood pressure targets in this trial were within the values considered as 'lower targets' in our systematic review. |
| Steno‐2 2003 | This trial was not included because the multifactorial intervention prevented any inference as to whether any difference in clinical outcomes could be attributed to a lower blood pressure target or to any of the other combined interventions. |
| UKPDS 38 1998 | This study was excluded from the meta‐analysis because the target for systolic blood pressure in the 'tight control' group was higher than that stated in our protocol. In addition and more importantly the targets for both systolic and diastolic blood pressure in the 'less tight control group' were much higher than specified in the protocol for this systematic review. |
Differences between protocol and review
No differences.
Contributions of authors
Jose Agustin Arguedas developed the basis for the protocol. He was primarily responsible for searching, identifying and assessing studies, data extraction and analyses, and for writing the review. Viriam Leiva independently verified the trials for inclusion and the data entry. James Wright helped formulate the idea for the protocol and assisted with methodologic issues, interpretation of the findings and in writing the review.
Sources of support
Internal sources
-
Universidad de Costa Rica, Costa Rica.
salary support and IT infrastructure
-
University of British Columbia, Canada.
salary support and IT infrastructure
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
ABCD‐2V 2006 {published data only}
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. American Journal of Hypertension 2006;19(12):1241‐8. [DOI] [PubMed] [Google Scholar]
ABCD‐H 1998 {published data only}
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff Sl, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin‐dependent diabetes and hypertension. New England Journal of Medicine 1998;338(10):645‐52. [DOI] [PubMed] [Google Scholar]
ABCD‐N 2002 {published data only}
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney International 2002;61(3):1086‐97. [DOI] [PubMed] [Google Scholar]
ACCORD BP 2010 {published data only}
- The ACCORD Study Group. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. New England Journal of Medicine 2010;362(17):1575‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
HOT 1998 {published data only}
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt B, Julius S, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. HOT Stdy Group. Lancet 1998;351(9118):1755‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
HDS 1996 {published data only}
- Anon. Hypertension in Diabetes Study IV. Therapeutic requirements to maintain tight blood presure control [Erratum]. Diabetologia 1997;40(3):366. [DOI] [PubMed] [Google Scholar]
- Hypertension in Diabetes Study Group. Hypertension in Diabetes Study IV. Therapeutic requirements to maintain tight blood presure control. Diabetologia 1996;39(12):1554‐61. [DOI] [PubMed] [Google Scholar]
Lewis 1999 {published data only}
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect on intensive blood pressure control on the course of type 1 diabetic nephropathy. American Journal of Kidney Diseases 1999;34(5):809‐17. [DOI] [PubMed] [Google Scholar]
SANDS 2008 {published data only}
- Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA 2008;299(14):1678‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steno‐2 2003 {published data only}
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 2003;348(5):383‐93. [DOI] [PubMed] [Google Scholar]
UKPDS 38 1998 {published data only}
- Anon. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38 [Erratum]. BMJ 1999;318(7175):29. [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317(7160):703‐13. [PMC free article] [PubMed] [Google Scholar]
Additional references
ABCD 2007
- Schrier RW, Estacio RO, Mehler PS, Hiatt WR. Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: a summary of the ABCD trial. Nature Clinical Practice: Nephrology 2007;3(8):428‐38. [DOI] [PubMed] [Google Scholar]
ABCD‐H 2000
- Estacio RO, Gifford N, Jeffers BW, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23(Suppl 2):B54‐64. [PubMed] [Google Scholar]
ADA 2012
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2012;35(Suppl 1):S11‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 2013
- American Diabetes Association. Standards of medical care in diabetes 2013. Diabetes Care 2013;36(Suppl 1):S11‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adler 2000
- Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews CR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321(7258):412‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
AHA 2007
- Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL Jr, et al. Treatment of hypertension in the prevention and management of ischemic heart disease. A scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007;115(21):2761‐88. [DOI] [PubMed] [Google Scholar]
Arguedas 2009
- Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD004349.pub2] [DOI] [PubMed] [Google Scholar]
Arguedas 2010
- Arguedas JA. Blood pressure targets: are clinical guidelines wrong?. Current Opinion in Cardiology 2010;25(4):350‐4. [DOI] [PubMed] [Google Scholar]
Bangalore 2010
- Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, et al. J‐curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. European Heart Journal 2010;31(23):2897‐908. [DOI] [PubMed] [Google Scholar]
Bangalore 2011
- Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose. Observations from traditional and Bayesian random‐effects meta‐analyses of randomized trials. Circulation 2011;123(24):2799‐810. [DOI] [PubMed] [Google Scholar]
BHS 2004
- Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS‐IV): summary. BMJ 2004;328(7440):634‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 2005
- Boissel JP, Gueyffier F, Boutitie F, Pocock S, Fagard R. Apparent effect on blood pressure is only partly responsible for the risk reduction due to antihypertensive treatments. Fundamental & Clinical Pharmacology 2005;19(5):579‐84. [DOI] [PubMed] [Google Scholar]
