Abstract
hERG potassium channels are critical for cardiac excitability. hERG channels have a Per-Arnt-Sim (PAS) domain at their N-terminus, and here, we examined the mechanism for PAS domain regulation of channel opening and closing (gating). We used TAG codon suppression to incorporate the noncanonical amino acid 4-benzoyl-L-phenylalanine (BZF), which is capable of forming covalent cross-links after photoactivation by ultraviolet (UV) light, at three locations (G47, F48, and E50) in the PAS domain. We found that hERG-G47BZF channels had faster closing (deactivation) when irradiated in the open state (at 0 mV) but showed no measurable changes when irradiated in the closed state (at −100 mV). hERG-F48BZF channels had slower activation, faster deactivation, and a marked rightward shift in the voltage dependence of activation when irradiated in the open (at 0 mV) or closed (at −100 mV) state. hERG-E50BZF channels had no measurable changes when irradiated in the open state (at 0 mV) but had slower activation, faster deactivation, and a rightward shift in the voltage dependence of activation when irradiated in the closed state (at −100mV), indicating that hERG-E50BZF had a state-dependent difference in UV photoactivation, which we interpret to mean that PAS underwent molecular motions between the open and closed states. Moreover, we propose that UV-dependent biophysical changes in hERG-G47BZF, F48BZF, and E50BZF were the direct result of photochemical cross-linking that reduced dynamic motions in the PAS domain and broadly stabilized the closed state relative to the open state of the channel.
Significance
The opening and closing (gating) of voltage-activated human ether-á-go-go-related gene (hERG) potassium channels drives termination of the cardiac action potential. hERG contains cytosolic, N-terminal Per-Arnt-Sim (PAS) domains that tune hERG gating for its role in the heart, but the mechanism for PAS domain regulation of hERG is unclear. In this study, we used a photo-activatable noncanonical amino acid incorporated into the PAS domain to lock the channel into discrete states with ultraviolet light irradiation. We show that photoinhibition of the PAS domain was state dependent, suggesting that PAS dynamically rearranges to regulate hERG channel gating.
Introduction
The KCNH family of voltage-gated, potassium-selective channels comprises the EAG (ether-à-go-go, KCNH1), ERG (ether-à-go-go related, KCNH2), and ELK (ether-à-go-go like; KCNH8) channels. KCNH channels have six transmembrane segments (Fig. 1 A) where the S1–S3 helices (Fig. 1 A, light gray) and positively charged S4 helix (Fig. 1 A, dark gray) form the voltage sensing domain (VSD), and the S5 helix, loop and S6 helix form the pore (Fig. 1 A, black). Intracellularly, KCNH channels have an N-terminal PAS-Cap (Fig. 1 A, light green), Per-Arnt-Sim (PAS) domain, including the alpha A helix (Fig. 1 A, green), an N-linker (Fig. 1 A, black) that connects the PAS to the VSD, and a C-terminal C-linker (Fig. 1 A, blue) cyclic nucleotide-binding homology domain (CNBHD) (Fig. 1 A, purple), within which a small intrinsic ligand (IL) motif is nested (Fig. 1 A, orange hexagon) and a C-terminal region (Fig. 1 A, black line) (1,2,3). As shown by CryoEM, four individual subunits assemble to make a tetrameric channel (Fig. 1 B) (2). KCNH channels are more closely related to the HCN (hyperpolarization-activated cyclic nucleotide-regulated) and CNG (cyclic nucleotide-gated) channels than to other voltage-gated potassium channels (Fig. 1 C) (4,5). KCNH channels, unlike HCN and CNG, are not directly activated by cyclic nucleotides (6,7). Instead, in KCNH channels, the IL motif resides within a pocket of the CNBHD that is homologous to the nucleotide biding site of HCN and CNG channels (8,9,10). The KCNH family member human ERG (hERG, KCNH2) is critical for cardiac function and encodes the alpha subunits of the rapid component of the delayed-rectifier K current (IKr) that drives action potential repolarization in the heart (11,12,13). Inherited mutations in hERG cause familial type 2 long QT syndrome, a predisposition to cardiac arrhythmias and sudden death (14). The primary mechanism for an acquired form of long QT is inhibition of hERG by the off-target effects of pharmaceuticals, which is a common clinical problem (15).
