Abstract
Background
Pregnancy complications such as pre‐eclampsia and eclampsia, intrauterine growth restriction and placental abruption are thought to have a common origin related to abnormalities in the development and function of the placenta.
Objectives
To compare, using the best available evidence, the benefits and harms of antenatal antithrombotic therapy to improve maternal or infant health outcomes in women considered at risk of placental dysfunction, when compared with other treatments, placebo or no treatment.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (17 July 2012).
Selection criteria
Randomised controlled trials comparing antenatal antithrombotic therapy (either alone or in combination with other agents) with placebo or no treatment, or any other treatment in the antenatal period to improve maternal or infant health outcomes in women considered at risk of placental dysfunction.
Data collection and analysis
Two review authors evaluated trials under consideration for appropriateness for inclusion and methodological quality without consideration of their results according to the prestated eligibility criteria. We used a fixed‐effect meta‐analysis for combining study data if the trials were judged to be sufficiently similar. We investigated heterogeneity by calculating I² statistic, and if this indicated a high level of heterogeneity among the trials included, we used a random‐effects model.
Main results
Our search strategy identified 18 reports of 14 studies for consideration. The original review included five studies (484 women) which met the inclusion criteria, with a further five studies included in the updated review, involving an additional 655 women. The overall quality of the included trials was considered fair to good.
Nine studies compared heparin (alone or in combination with dipyridamole or low‐dose aspirin) with no treatment; and one compared trapidil (triazolopyrimidine).
While this review identified the use of heparin to be associated with a statistically significant reduction in risk of perinatal mortality (six studies; 653 women; risk ratio (RR) 0.40; 95% confidence intervals (CI) 0.20 to 0.78), preterm birth before 34 (three studies; 494 women; RR 0.46; 95% CI 0.29 to 0.73) and 37 (five studies; 621 women; RR 0.72; 95% CI 0.58 to 0.90) weeks' gestation, and infant birthweight below the 10th centile for gestational age (seven studies; 710 infants; RR 0.41; 95% CI 0.27 to 0.61), there is a lack of reliable information available related to clinically relevant, serious adverse infant health outcomes, which have not been reported to date.
Authors' conclusions
While treatment with heparin for women considered to be at particularly high risk of adverse pregnancy complications secondary to placental insufficiency was associated with a statistically significant reduction in risk of perinatal mortality, preterm birth before 34 and 37 weeks' gestation, and infant birthweight below the 10th centile for gestational age when compared with no treatment for women considered at increased risk of placental dysfunction, to date, important information about serious adverse infant and long‐term childhood outcomes is unavailable.
Keywords: Female; Humans; Infant; Infant, Newborn; Pregnancy; Aspirin; Aspirin/therapeutic use; Dipyridamole; Dipyridamole/therapeutic use; Eclampsia; Eclampsia/prevention & control; Fibrinolytic Agents; Fibrinolytic Agents/therapeutic use; Heparin; Heparin/therapeutic use; Infant, Low Birth Weight; Placenta Diseases; Placenta Diseases/prevention & control; Pre‐Eclampsia; Pre‐Eclampsia/drug therapy; Pre‐Eclampsia/prevention & control; Randomized Controlled Trials as Topic; Thrombosis; Thrombosis/prevention & control; Trapidil; Trapidil/therapeutic use; Treatment Outcome
Plain language summary
Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction
Pregnancy complications such as pre‐eclampsia and eclampsia, intrauterine fetal growth restriction and placental abruption are thought to be related to abnormalities in the development and function of the placenta. Treatment with heparin to prevent the development of blood clots within the placenta appears to be a promising intervention to prevent these complications. The numbers of pregnant women with pre‐eclampsia, preterm birth, perinatal death and a low birthweight infant (weighing less than the 10th centile for gestational age) were reduced with this treatment. Ten randomised trials involving 1139 women met the inclusion criteria for the review. Nine studies compared heparin (alone or in combination with dipyridamole) with no treatment; and one compared triazolopyrimidine with placebo. The most commonly recognised side effect for women related to this treatment was mild skin bruising. To date, important information about serious adverse infant and long‐term childhood outcomes with using anti‐clotting medications is unavailable. Further research is required.
Background
Description of the condition
Pregnancy complications such as pre‐eclampsia and eclampsia, restricted growth of the baby in the uterus (intrauterine fetal growth restriction (IUGR)) and placental abruption are thought to be related to problems with the development and function of the placenta. Pre‐eclampsia and its complications remain a common cause of maternal and infant morbidity and mortality throughout the world (Sibai 2005). Infants born to women with pre‐eclampsia have an increased risk of IUGR, preterm birth and perinatal mortality (Laws 2004). Infants who are small‐for‐gestational age (SGA) at birth are recognised to be at increased risk of adverse health outcomes (Bernstein 2000; McIntire 1999), including mortality (Cnattingius 1998; Kok 1998), birth hypoxia (Cnattingius 1998), and developmental problems during infancy and childhood (Kok 1998; Roth 1999). The risk of infant mortality is further increased in both term and preterm infants in the presence of growth restriction (Tan 2005). Fetal growth restriction is estimated to complicate 5% to 10% of perinatal deaths, with over 75% of stillborn infants being of low birthweight (Laws 2004). For women and their families, the loss of a pregnancy through intrauterine fetal death is an emotionally devastating event.
Central to the development and continuation of a normal healthy pregnancy is the optimal development of the placenta. The placenta is required to form an effective exchange system for the transfer of oxygen, nutrients and waste products between the mother and the fetus (Khong 2004; Robson 2002). Crucial to this development is the transformation of the usually high resistance spiral arteries into large calibre, low resistance uteroplacental vessels by a process of extra‐villous trophoblast infiltration (Burton 2009; Khong 2004; Robson 2002). When this process does not occur, the placenta that develops is small (Egbor 2006; Khong 2004; Sander 2005; Toal 2008), and there is a poor maternal cardiovascular response to pregnancy, resulting in haemoconcentration, a lack of second trimester blood pressure reduction (Dekker 2005a; Sibai 2005). These changes in the placenta precede the development of severe early‐onset pre‐eclampsia (Dekker 2001; Dekker 2005a; Dekker 2005b; Sibai 2005). Pathological examination of the placenta after birth, which has occurred secondary to pre‐eclampsia or IUGR occurring in early pregnancy, may identify the presence of ischaemic thrombotic lesions, including infarction (or damage to the placental tissue due to clots forming in the placental blood vessels on the maternal side) (Ferrazzi 1999; Franco 2011; Viero 2004; Walker 2012).
Description of the intervention
An intervention that may be able to prevent the development of vascular pathology (specifically to prevent the development of blood clots within the placenta and the subsequent death or infarction of placental tissue) may be effective in reducing the risk or preventing the development of clinical complications such as fetal death, pre‐eclampsia or IUGR. Therapies that may be effective include the use of antithrombotic medications, which act by preventing the formation of blood clots, in addition to some immune mediating functions. These medications include unfractionated heparin (UFH), and low molecular weight heparin (LMWH), and are administered by injection below the skin of the abdomen (called subcutaneous injection).