BPLTTC 2003
- Turnbull F, Blood Pressure Lowering Treatment Trialists´ Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet 2003;362(9395):1527‐35. [DOI] [PubMed] [Google Scholar]
CHEP 2011
- Rabi DM, Daskalopoulou SS, Padwal RS, Khan NA, Grover SA, Hackam DG, et al. The 2011 Canadian Hypertension Education Program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Canadian Journal of Cardiology 2011;27(4):415‐33. [DOI] [PubMed] [Google Scholar]
Cooper‐DeHoff 2010
- Cooper‐DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304(1):61‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deedwania 2011
- Deedwania PC. Blood pressure control in diabetes mellitus. Is lower always better, and how low it should go?. Circulation 2011;123(24):2776‐8. [DOI] [PubMed] [Google Scholar]
Dorresteijn 2012
- Dorresteijn JA, Graaf Y, Spiering W, Grobbee DE, Bots ML, Visseren FL, Secondary Manifestations of Arterial Disease Study Group. Relation between blood pressure and vascular events and mortality in patients with manifest vascular disease. J‐curve revisited. Hypertension 2012;59(1):14‐21. [DOI] [PubMed] [Google Scholar]
ESH‐ESC 2007
- Mancia G, Backer G, Domiiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Journal of Hypertension 2007;25(6):1105‐87. [DOI] [PubMed] [Google Scholar]
ESH/ESC2013
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2013;34(28):2159‐219. [DOI] [PubMed] [Google Scholar]
Farnett 1991
- Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J‐curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous?. JAMA 1991;265(4):489‐95. [PubMed] [Google Scholar]
Filippone 2011
- Filippone EJ, Foy A, Newman E. Goal‐directed antihypertensive therapy: lower may not always be better. Cleveland Clinic Journal of Medicine 2011;78(2):123‐33. [DOI] [PubMed] [Google Scholar]
Grossman 2011
- Grossman E. Blood pressure: the lower, the better. The con side. Diabetes Care 2011;34(Suppl 2):308‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
JNC 7 2003
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. The JNC 7 report. JAMA 2003;289(19):2560‐72. [DOI] [PubMed] [Google Scholar]
Kannel 1996
- Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA 1996;275(20):1571‐6. [PubMed] [Google Scholar]
LA 2009
- Sánchez RA, Ayala M, Baglivo H, et al. Latin American Expert Group. Latin American Guidelines on Hypertension. J Hypertens 2009;27:905‐22. [DOI] [PubMed] [Google Scholar]
Laurent 2004
- Laurent S. Guidelines from the British Hypertension Society. The lower the pressure the better. BMJ 2004;328(7440):593‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacMahon 1990
- MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke and CHD. Part 1. Prolonged differences in blood pressure: prospective observational studies collected for the regression dilution blas. Lancet 1990;335(8692):765‐74. [DOI] [PubMed] [Google Scholar]
Mancia 2011
- Mancia G, Grassi G, Zanchetti A. Antihypertensive treatment and blood pressure in diabetic and nondiabetic patients. The lower, the better?. Diabetes Care 2011;34(Suppl 2):304‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McBrien 2012
- McBrien K, Rabi DM, Campbell N, Barnieh L, Clement F, Hemmelgarn BR, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus. Systematic review and meta‐analysis. Archives of Internal Medicine 2012;172(17):1296‐303. [DOI] [PubMed] [Google Scholar]
Messerli 2006
- Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous?. Annals of Internal Medicine 2006;144(12):884‐93. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Hypertension. Clinical management of primary hypertension in adults. NICE clinical guideline 127 2011:1‐36.
Oparil 2003
- Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Annals of Internal Medicine 2003;139(9):761‐76. [DOI] [PubMed] [Google Scholar]
Parati 2011
- Parati G, Bilo G, Ochoa JE. Benefits of tight blood pressure control in diabetic patients with hypertension. Importance of early and sustained implementation of effective treatment strategies. Diabetes Care 2011;34(Suppl 2):S297‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prospective Studies 2002
- Lewington S, Clarke R, Qisilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903‐13. [DOI] [PubMed] [Google Scholar]
Reboldi 2011
- Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P, et al . Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta‐analysis in 73913 patients. Journal of Hypertension 2011;29(7):1253‐69. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sleight 2009
- Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial study. Journal of Hypertension 2009;27(7):1360‐9. [DOI] [PubMed] [Google Scholar]
Stamler 1993
- Stamler J, Stamler R, Neaton JD. Blood Pressure, systolic and diastolic and cardiovascular risk. US population data. Archives of Internal Medicine 1993;153(5):598‐615. [DOI] [PubMed] [Google Scholar]
Stamler 1993b
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434‐44. [DOI] [PubMed] [Google Scholar]
Vokó 1999
- Vokó Z, Bots ML, Hofman A, Koudstaal PJ, Witteman JC, Breteler MM. J‐shaped relation between blood pressure and stroke in treated hypertensives. Hypertension 1999;34(6):1181‐7. [DOI] [PubMed] [Google Scholar]
WHO/ISH 2003
- Whitworth JA, World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO/International Society of Hypertension (ISH) statement on management of hypertension. Journal of Hypertension 2003;21(11):1983‐92. [DOI] [PubMed] [Google Scholar]
Zanchetti 2003
- Zanchetti A, Hansson L, Clement D, Elmfeldt D, Julius S, Rosenthal T, et al. HOT Study Group. Benefits and risks of more intensive blood pressure lowering in hypertensive patients of the HOT study with different risk‐profiles: does a J‐shaped curve exist in smokers?. Journal of Hypertension 2003;21(4):797‐804. [DOI] [PubMed] [Google Scholar]
Zanchetti 2009
- Zanchetti A. Bottom blood pressure or bottom cardiovascular risk? How far can cardiovascular risk be reduced?. Journal of Hypertension 2009;27(8):1509‐20. [DOI] [PubMed] [Google Scholar]