Figure 1.
Incorporation of the noncanonical amino acid BZF into the PAS domain of hERG K channels. (A) Schematic of two (of four) hERG subunits showing key functional domains including the N-terminal (Per-Arnt-Sim) PAS-Cap domain (light green), PAS domain and alpha A helix (green), N-linker region (black), voltage sensor domain S1–S3 helices (gray) and S4 helix (dark gray), pore domain composed of the S5 helix, loop, and S6 helix (black), C-linker (blue), cyclic nucleotide-binding homology domain (CNBHD) (purple), intrinsic ligand (orange), and C-terminal region (black). (B) hERG tetramer modeled from structurally determined coordinates with similar color-coding of functional domains as in (A). (PDB: 5VA2). (C) Phylogenetic tree of hERG and related ion channels. (D) For amber codon suppression, a Xenopus oocyte (large circle) was injected in the nucleus with a plasmid that encodes both the transfer RNA (tRNABZF) and RNA synthetase (RSBZF), also known as the orthogonal set, which binds and then charges tRNA with BZF. The charged tRNABZF is used by the ribosome (gray schematic) to read through a genetically modified version of hERG mRNA containing a TAG stop (amber) codon and inserts BZF into the nascent peptide that is translocated to the membrane as a full-length channel (purple) with BZF incorporated (red circle). (E) BZF incorporated into a protein (top) will be part of the primary peptide chain with the amino group forming a peptide bond to the upstream endogenous amino acid (R1) and the carboxyl group bound to the endogenous downstream amino acid (R2) and harbors the functional group (benzophenone) attached to Cα. When exposed to UV light, BZF forms a double radical capable of eliminating water and forming a new carbon-carbon bond depicted here with a protein, where R3 is the moiety disrupted by new bond formation and is dependent on localized chemistry. (F) Structure of PAS (green) and CNBHD (purple) from adjacent hERG subunits (PDB: 5VA2) and the locations of residues G47, F48, and E50 in the PAS alpha A helix with side chains shown as sticks. (G) Plot of peak current measured at 40 mV from two-electrode voltage-clamp recordings from oocytes after expression of hERG (as a negative control) or hERG-G47TAG, hERG-F48TAG, and hERG-E50TAG mutants with the orthogonal set and either without BZF (+/−) (black hollow symbols) or with BZF (+/+) (black solid symbols) added to the bath solution. n ≥ 3, ∗∗p ≤ 0.01.
hERG channels have characteristic slow closing (deactivation) kinetics that are regulated by the PAS domain and PAS-Cap (16,17,18,19,20,21) by a mechanism in which the PAS domain of one subunit makes a direct interaction with the CNBHD of a neighboring subunit (22,23,24,25,26,27,28). But, how intracellular PAS domains might rearrange during channel gating is unclear. Historically, work to study electromechanical coupling in voltage-gated K channels utilized high-affinity metal bridges that require dual cysteine mutations within favorable structural proximity that when exposed to cadmium form a metal bridge, thereby locking a channel in a particular conformational state (29). This approach is limited as it requires removal of native cysteines, cytosolic placement of several permissive cysteine mutations for metal ion accessibility, and subsequent application of metal ions.