How the intervention might work
There have been a number of reports in the literature related to the use of antithrombotic medications in women with a history of placental infarction in a previous pregnancy, as a means of preventing placental dysfunction in a subsequent pregnancy, and improving infant outcomes (Alkazaleh 2004; Bonnar 1975; Buyse 1974; Chupin 1978; Fuke 1994Moe 1982). These studies are in the form of case series and cohort studies, which have inherent bias, involve a small number of pregnant women only, and variably report important clinical outcomes. Furthermore, two reports (Bonnar 1975; Buyse 1974) involved the use of warfarin from the second trimester of pregnancy before converting to heparin therapy from 36 weeks' gestation. Warfarin is an oral anticoagulant medication that is currently not recommended for use in pregnancy as it is associated with the development of fetal anomalies when used in the first trimester of pregnancy. The results of these case series and cohort studies should be interpreted with caution.
The use of antithrombotic medications, while potentially beneficial in reducing the occurrence of adverse pregnancy outcome, are not without potential harm. The most commonly recognised minor side effects for the woman related to antithrombotic therapy include local skin reactions and skin bruising (Greer 2006). More serious complications for the woman are uncommon with the relatively short‐term use of medication as seen during pregnancy (Greer 2006). Other potential complications include bone loss (called osteopaenia), and subsequent osteoporotic bone fractures. These complications occur in less than 1% of patients exposed to long‐term heparin therapy (rather than the short duration associated with use during pregnancy), and predominantly occur with the use of UFH (rather than LMWH) (Greer 2006). Heparin‐induced thrombocytopenia (or a fall in platelet count; platelets are components of the blood which assist in clotting) is estimated to occur in less than 1% of women exposed to long‐term heparin therapy, is reversible with cessation of medication, and again, is more common when UFH is used (Greer 2006). Placental bleeding or abruption has been reported to occur in 0.04% of women using long‐term heparin therapy during pregnancy (Greer 2006). Furthermore, the use of heparin may be associated with an increased risk of bleeding at the time of epidural or spinal anaesthesia, as well as increasing risks of post‐operative bleeding (Greer 2006). Antithrombotic medications, such as UFH and LMWH, do not cross the placenta, and are therefore, safe for the fetus when used during pregnancy (Greer 2006).
Why it is important to do this review
While there may be benefits in the use of antenatal antithrombotic therapy for women considered to be at risk of complications related to placental dysfunction, in terms of improved maternal and infant health outcomes, there may also be harms related to the potential side effects of medication. The aim of this review is to use the best available evidence to assess the benefits and harms of antenatal antithrombotic therapy for women where the intention is to improve maternal or infant health outcomes associated with placental dysfunction when compared with other treatments, placebo or no treatment.
This review will not consider the use of heparin in pregnant women with acquired or inherited thrombophilias (predisposition to thrombosis) (Walker 2003), its use in recurrent miscarriage or recurrent pregnancy loss in women with (Empson 2005) or without (Kaandorp 2009) antiphospholipid syndrome, or as prophylaxis for venous thromboembolic disease in pregnancy and the postpartum period (Gates 2002) as the use of antithrombotic agents for these indications are covered in other Cochrane reviews.
Objectives
To compare, using the best available evidence, the benefits and harms of antenatal antithrombotic therapy to improve maternal or infant health outcomes in women considered at risk of placental dysfunction, when compared with other treatments, placebo, or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion all published, unpublished, and ongoing randomised controlled trials (RCTs) comparing antenatal antithrombotic therapy (either alone or in combination with other agents) with placebo or no treatment, or any other treatment in the antenatal period to improve maternal or infant health outcomes in women considered at risk of placental dysfunction.
We excluded trials assessing the role of heparin in pregnant women with acquired or inherited thrombophilias, its use in recurrent miscarriage or recurrent pregnancy loss in women with or without antiphospholipid syndrome, or as prophylaxis for venous thromboembolic disease in pregnancy and the postpartum period. We excluded quasi‐randomised trials (e.g. those randomised by date of birth or hospital number). We also excluded studies where low‐dose aspirin was used alone. We included studies reported only in abstract form in the Studies awaiting classification category; if relevant we will include these in the analyses when published as full reports.
Types of participants
Women undergoing antenatal treatment with antithrombotic therapy where the intention is to improve maternal or infant health outcomes in women considered at particularly high risk of complications related to placental dysfunction.
Types of interventions
Antenatal antithrombotic therapy (alone or in combination with other agents) versus placebo or no treatment, or any other treatment in the antenatal period to improve maternal or infant health outcomes in women considered at risk of placental dysfunction. We considered for inclusion studies reporting comparisons between different antithrombotic agents or different doses of antithrombotic agents.
Types of outcome measures
Primary outcomes
Perinatal mortality
Preterm birth (less than 34 weeks' gestation)
Major neurodevelopmental handicap at childhood follow‐up
Secondary outcomes
Maternal
Pre‐eclampsia or eclampsia
Placental abruption
Antepartum haemorrhage (beyond 20 weeks and requiring hospital admission)
Length of antenatal stay
Use of antenatal corticosteroids for fetal lung maturation
Antibiotic use after birth
Postpartum haemorrhage (defined as blood loss greater than 500 mL at vaginal birth or greater than 1000 mL at caesarean birth)
Need for blood transfusion
Anaesthetic complications (as defined by trial authors)
Adverse drug reaction (including bruising, local skin reaction, minor haemorrhage, heparin‐induced thrombocytopaenia, osteopaenia, bone fractures)
Maternal death
Infant
Birth before 37, 32, and 28 completed weeks
Birthweight less than the 10th centile for gestational age
Birthweight less than 2500 g
Apgar score of less than seven at five minutes
Respiratory distress syndrome
Use of mechanical ventilation
Duration of mechanical ventilation
Intraventricular haemorrhage ‐ Grades III or IV
Periventricular leucomalacia
Retinopathy of prematurity
Retinopathy of prematurity ‐ Grades III or IV
Chronic lung disease
Necrotising enterocolitis
Neonatal sepsis
Fetal death
Neonatal death
Admission to neonatal intensive care unit
Neonatal length of hospital stay
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (17 July 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 1.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We assessed methods as:
low risk of bias (e.g. no or low (less than 20%) missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference for outcomes measured in the same way between trials. In future updates, if appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
No cluster‐randomised trials were included in this 2013 updated. In future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook Section 16.3.4 using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not an appropriate study design in this setting and will not be included.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. This has not been performed for this update.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We did not carry out any subgroup analysis in this 2013 update.
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
Antithrombotic agent administered (UFH versus LMWH versus other).
Gestational age treatment commenced (first trimester versus second trimester versus third trimester).
The primary outcomes will be used in subgroup analysis (perinatal mortality; preterm birth less than 34 weeks' gestation; and major neurodevelopmental delay at childhood follow‐up).
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2011). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
Sensitivity analyses will be conducted to evaluate the effect of trial quality in future updates.