Here, we took an updated approach to investigating electromechanical coupling, which improved upon some of the previous shortcomings of the metal bridge method such as cysteine mutagenesis, probe accessibility, and post hoc metal labeling of proteins, by combining patch-clamp electrophysiology with ultraviolet (UV) light photoactivation of a genetically encoded chemical cross-linker. To carry out these experiments, we first incorporated the noncanonical amino acid (ncAA) benzoyl-L-phenylalanine (BZF) using TAG codon suppression into hERG-TAG constructs to generate hERG-BZF channels (Fig. 1 D). A pulse of UV light applied to BZF transforms it into a reversible double radical capable of forming a new carbon-carbon bond to neighboring atoms (Fig. 1 E) (30,31). In our experiments, patch-clamp electrophysiology was used to hold the channel in different conformational (e.g., open or closed) states, and simultaneous UV irradiation was used to activate and cross-link BZF, thereby capturing or locking a conformational state of the BZF-labeled channel domain. Subsequent measurement of channel biophysical properties with patch-clamp recordings of cross-linked channels was used to reveal the allosteric effects of intracellular domains on channel gating and voltage-activation relationships (32).
hERG channels with BZF incorporated into the alpha A helix of the PAS domain (Fig. 1 F) were photo-cross-linked by application of UV light in simultaneous patch-clamp recordings. We report 1) a state-independent modification of activation, deactivation, and steady-state activation of hERG-F48BZF channels, 2) open state-dependent acceleration of deactivation in hERG-G47BZF, and 3) closed state-dependent slowing of activation, speeding of deactivation, and rightward shift in steady-state activation of hERG-E50BZF channels. We show that the conformation of the PAS domain allosterically regulates hERG channel gating, and thus the action of the transmembrane domains, and furthermore, our results that show a state dependence to cross-linking that indicated that that the PAS domain moved during gating.
Materials and Methods
Molecular biology
A full-length human ERG (NCBI protein accession number: NP_000229) expression clone with inactivation mostly removed by a point mutation (S620T) was subcloned into a modified pcDNA3 vector that contained a T7 promoter and 3′ and 5′ untranslated region of a Xenopus B-globin gene, which is denoted as hERG in this manuscript. Point mutations to insert TAG codons into the hERG gene were generated with site-directed mutagenesis by BioInnovatise, Rockville, MD, and validated by Sanger sequencing. All hERG and hERG-BZF constructs in this manuscript had a C-terminal Citrine fusion tag. The orthogonal set encoding the BZF-tRNA and BZF RNA synthetase (RS) was a generous gift from Thomas P. Sakmar at The Rockefeller University. hERG channel RNA was synthesized in vitro using the mMESSAGE mMACHINE T7 transcription kit (Thermo Fisher, Waltham, MA) from the linearized cDNA.
Oocyte harvest, injection, and incubation
Oocytes were harvested from Xenopus laevis as previously described or purchased from Ecocyte Biosciences. Channel mRNA (∼50 nl) was injected into the cytosolic region of the oocyte . The plasmids that encoded the orthogonal tRNA and aminoacyl-tRNA synthetase specific to TAG codon suppression and incorporation of BZF were blind injected into the oocyte nucleus (10 nL at a ratio of 40 ng/uL RS:120 ng/uL tRNA). Oocytes were incubated at 16°C in ND96 with 50 μg/mL gentamycin and 2.5 mM sodium pyruvate for 2–4 days with gentle agitation. BZF (Apollo Scientific or BaChem) was dissolved in 1 M NaOH and added to the bath at 1 mM (pH adjusted to 7.6 with 1 N HCl).
Channel expression screening
To incorporate BZF into hERG channels for cross-linking studies, we utilized TAG codon suppression technology that uses the least common stop codon (TAG or amber stop) (Fig. 1 D) (33). In brief, a TAG codon was placed into the hERG gene at the location of interest for BZF incorporation. The TAG codon-containing cDNA was transcribed to RNA and injected into the cytosol of the oocyte. Orthogonal machinery encoding a tRNA/synthetase set was injected into the nucleus of the oocyte. The oocyte was incubated with BZF in the medium. The orthogonal tRNA synthetase recognized and attached BZF to the orthogonal tRNA, and the BZF was incorporated by the ribosome by suppression of the TAG codon in hERG channel mRNA and made a full-length hERG channel with BZF incorporated at the TAG codon and into the site of interest (Fig. 1 D).
To rule out any nonspecific protein expression with the hERG-TAG constructs, such as 1) nonspecific read through by skipping the TAG codon or 2) nonspecific charging of orthogonal or endogenous-tRNAs by the orthogonal or endogenous tRNA synthetases, we expressed all channels (hERG and hERG-TAG) in the presence of the orthogonal set and the absence (+/−) of the ncAA BZF or in the presence of the orthogonal set and in the presence of ncAA BZF (+/+).
To validate hERG-G47TAG, hERG-F48TAG, and hERG-E50TAG constructs for TAG codon suppression and BZF incorporation, oocytes injected with hERG-TAG mRNAs and the orthogonal set plasmid were incubated in the absence (+/−) or presence (+/+) or BZF (34) and were assayed using two-electrode voltage-clamp (see Fig. 1). Only TAG constructs for which there were robust currents in the presence of BZF, but not the absence of BZF (as in Fig. 1 G), were used for subsequent photoactivation experiments.
Electrophysiology and photo-cross-linking
Excised, inside-out patch-clamp recordings from oocytes expressing hERG and hERG-BZF channels were performed 3–4 days post injection (dpi) using a Nikon Ti2 microscope with a 40× water immersion objective (N.A. 1.15) on glass-bottom (Mattek) dishes. BZF was excited with wide-field excitation using a Nikon Elements controlled SOLA SM II and an ET-DAPI filter cube with excitation 350/50 nm and dichroic 400 nm (Chroma). Excised patch membranes were exposed to UV light at discrete time intervals and holding voltages (−100 mV or 0 mV). After each discrete time interval of UV exposure, biophysical recordings were performed. The bath solution contained 130 mM KCl, 0.2 mM EDTA, and 10 mM HEPES (pH 7.4). The internal solution contained 4 mM KCl, 126 mM NaCl, 0.2 mM EDTA 0.2, and 10 mM HEPES (pH 7.2). The initial pipette resistance was 0.5–0.9 MOhms for oocyte recordings. Borosilicate patch electrodes were made using a P97 micropipette puller (Sutter Instrument, Novato, CA) and polished with an MF2 microforge (Narishige). Recordings were made at 22°C–24°C. Our method for UV photoactivation of hERG was based in part on those for glutamate receptors (35).
The channel conductance-voltage relationship (G-V curve) was measured from tail currents at −100 mV. It was fitted with a sigmoid function in Igor Pro, and the voltage at 50% conductance was reported (V0.5). Channel activation was measured as apparent time to half-maximum current of the trace invoked by a −60-mV potential. The channel deactivation rate was measured from current relaxations with repolarizing voltage steps (−100 mV) and fit with an exponential function (y = Aeˆ(−t/tau-fast) + Beˆ(−t/tau-slow)) where t is time and tau is the time constant. Changes in the biophysical properties of hERG and hERG-BZF channels reported in Fig. S1 were calculated by the difference between values measured at 15 s UV irradiation and before UV irradiation (0 s) for the same membrane patch. Data was plotted in Igor Pro or GraphPad Prism.
Statistics
Statistics reported here were calculated from a Student's t-test or one-way ANOVA where appropriate. p values were denoted as follows: (p ≤ 0.05, ∗), (p ≤ 0.01, ∗∗), (p ≤ .005 ∗∗∗), and (p ≤ 0.001, ∗∗∗∗). Plots were generated in Graph Pad. Errors reported in the tables were the SE.
Results
Incorporation of BZF into the hERG channel PAS domain using amber codon suppression
To carry out these experiments, we used TAG codon suppression technology in Xenopus oocytes to incorporate BZF into hERG channels (Fig. 1 D). To achieve this, we placed TAG codons in the hERG genetic sequence at sites that, due to their position, we hypothesized were involved in gating, corresponding to the amino acids G47, F48, and E50 in a small, solvent exposed helix (the alpha A helix) within the PAS domain (Fig. 1, A–F). We next tested for expression of hERG and hERG-TAG constructs in the presence of the orthogonal machinery and BZF (+/+) or the presence of orthogonal machinery and absence of BZF (+/−) 3 3 dpi using two-electrode voltage-clamp (Fig. 1 G). In a control experiment, we observed no measurable change in currents from wild-type hERG channels under either (+/−) or (+/+) conditions (Fig. 1 G). For hERG-TAG constructs, we observed no meaningful expression in (+/−) conditions, but robust expression in (+/+) conditions, indicating incorporation of BZF and formation of hERG-G47BZF, hERG-F48BZF, and hERG-E50BZF channels (Fig. 1 G).