Results
Description of studies
Results of the search
The search of the Pregnancy and Childbirth Group's Trials Register found 22 reports of 15 studies. The original review included five studies, and this updated review has included an additional five studies; for further details, see Characteristics of included studies. Two studies were excluded (Airoldi 1988; Eid 2006) as they were not randomised (see Characteristics of excluded studies). Four reports of two studies (Bonnar 1980; Ferrier 2000) have been reported in abstract form only, and further information is required to assess their methodological quality and relevance to this review. One further report is also awaiting classification (Yu 2004b) (see Studies awaiting classification).
Included studies
Our search strategy identified 22 reports of 15 studies for consideration. The original review included five studies (484 women) which met the inclusion criteria (Kincaid‐Smith 1995; Mello 2005; Nieder 1995; Rey 2009; Yu 2004a). The updated review has included a further five studies (Gris 2010; Gris 2011; Kingdom 2011; Martinelli 2012; Yu 2010), involving an additional 655 women.
Nine studies compared heparin (alone or in combination with dipyridamole or low‐dose aspirin) with no treatment (Gris 2010; Gris 2011; Kincaid‐Smith 1995; Kingdom 2011; Martinelli 2012; Mello 2005; Rey 2009; Yu 2004a; Yu 2010). The study by Yu and colleagues involved a comparison between UFH and LMWH also (Yu 2004a). A single study compared trapidil (triazolopyrimidine) with placebo (Nieder 1995).
Participant population
The included studies recruited women considered to be at particularly high risk of adverse outcomes from placental insufficiency, predominantly based on a past history (particularly pre‐eclampsia, eclampsia, renal disease, placental abruption, fetal growth restriction, or fetal death).
Reported outcomes
There was variable reporting of the pre‐specified outcomes.
Refer to Characteristics of included studies for further information.
Excluded studies
Two studies were excluded (Airoldi 1988; Eid 2006) as they were not randomised (see Characteristics of excluded studies).
Risk of bias in included studies
The overall quality of the included trials was considered fair to good.
Please refer to the table Characteristics of included studies for further details and Figure 1 and Figure 2.
1.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All trials were stated to be randomised, with Gris 2010, Gris 2011, Kingdom 2011, Martinelli 2012, Mello 2005 and Rey 2009 utilising computer‐generated random number tables. The precise method of randomisation was unclear or not stated in Kincaid‐Smith 1995, Nieder 1995, Yu 2004a and Yu 2010. Allocation concealment utilised a central telephone randomisation service in Kingdom 2011 and Martinelli 2012, while sealed opaque envelopes were utilised by Gris 2010, Gris 2011, Kincaid‐Smith 1995 and Rey 2009. The method of allocation concealment was unclear or not stated for Mello 2005, Nieder 1995, Yu 2004a, and Yu 2010.
Blinding
The study by Rey 2009 was the only one to ensure blinding of outcome assessors; blinding of participants and caregivers was not achieved or not stated in any of the included trials (Gris 2010; Gris 2011; Kingdom 2011; Kincaid‐Smith 1995; Martinelli 2012; Mello 2005; Nieder 1995; Rey 2009; Yu 2004a; Yu 2010).
Incomplete outcome data
The majority of the studies appeared to be at low risk of attrition bias, with low levels of missing data.
Selective reporting
The majority of the studies appeared to be at low risk of selective reporting, although without access to a pre‐specified trial protocol, this is difficult to assess.
Other potential sources of bias
The baseline characteristics of participants in the included studies appeared comparable at the time of trial entry. However, the trials by Martinelli 2012 and Rey 2009 were both halted prior to achieving their intended sample size for reasons of slow recruitment and futility.
Effects of interventions
Heparin (alone or with other medications) versus no treatment
We included nine studies involving 979 women.
Primary outcomes
For the primary outcomes, women who were administered heparin during pregnancy were at significantly lower risk of perinatal death (six studies; 653 women; risk ratio (RR) 0.40; 95% confidence intervals (CI) 0.20 to 0.78; Analysis 1.1), or of giving birth prior to 34 weeks' gestation (three studies; 494 women; RR 0.46; 95% CI 0.29 to 0.73; Analysis 1.2). There was no available information for the outcome major neurodevelopmental delay at child follow‐up (one study; 107 infants; RR not estimable).
1.1. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 1 Perinatal mortality.
1.2. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 2 Preterm birth less than 34 weeks' gestation.
Secondary outcomes
Women administered heparin during pregnancy were at significantly lower risk of developing pre‐eclampsia (seven studies; 761 women; RR 0.43; 95% CI 0.28 to 0.65). However a high degree of heterogeneity was identified, and when a random‐effects model was used, the results were no longer statistically significant (seven studies; 761 women; average RR 0.47; 95% CI 0.22 to 1.03; Analysis 1.4; random‐effects model; Heterogeneity: Tau² = 0.58; Chi² = 14.24, df = 6 (P = 0.03); I² = 58%). The use of heparin was not associated with a significant reduction in eclampsia (one study; 135 women; RR not estimable; Analysis 1.5). While the use of heparin was associated with a significant difference in mean length of antenatal hospitalisation (one study; 20 women; mean difference (days) (MD) ‐9.00; 95% CI ‐15.14 to ‐2.86; Analysis 1.8), and birth prior to 37 weeks' gestation (five studies; 621 women; RR 0.72; 95% CI 0.58 to 0.90; Analysis 1.10), there were no significant differences in the risk of placental abruption (four studies; 551 women; RR 0.38; 95% CI 0.10 to 1.40; Analysis 1.6).
1.4. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 4 Pre‐eclampsia.
1.5. Analysis.
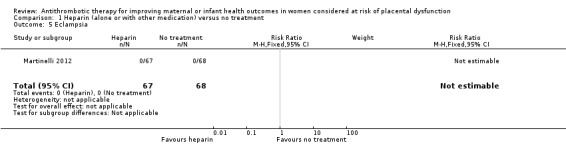
Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 5 Eclampsia.
1.8. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 8 Mean length of antenatal hospital stay.
1.10. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 10 Preterm birth less than 37 weeks' gestation.
1.6. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 6 Placental abruption.
Infants born to women following heparin administration were significantly less likely to have birthweight below the 10th centile (seven studies; 710 infants; RR 0.41; 95% CI 0.27 to 0.61; Analysis 1.12), and Apgar score of less than seven at five minutes of age (three studies; 519 infants; RR 0.42; 95% CI 0.29 to 0.60; Analysis 1.13). Infants were less likely to require admission to the neonatal intensive care unit (three studies; 416 infants; RR 0.53; 95% CI 0.35 to 0.79), although a high degree of heterogeneity was identified, and when a random‐effects model was used, the results were no longer statistically significant (three studies; 416 infants; average RR 0.62; 95% CI 0.25 to 1.53; Analysis 1.14; random‐effects model; Heterogeneity: Tau² = 0.51; Chi² = 9.85, df = 2 (P = 0.007); I² = 80%). There were no statistically significant differences identified in risk of either intrauterine fetal death (three studies; 519 women; RR 0.58; 95% CI 0.23 to 1.46; Analysis 1.15), or neonatal death (two studies; 384 infants; RR 0.29; 95% CI 0.06 to 1.36; Analysis 1.16).
1.12. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 12 Infant birthweight less than 10th centile for gestational age.
1.13. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 13 Apgar score less than 7 at 5 minutes age.