UV-dependent photochemical cross-linking in the closed state changes hERG-F48BZF and hERG-E50BZF channel properties
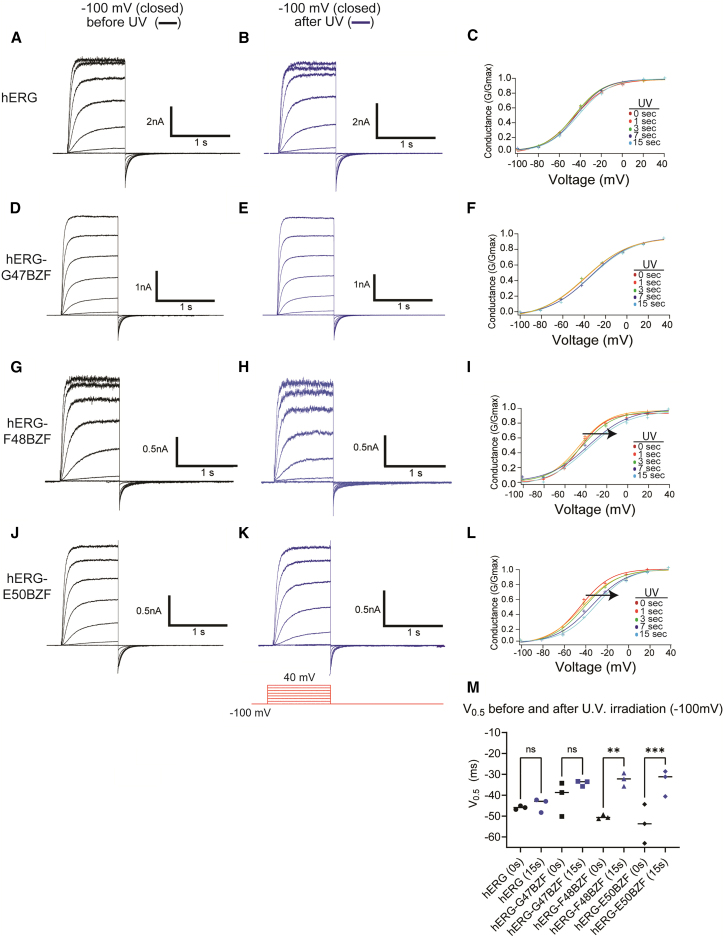
As a negative control, to examine whether hERG channels were photosensitive to UV light, we excised membrane patches from oocytes expressing hERG after co-expression with the orthogonal set and BZF and subjected patches to UV irradiation and current recordings. Excised patches were voltage-clamped at a holding voltage (−100 mV), which closed hERG channels, and a family of currents were recorded without UV irradiation (UV 0 s, black traces) (Fig. 2 A) using a standard voltage protocol (inset). Next, patches were exposed to short, discrete pulses of UV irradiation, while held in the closed state between recordings at −100 mV, for total cumulative exposure times of 1 s, 3 s, 7 s, and 15 s, and a family of channel currents was recorded after each UV pulse. The current family after final UV irradiation (UV 15 s, blue traces) is shown for comparison (Fig. 2 B). The G-V relationship was plotted for all currents recorded at the discrete UV irradiation intervals, as indicated (Fig. 2 C), and the average midpoints (V0.5) in the G-V relationship under these conditions were −46 ± 1 mV before UV irradiation and −44 ± 3 mV after 15 s of UV irradiation, which was not significantly different (Fig. 2 M), indicating that control hERG channels were not measurably affected by UV light in the closed state (Table 1; Fig. S1 A; Tables S1 and S2.)