1.14. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 14 NICU admission.
1.15. Analysis.
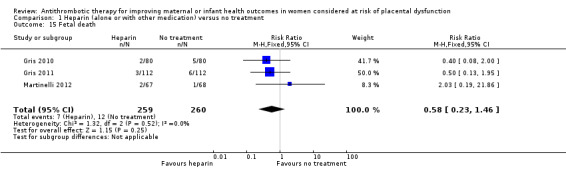
Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 15 Fetal death.
1.16. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 16 Neonatal death.
Triazolopyrimadine versus placebo
We included a single study involving 160 women at risk of developing pre‐eclampsia.
Primary outcomes
None of the pre‐specified primary outcomes were reported.
Secondary outcomes
There were no statistically significant differences identified for the outcome pre‐eclampsia (one study; 160 women; RR 0.38; 95% CI 0.12 to 1.16; Analysis 2.1).
2.1. Analysis.

Comparison 2 Triazolopyrimidine (Trapidil) versus placebo, Outcome 1 Pre‐eclampsia.
Unfractionated heparin versus low molecular weight heparin
We included a single study involving 68 women with established fetal growth restriction.
Primary outcomes
There was no available information for the outcome major neurodevelopmental delay at child follow‐up (one study; 68 infants; RR not estimable; Analysis 3.1).
3.1. Analysis.

Comparison 3 Unfractionated heparin versus low molecular weight heparin, Outcome 1 Major neurodevelopmental delay at child follow‐up.
Secondary outcomes
There was no available information for the outcomes antepartum haemorrhage or thrombocytopaenia (one study; 68 infants; RR not estimable; Analysis 3.2; Analysis 3.3). There were no statistically significant differences identified for the outcomes preterm birth before 37 weeks' gestation (one study; 68 women; RR 1.26; 95% CI 0.22 to 7.05; Analysis 3.4), or infant birthweight less than the 10th centile (one study; 68 infants; RR 0.84; 95% CI 0.13 to 5.61; Analysis 3.5).
3.2. Analysis.

Comparison 3 Unfractionated heparin versus low molecular weight heparin, Outcome 2 Antepartum haemorrhage (after 20 weeks and requiring hospitalisation).
3.3. Analysis.

Comparison 3 Unfractionated heparin versus low molecular weight heparin, Outcome 3 Thrombocytopaenia.
3.4. Analysis.

Comparison 3 Unfractionated heparin versus low molecular weight heparin, Outcome 4 Preterm birth prior to 37 weeks' gestation.
3.5. Analysis.

Comparison 3 Unfractionated heparin versus low molecular weight heparin, Outcome 5 Infant birthweight less than 10th centile for gestational age.
Discussion
Summary of main results
This review identified a statistically significant reduction in risk of perinatal mortality, preterm birth before 34 and 37 weeks' gestation, and infant birthweight below the 10th centile for gestational age, when comparing antenatal heparin administration with no treatment for women considered at to be at particularly high risk of adverse outcomes and placental dysfunction.
Overall completeness and applicability of evidence
The findings of this review are based on 10 included randomised studies and 1139 participants. However, for the majority of outcomes, the available meta‐analysis involved a smaller number of studies and participants (ranging from 20 to 710). This review identified a statistically significant reduction in risk of perinatal mortality, preterm birth before 34 and 37 weeks' gestation, and infant birthweight below the 10th centile for gestational age. The effect of treatment with antithrombotic agents on a woman's risk of pre‐eclampsia and eclampsia remain uncertain, with considerable heterogeneity across the identified trials to date, reflecting differences in the included patient populations. There is a lack of reliable information available related to clinically relevant infant health outcomes, which have not been reported to date, including childhood growth and development. Similarly, there is a lack of information available related to maternal side effects from medication, and maternal psychological wellbeing associated with prolonged daily treatment during pregnancy.
The proposed mechanism whereby heparin mediates beneficial effects on maternal and perinatal outcome is based on the assumption that heparin is a placental anticoagulant. However, to date only one trial (Kingdom 2011) has incorporated placental pathology analysis, noting no differences in the prevalence of placental infarction between women exposed to heparin during pregnancy and women who were not. Any subsequent trials evaluating the role of heparin in women with evidence of placental dysfunction are encouraged to incorporate placental pathology assessments blinded to treatment allocation.
Heparin appears to reduce the risk of developing pre‐eclampsia. In‐vitro, heparin effectively reverses the anti‐angiogenic response of placenta villi (Sobel 2011). Severe forms of pre‐eclampsia are associated with increased circulating levels of the anti‐angiogenic protein sFlt1 and reduced levels of placenta growth factor (PlGF) (Rana 2012). It is of concern therefore that heparin increases placental production of sFlt‐1, and thus circulating levels during pregnancy (Drewlo 2011). Further research is therefore required to understand the interactions between heparin and the placenta that mediate a reduction in the risk of developing severe pre‐eclampsia.
Quality of the evidence
The overall quality of the included trials was considered fair to good. All trials were stated to be randomised, with six utilising computer‐generated random number tables. Two trials utilised a central telephone randomisation service to maintain allocation concealment, while four trials used sealed opaque envelopes. Blinding of outcome assessors was achieved in one trial only, with the remainder either not stating or not achieving blinding of participants or caregivers, increasing the potential for bias. While there did not appear to be evidence of selective reporting or incomplete data, two trials were halted prior to achieving their estimated sample size due to reasons of difficulties with recruitment and futility.
Potential biases in the review process
Methodologically, six of the 10 included trials were considered to be at low risk of bias. However, blinding of outcome assessors was achieved in only one study, and participants and caregivers were aware of treatment allocation in all of the included studies.
Authors' conclusions
Implications for practice.
Treatment with heparin for women considered at particularly high risk of adverse pregnancy complications secondary to placental insufficiency was associated with a statistically significant reduction in risk of perinatal mortality, preterm birth before 34 and 37 weeks' gestation, and infant birthweight below the 10th centile for gestational age, when compared with no treatment. However, important information about infant and long‐term childhood growth and development is currently unavailable. Furthermore, the interpretation of these findings and potential clinical use of heparin should be confined to women who are considered to be at particularly high risk of adverse pregnancy outcomes, often based on past pregnancy history.
Implications for research.
Further well designed randomised trials that are sufficiently powered to detect differences in important maternal outcomes (including both pre‐eclampsia and eclampsia, as well as potential complications from antithrombotic medications), and infant and childhood growth and neurodevelopmental outcomes are required, with standardised reporting of outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 28 August 2012 | New search has been performed | Search updated July 2012. |
| 28 August 2012 | New citation required and conclusions have changed | The updated review includes an additional five randomised trials which has changed the conclusions of the review. In this 2013 update, there is no evidence of a difference in pre‐eclampsia and evidence for a reduction in perinatal mortality and preterm birth before 34 and 37 weeks. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 17 July 2012 | Amended | Search updated. Eight reports of seven trials added to Studies awaiting classification (Gris 2010a; Gris 2011a; Kingdom 2011a; Martinelli 2012a; Walker 2011a; Yu 2004b; Yu 2010a). |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Kincaid‐Smith 1995; Mello 2005; Nieder 1995; Rey 2009; Yu 2004a.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors (JD and RW) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2008) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we will re‐include missing data in the analyses which were undertaken. We considered more than 20% incomplete data to be inadequate. We assessed methods as:
adequate;
inadequate:
unclear.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2008). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any eligible cluster‐randomised trials.
Cross‐over trials
We did not consider cross‐over trials an appropriate study design for the evaluation of this intervention and we have not included them.
Dealing with missing data
For included studies, we have noted levels of attrition. We have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes we have carried out analyses, as far as possible, on an intention‐to‐treat basis; i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we had identified substantial heterogeneity (greater than 50%) in a fixed‐effect meta‐analysis, we would have noted this and repeated the analysis using a random‐effects method.
Assessment of reporting biases
Where we suspected reporting bias (see ‘Selective reporting bias’ above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we have explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect inverse variance meta‐analysis for combining data where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If we had identified clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials we would have used random‐effects meta‐analysis.
If we had identified substantial heterogeneity in a fixed‐effect meta‐analysis we would have noted this and repeated the analysis using a random‐effects method.
Subgroup analysis and investigation of heterogeneity
We had planned to carry out the following subgroup analyses had we identified substantial heterogeneity:
antithrombotic agent administered (unfractionated heparin versus low molecular weight heparin versus other);
gestational age treatment commenced (first trimester versus second trimester versus third trimester).
We would have used the primary outcomes in subgroup analysis.
For fixed‐effect meta‐analyses we conducted planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001. For random‐effects meta‐analyses we assessed differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
We did not carry out sensitivity analysis.
Data and analyses
Comparison 1. Heparin (alone or with other medication) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 6 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.20, 0.78] |
| 2 Preterm birth less than 34 weeks' gestation | 3 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.29, 0.73] |
| 3 Major neurodevelopmental delay at child follow‐up | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Pre‐eclampsia | 7 | 761 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.22, 1.03] |
| 5 Eclampsia | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Placental abruption | 4 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.10, 1.40] |
| 7 Antepartum haemorrhage (after 20 weeks requiring hospitalisation) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Mean length of antenatal hospital stay | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐15.14, ‐2.86] |
| 9 Thrombocytopaenia | 2 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Preterm birth less than 37 weeks' gestation | 5 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.58, 0.90] |
| 11 Infant birthweight less than 2500 grams | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.82] |
| 12 Infant birthweight less than 10th centile for gestational age | 7 | 710 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.27, 0.61] |
| 13 Apgar score less than 7 at 5 minutes age | 3 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.29, 0.60] |
| 14 NICU admission | 3 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.25, 1.53] |
| 15 Fetal death | 3 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.46] |
| 16 Neonatal death | 2 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.36] |
1.3. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 3 Major neurodevelopmental delay at child follow‐up.
1.7. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 7 Antepartum haemorrhage (after 20 weeks requiring hospitalisation).
1.9. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 9 Thrombocytopaenia.
1.11. Analysis.

Comparison 1 Heparin (alone or with other medication) versus no treatment, Outcome 11 Infant birthweight less than 2500 grams.
Comparison 2. Triazolopyrimidine (Trapidil) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.12, 1.16] |
Comparison 3. Unfractionated heparin versus low molecular weight heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major neurodevelopmental delay at child follow‐up | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Antepartum haemorrhage (after 20 weeks and requiring hospitalisation) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Thrombocytopaenia | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Preterm birth prior to 37 weeks' gestation | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.22, 7.05] |
| 5 Infant birthweight less than 10th centile for gestational age | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.13, 5.61] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gris 2010.
| Methods | Trial conducted in France between January 2000 and 2009. | |
| Participants | 160 women with placental abruption in a previous pregnancy. | |
| Interventions | Women were randomised to (1) subcutaneous enoxaparin or (2) no treatment. | |
| Outcomes | Perinatal mortality; pre‐eclampsia; preterm birth less than 37 and 34 weeks; NICU admission. | |
| Notes | Method of randomisation: computer‐generated. Allocation concealment: sealed opaque envelopes. Blinding of participants, caregivers: no; outcome assessors: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants, caregivers: no. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors: not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears complete. |
| Selective reporting (reporting bias) | Low risk | Appears complete. |
| Other bias | Low risk | No other potential bias identified. |
Gris 2011.
| Methods | Trial conducted in France between January 2000 and 2010. | |
| Participants | 224 women with severe pre‐eclampsia in a previous pregnancy. | |
| Interventions | Women were randomised to (1) subcutaneous enoxaparin and aspirin or (2) aspirin alone. | |
| Outcomes | Perinatal mortality; pre‐eclampsia; preterm birth less than 37 and 34 weeks; NICU admission. | |
| Notes | Method of randomisation: computer‐generated. Allocation concealment: sealed opaque envelopes. Blinding of participants, caregivers: no; outcome assessors: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants, caregivers: no. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors: not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears complete. |
| Selective reporting (reporting bias) | Low risk | Appears complete. |
| Other bias | Low risk | No other potential bias identified. |
Kincaid‐Smith 1995.
| Methods | Trial conducted in Australia. | |
| Participants | 21 women with primary glomerulonephritis or reflux nephropathy who were considered on clinical grounds to be at high risk of developing pre‐eclampsia; randomised from 14 weeks' gestation. | |
| Interventions | Women were randomised to (1) subcutaneous heparin and dipyridamole or (2) no treatment. | |
| Outcomes | Perinatal mortality; pre‐eclampsia; length of antenatal stay; preterm birth less than 37 weeks; infant birthweight < 2500 g; infant birthweight less than 10th centile for gestational age. | |
| Notes | Method of randomisation: stated "randomized by the envelope method". Allocation concealment: "envelope method". Blinding of participants, caregivers and outcome assessors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized by the envelope method". |
| Allocation concealment (selection bias) | Low risk | "Envelope method." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants, caregivers, or outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of participants, caregivers, or outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears complete. |
| Selective reporting (reporting bias) | Low risk | Appears complete. |
| Other bias | Low risk | No other potential bias identified. |
Kingdom 2011.
| Methods | Trial conducted in Toronto, Canada between March 2007 and May 2010. | |
| Participants | 32 women with a singleton pregnancy and negative thrombophilia screen, who had 2 or more of the following: abnormal first or second trimester maternal serum screening (in the absence of a chromosomal fetal anomaly); abnormal placental morphology detected on ultrasound; abnormal maternal uterine artery Doppler waveform prior to 24 weeks' gestation. | |
| Interventions | Women were randomised to (1) subcutaneous heparin 7500 IU twice daily and medical surveillance or (2) medical surveillance only. 8 of 16 women in the control group received low‐dose aspirin alone. |
|
| Outcomes | Primary outcomes related to feasibility of recruitment and maternal emotional wellbeing. | |
| Notes | Method of randomisation: computer‐generated. Allocation concealment: central telephone randomisation. Blinding of participants, caregivers and outcome assessors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants, caregivers and outcome assessors: no. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of participants, caregivers and outcome assessors: no. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported. |
| Other bias | Low risk | No. |
Martinelli 2012.
| Methods | Trial conducted in 8 centres in Italy, between April 2007 and April 2010. | |
| Participants | 135 women with a history of pre‐eclampsia, eclampsia, HELLP syndrome, previous fetal loss or fetal growth restriction, with gestational age less than 12 weeks' gestation. | |
| Interventions | Women were randomised to (1) low molecular weight heparin and medical surveillance or (2) medical surveillance only. | |
| Outcomes | Pre‐eclampsia; eclampsia, HELLP syndrome, placental abruption, fetal growth restriction, or fetal death. | |
| Notes | Method of randomisation: computer‐generated random number sequence. Allocation concealment: central telephone randomisation. Blinding of participants and caregivers: no; outcome assessors: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and caregivers: no. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors: not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 135 women randomised; 128 women analysed. |
| Selective reporting (reporting bias) | Low risk | Appears complete. |
| Other bias | High risk | Trial stopped after 135 women randomised due to futility (estimated sample size 266 women). |
Mello 2005.
| Methods | Trial conducted in Italy, between January 2001 and December 2002. | |
| Participants | 80 women with a history of pre‐eclampsia who were negative on thrombophilia testing; treatment was started as soon as pregnancy was confirmed. | |
| Interventions | Women were randomised to (1) low molecular weight heparin or (2) no treatment. | |
| Outcomes | Pre‐eclampsia; infant birthweight less than 10th centile for gestational age. | |
| Notes | Method of randomisation: computer‐generated random number sequence. Allocation concealment: not stated. Blinding of participants, caregivers, and outcome assessors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants, caregivers, or outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of participants, caregivers, or outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears complete. |
| Selective reporting (reporting bias) | Low risk | Appears complete. |
| Other bias | Low risk | No other potential bias identified. |
Nieder 1995.
| Methods | Trial conducted in Germany. | |
| Participants | 160 women at increased risk of developing pre‐eclampsia, between 22 and 38 weeks' gestation. | |
| Interventions | Women were randomised to (1) triazolopyrimidine or (2) placebo. | |
| Outcomes | Rate of pre‐eclampsia; rate of preterm birth. | |
| Notes | Method of randomisation: not stated. Allocation concealment: not stated. Blinding of participants, caregivers, and outcome assessors: not stated. Study stated to be "randomised, double blind, placebo control". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | No other potential bias identified. |
Rey 2009.
| Methods | Trial conducted in Canada between August 2000 and June 2007. | |
| Participants | 116 women with past history of severe pre‐eclampsia, fetal growth restriction (infant birthweight less than 5th centile), unexplained fetal death or abruption, in the absence of a known thrombophilia; women randomised from 17 weeks' gestation. Original sample size 276 women; trial stopped after an interim analysis due to slow recruitment. |
|
| Interventions | Women were randomised to (1) low molecular weight heparin or (2) no treatment. | |
| Outcomes | Primary composite of severe pre‐eclampsia, infant birthweight less than 5th centile, abruption requiring birth less than 34 weeks' gestation, or fetal death after 20 weeks. | |
| Notes | Method of randomisation: computer‐generated random number table. Allocation concealment: sealed opaque envelopes. Blinding of participants and caregivers: no. Blinding of outcome assessors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants or caregivers. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears complete. |
| Selective reporting (reporting bias) | Low risk | Appears complete. |
| Other bias | High risk | Trial stopped early after interim analysis due to slow recruitment. |
Yu 2004a.
| Methods | Trial conducted in China. | |
| Participants | 107 women with established fetal growth restriction. | |
| Interventions | Women were randomised to (1) standard heparin infusion, (2) low molecular weight heparin, or (3) dextran infusion. | |
| Outcomes | Major neurodevelopmental handicap at child follow‐up; antepartum haemorrhage, thrombocytopaenia, preterm birth less than 37 weeks' gestation, infant birthweight less than 10th centile. | |
| Notes | Method of randomisation: stated "women were randomised into three groups". Allocation concealment: not stated. Blinding of participants, caregivers, and outcome assessors: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation: stated "women were randomised into three groups". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | No other potential bias identified. |
Yu 2010.
| Methods | Trial conducted in China. | |
| Participants | 104 women, 73 with established fetal growth restriction, and 31 with established pre‐eclampsia. | |
| Interventions | Women were randomised to (1) standard heparin infusion, or (2) the control group (no details provided as to what this comprised). | |
| Outcomes | Ultrasound and haematological measures; no prespecified review outcomes were reported. | |
| Notes | Method of randomisation: not stated. Allocation concealment: not stated. Blinding of participants, caregivers, and outcome assessors: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | No other potential bias identified. |
HELLP: haemolysis, elevated liver enzymes, low platelet count IU: international units NICU: neonatal intensive care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Airoldi 1988 | Not a randomised controlled trial. |
| Eid 2006 | Not a randomised controlled trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Bonnar 1980.
| Methods | Study conducted in Ireland. |
| Participants | 40 women at high risk of fetal growth restriction. |
| Interventions | Treatment with heparin and dipyridamole or no treatment. |
| Outcomes | Infant growth restriction. |
| Notes | There is insufficient information presented in the abstract to evaluate study methodology; no data are presented. |
Ferrier 2000.
| Methods | Trial conducted in Switzerland. |
| Participants | 24 women with a history of pre‐eclampsia, renal disease, or hypertension. |
| Interventions | Treatment with heparin and low‐dose aspirin or low‐dose aspirin alone. |
| Outcomes | Hypertension, fetal loss, uric acid renal clearance. |
| Notes | There is insufficient information presented in the abstract to evaluate study methodology. |
Yu 2004b.
| Methods | Trial conducted in China. |
| Participants | Women with established fetal growth restriction. |
| Interventions | Women were randomised to (1) standard heparin infusion, (2) low molecular weight heparin, or (3) dextran infusion. |
| Outcomes | Doppler ultrasound parameters reported. |
| Notes | Method of randomisation: stated "women were randomised into three groups".
Allocation concealment: not stated.
Blinding of participants, caregivers, and outcome assessors: not stated. This could be an additional report of Yu 2004a. Awaiting further clarification from authors. |
Differences between protocol and review
Methods updated to current Cochrane Pregnancy and Childbirth Group standards.
Contributions of authors
J Dodd prepared this updated review. J Dodd and R Windrim assessed studies for inclusion and conducted data extraction. All review authors reviewed and commented critically on all versions of the updated review and agreed to the final version submitted.
Sources of support
Internal sources
Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
External sources
Neil Hamilton Fairley Fellowship supported by the NHMRC (ID 399224), Australia.
Declarations of interest
John Kingdom, Jodie Dodd, Anne McLeod and Rory Windrim are authors of one of the included studies (Kingdom 2011). This trial assessment and data extraction was conducted by an independent assessor, with experience in the conduct of Cochrane systematic reviews.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Gris 2010 {published data only}
- Gris JC, Chauleur C, Faillie JL, Baer G, Mares P, Fabbro‐Peray P, et al. Enoxaparin for the secondary prevention of placental vascular complications in women with abruptio placentae: the pilot randomised controlled NOH‐AP trial. Thrombosis and Haemostasis 2010; Vol. 104, issue 4:771‐9. [DOI] [PubMed]
Gris 2011 {published data only}
- Gris JC, Chauleur C, Molinari N, Mares P, Fabbro‐Peray P, Quere I, et al. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre‐eclampsia: The pilot randomised controlled NOH‐PE trial. Thrombosis and Haemostasis 2011;106(6):1053‐61. [DOI] [PubMed] [Google Scholar]
Kincaid‐Smith 1995 {published data only}
- Kincaid Smith P, North RA, Fairley KF, Kloss M, Ihle BU. Prevention of pre‐eclampsia in high risk women with renal disease: a prospective randomized trial of heparin and dipyridamole. Nephrology 1995;1:297‐300. [Google Scholar]
Kingdom 2011 {published data only}
- Kingdom J D Current. Does heparin improve pregnancy outcomes for women with evidence of placental dysfunction?. Controlled Trials (www.controlled‐trials.com) (accessed 21 June 2007).
- Kingdom JCP, Walker M, Proctor LK, Keating S, Shah PS, McLeod A, et al. Unfractionated heparin for second trimester placental insufficiency: A pilot randomized trial. Journal of Thrombosis and Haemostasis 2011;9(8):1483‐92. [DOI] [PubMed] [Google Scholar]
- Walker M, Proctor L, Dodd J, Keunen H, Keating S, McLeod A, et al. Heparin to prevent placental insufficiency (HEPRIN): A pilot randomized controlled trial. Reproductive Sciences 2011;3(Suppl 1):361A‐2A. [Google Scholar]
Martinelli 2012 {published data only}
- Martinelli I. Low molecular weight heparin in pregnant women with previous obstetrical complications. A multicenter, randomized trial. Pathophysiology of Haemostasis and Thrombosis 2010;37 Suppl 1:A3. [Google Scholar]
- Martinelli I, Ruggenenti P, Cetin I, Pardi G, Perna A, Vergani P, et al. Heparin in pregnant women with previous placenta‐mediated pregnancy complications: A prospective, randomized, multicenter, controlled clinical trial. Blood 2012;119(14):3269‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mello 2005 {published data only}
- Mello G, Parretti E, Fatini C, Riviello C, Gensini F, Marchionni M, et al. Efficacy of LMWH in lowering the recurrence rate of preeclampsia and in restoring the physiological vascular changes in ACE DD genotype women [abstract]. Hypertension in Pregnancy 2004;23(Suppl 1):162. [Google Scholar]
- Mello G, Parretti E, Fatini C, Riviello C, Gensini F, Marchionni M, et al. Low‐molecular‐weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin‐converting enzyme DD women. Hypertension 2005;45(1):86‐91. [DOI] [PubMed] [Google Scholar]
Nieder 1995 {published data only}
- Nieder J, Claus P, Augustin W. Prevention of pre‐eclampsia and fetal growth retardation by trapidil. Zentralblatt fur Gynakologie 1995;117:23‐8. [PubMed] [Google Scholar]
Rey 2009 {published data only}
- Rey E. Dalteparin in prevention of recurrence of severe obstetrical complications in women without thrombophilia. JOGC: Journal of Obstetrics and Gynaecology Canada 2007;29(6 Suppl 1):S46. [Google Scholar]
- Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, et al. Dalteparin for the prevention of recurrence of placental‐mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. Journal of Thrombosis and Haemostasis 2009;7(1):58‐64. [DOI] [PubMed] [Google Scholar]
Yu 2004a {published data only}
- Yu YH, Shen LY, Zhong M, Zhang Y, Su GD, Gao YF, et al. Effect of heparin on fetal growth restriction. Chung‐Hua Fu Chan Ko Tsa Chih 2004;39(12):793‐6. [PubMed] [Google Scholar]
Yu 2010 {published data only}
- Yu YH, Shen LY, Zou H, Wang ZJ, Gong SP. Heparin for patients with growth restricted fetus: a prospective randomized controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(9):980‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Airoldi 1988 {published data only}
- Airoldi ML, Capetta P, Tasca A, Bertulessi C, Rossi E, Polvani F. Role of early treatment with heparin and dipyridamole in the prevention of pre‐eclampsia and placental insufficiency. 6th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:233.
Eid 2006 {published data only}
- Eid M, Taye A. Tinzaparin sodium supplementation improves the obstetric outcomes in singleton gestations following ART. Human Reproduction 2006;21(Suppl):i70. [Google Scholar]
References to studies awaiting assessment
Bonnar 1980 {published data only}
- Bonnar J, Gitt BP. Prevention of fetal growth retardation by antithrombotic therapy. 2nd Congress of the International Society for the Study of Hypertension in Pregnancy; 1980 Dec 1‐4; Cairo Egypt. 1980:60.
- Gill BP, Bonnar J. Controlled study of subcutaneous heparin and dipyridamole in women at risk to fetal growth retardation. 1st Congress of the International Society of Hypertension in Pregnancy; 1978 September 27‐29; Dublin, Ireland. 1978:97.
Ferrier 2000 {published data only}
- Ferrier C, Koeferl U, Duerig P, Schneider H. Effects of LMW‐heparin and low‐dose aspirin on renal uric acid handling in high‐risk pregnancies. Hypertension in Pregnancy 2000;19(Suppl 1):O68. [Google Scholar]
- Ferrier C, Koferl U, Durig P, Schneider H. LMW‐heparin and low‐dose aspirin for prevention of preeclampsia: preliminary data of a randomized prospective study [abstract]. Hypertension in Pregnancy 2000;19(1):82. [Google Scholar]
Yu 2004b {published data only}
- Yu YH, Shen LY, Wang ZJ, Zhang Y, Su GD. [Effect of heparin on umbilical blood flow in patients with fetal growth retardation]. [Chinese]. Di Yi Junyi Daxue Xuebao 2004; Vol. 24, issue 4:423‐5. [PubMed]
Additional references
Alkazaleh 2004
- Alkazaleh F, Viero S, Simchen M, Walker M, Smith G, Laskin C, et al. Ultrasound diagnosis of severe thrombotic placental damage in the second trimester: an observational study. Ultrasound in Obstetrics and Gynecology 2004;23(5):472‐6. [DOI] [PubMed] [Google Scholar]
Bernstein 2000
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A, for the Vermont Oxford Network. Morbidity and mortality among very‐low‐birth‐weight neonates with intrauterine growth restriction. American Journal of Obstetrics and Gynecology 2000;182(1):198‐206. [DOI] [PubMed] [Google Scholar]
Bonnar 1975
- Bonnar J, Redman CW, Sheppard BL. Treatment of fetal growth retardation in utero with heparin and dipyridamole. European Journal of Obstetrics & Gynecology and Reproductive Biology 1975;5(1‐2):123‐34. [DOI] [PubMed] [Google Scholar]
Burton 2009
- Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009;30(6):473‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buyse 1974
- Buyse FG, Wormgoor BH, Bernard JT, Koudstaal J. Anticoagulant therapy of patients with repeated placental infarction. Obstetrics & Gynecology 1974;43(6):844‐8. [PubMed] [Google Scholar]
Chupin 1978
- Chupin L, Herrmann JM, Maillet R, Gillet JY, Colette C, Gibey R, et al. The effects of heparin in underdevelopment of the fetus due to maternal vascular conditions. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1978;7(4):849‐54. [PubMed] [Google Scholar]
Cnattingius 1998
- Cnattingius S, Haglund B, Kramer MS. Differences in late fetal death rates in association with determinants of small for gestational age fetuses: population based cohort study. BMJ 1998;316:1483‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. London: BMJ Books, 2001. [Google Scholar]
Dekker 2001
- Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre‐eclampsia. Lancet 2001;357(9251):209‐15. [DOI] [PubMed] [Google Scholar]
Dekker 2005a
- Dekker G. Prothrombotic mechanisms in preeclampsia. Thrombosis Research 2005;115:17‐21. [PubMed] [Google Scholar]
Dekker 2005b
- Dekker GA, Robillard PY. Preeclampsia: a couple's disease with maternal and fetal manifestations. Current Pharmaceutical Design 2005;11(6):699‐710. [DOI] [PubMed] [Google Scholar]
Drewlo 2011
- Drewlo S, Levytska K, Sobel M, Baczyk D, Lye SJ, Kingdom JC. Heparin promotes soluble VEGF receptor expression in human placental villi to impair endothelial VEGF signaling. Journal of Thrombosis and Haemostasis 2011;9(12):2486‐97. [DOI] [PubMed] [Google Scholar]
Egbor 2006
- Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early‐ and late‐onset pre‐eclampsia with and without fetal growth restriction. BJOG: an international journal of obstetrics and gynaecology 2006;113(5):580‐9. [DOI] [PubMed] [Google Scholar]
Empson 2005
- Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002859.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrazzi 1999
- Ferrazzi E, Bulfamante G, Mezzopane R, Barbera A, Ghidini A, Pardi G. Uterine Doppler velocimetry and placental hypoxic‐ischemic lesion in pregnancies with fetal intrauterine growth restriction. Placenta 1999;20(5‐6):389‐94. [DOI] [PubMed] [Google Scholar]
Franco 2011
- Franco C, Walker M, Robertson J, Fitzgerald B, Keating S, McLeod A, et al. Placental infarction and thrombophilia. Obstetrics and Gynecology 2011;117(4):929‐34. [DOI] [PubMed] [Google Scholar]
Fuke 1994
- Fuke Y, Aono T, Imai S, Suehara N, Fujita T, Nakayama M. Clinical significance and treatment of massive intervillous fibrin deposition associated with recurrent fetal growth retardation. Gynecologic and Obstetric Investigation 1994;38(1):5‐9. [DOI] [PubMed] [Google Scholar]
Gates 2002
- Gates S, Brocklehurst P, Davis LJ. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD001689] [DOI] [PubMed] [Google Scholar]
Greer 2006
- Greer IA. Anticoagulants in pregnancy. Journal of Thrombosis and Thrombolysis 2006;21(1):57‐65. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kaandorp 2009
- Kaandorp S, Nisio M, Goddijn M, Middeldorp S. Aspirin or anticoagulants for treating recurrent miscarriage in women without antiphospholipid syndrome. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004734.pub3] [DOI] [PubMed] [Google Scholar]
Khong 2004
- Khong TY. Placental vascular development and neonatal outcome. Seminars in Neonatology 2004;9(4):255‐63. [DOI] [PubMed] [Google Scholar]
Kok 1998
- Kok JH, Ouden AL, Verloove‐Vanhorick SP, Brand R. Outcome of very preterm small for gestational age infants: the first nine years of life. BJOG: an international journal of obstetrics and gynaecology 1998;105(2):162‐8. [DOI] [PubMed] [Google Scholar]
Laws 2004
- Laws PJ, Sullivan EA. Australia's mothers and babies, 2002. Sydney: Australian Institute of Health and Welfare, National Perinatal Statistics Unit, 2004. [Google Scholar]
McIntire 1999
- McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. New England Journal of Medicine 1999;340(16):1234‐8. [DOI] [PubMed] [Google Scholar]
Moe 1982
- Moe N. Anticoagulant therapy in the prevention of placental infarction and perinatal death. Obstetrics & Gynecology 1982;59(4):481‐3. [PubMed] [Google Scholar]
Rana 2012
- Rana S, Hacker MR, Modest AM, Salahuddin S, Lim KH, Verlohren S, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 2012;60(2):451‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robson 2002
- Robson SC, Simpson H, Ball E, Lyall F, Bulmer JN. Punch biopsy of the human placental bed. American Journal of Obstetrics and Gynecology 2002;187(5):1349‐55. [DOI] [PubMed] [Google Scholar]
Roth 1999
- Roth S, Chang TC, Robson S, Spencer JA, Wyatt JS, Stewart AL. The neurodevelopmental outcome of term infants with different intrauterine growth characteristics. Early Human Development 1999;55(1):39‐50. [DOI] [PubMed] [Google Scholar]
Sander 2005
- Sander CM, Gilliland D, Richardson A, Foley KM, Fredericks J. Stillbirths with placental hemorrhagic endovasculitis: a morphologic assessment with clinical implications. Archives of Pathology and Laboratory Medicine 2005;129(5):632‐8. [DOI] [PubMed] [Google Scholar]
Sibai 2005
- Sibai B, Dekker G, Kupferminc M. Pre‐eclampsia. Lancet 2005;365(9461):785‐99. [DOI] [PubMed] [Google Scholar]
Sobel 2011
- Sobel ML, Kingdom J, Drewlo S. Angiogenic response of placental villi to heparin. Obstetrics and Gynecology 2011;117(6):1375‐83. [DOI] [PubMed] [Google Scholar]
Tan 2005
- Tan TY, Yeo GS. Intrauterine growth restriction. Current Opinion in Obstetrics and Gynecology 2005;17(2):135‐42. [DOI] [PubMed] [Google Scholar]
Toal 2008
- Toal M, Keating S, Machin G, Dodd J, Adamson SL, Windrim RC, et al. Determinants of adverse perinatal outcome in high‐risk women with abnormal uterine artery Doppler images. American Journal of Obstetrics and Gynecology 2008;198(3):330.e1‐330.e7. [DOI] [PubMed] [Google Scholar]
Viero 2004
- Viero S, Chaddha V, Alkazaleh F, Simchen M, Malik A, Kelly E, et al. Prognostic value of placental ultrasound in pregnancies complicated by absent end‐diastolic flow velocity in the umbilical arteries. Placenta 2004;25(8‐9):735‐41. [DOI] [PubMed] [Google Scholar]
Walker 2003
- Walker MC, Ferguson SE, Allen VM. Heparin for pregnant women with acquired or inherited thrombophilias. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2012
- Walker MG, Fitzgerald B, Keating S, Ray JG, Windrim R, Kingdom JC. Sex‐specific basis of severe placental dysfunction leading to extreme preterm delivery. Placenta 2012;33(7):568‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dodd 2010
- Dodd JM, McLeod A, Windrim RC, Kingdom J. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD006780.pub2] [DOI] [PubMed] [Google Scholar]