Figure 2.
Currents and steady-state activation of hERG and hERG-BZF channels before and after UV irradiation in the closed state. Family of currents from (A and B) hERG, (D and E) hERG G47BZF, (G and H) hERG F48BZF, and (J and K) hERG E50BZF channels, recorded before (0 s) UV irradiation (black traces) or after 15 s UV irradiation (blue traces). UV light was applied in the closed state (−100 mV) in all experiments. Voltage protocol (red traces, inset) was 20-mV steps from a holding voltage of −100 mV. Scale bars are indicated for each current family. Conductance-voltage (G-V) plots for (C) hERG, (F) hERG G47BZF, (I) hERG F48BZF, and (L) hERG E50BZF, before (0 s) or after UV irradiation for durations of 0, 1, 3, 7 or 15 s. A right-shift in the GV (black arrow) was measured for hERG F48BZF and hERG E50BZF. (M) Scatter plot of the midpoint of the GV curves before (black symbols) or after (blue symbols) 15 s of UV irradiation. n = 3 for each experiment. ns denotes p > 0.05, ∗∗ denotes p ≤ 0.01, ∗∗∗ denotes p ≤ 0.001.
Table 1.
Biophysical Properties of hERG and hERG-BZF Channels Before and After UV Irradiation in the Closed State
| State: closed | ||||||||
|---|---|---|---|---|---|---|---|---|
| Channel | hERG | hERG | hERG-G47BZF | hERG-G47BZF | hERG-F48BZF | hERG-F48BZF | hERG-E50BZF | hERG-E50BZF |
| UV irradiation time (sec) | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 |
| V0.5 (mV) | −46 ± 1 | −44 ± 3 | −41 ± 8 | −34 ± 1 | −50 ± 1 | −32 ± 3 | −54 ± 9 | −33 ± 6 |
| Tau fast (ms) | 42 ± 1 | 44 ± 2 | 29 ± 5 | 27 ± 1 | 32 ± 2 | 21 ± 2 | 36 ± 4 | 21 ± 2 |
| Time to half max (sec) | 0.66 ± 0.02 | 0.65 ± 0.01 | 0.53 ± 0.03 | 0.59 ± 0.02 | 0.54 ± 0.02 | 0.68 ± 0.05 | 0.58 ± 0.02 | 0.75 ± 0.1 |
Using the same patch-clamp configuration and UV irradiation protocol as for control hERG channels, we measured a family of currents from hERG-G47BZF before (Fig. 2 D) and after 15 s of UV irradiation (Fig. 2 E). We observed no significant change in the G-V relationship after 15 s of UV irradiation, nor a change at the intermediate irradiation time points (Fig. 2 F). The average V0.5 for hERG-G47BZF was −41 ± 8 mV before UV exposure and −35 ± 1 mV after UV exposure, indicating no measurable effects of UV irradiation in the closed state for these channels (Fig. 2 M). In membrane patches expressing hERG-F48BZF channels, we recorded a family of currents before (Fig. 2 G) and after 15 s of UV irradiation (Fig. 2 H), and we recorded hERG-E50BZF before (Fig. 2 J) and after 15 s of UV irradiation (Fig. 2 K). We observed a robust and statistically significant right-shift in the G-V relationship after 15 s of UV irradiation for both hERG-F48BZF (Fig. 2 I) and hERG-E50BZF (Fig. 2 L). For hERG-F48BZF, the average V0.5 was −50 ± 1 mV before UV exposure and −32 ± 3 mV after UV exposure. For hERG-E50BZF, the average V0.5 was −54 ± 9 mV before UV exposure and −33 ± 6 mV after UV exposure (Fig. 2 M). These results show that cross-linking in hERG-F48BZF and hERG-E50BZF channels markedly shifted the steady-state activation, making the channel harder to open with depolarization (Table 1; Fig. S1 A; Tables S1 and S2).
UV-dependent photochemical cross-linking in the open state changes hERG F48BZF channel properties
As a negative control, to test if hERG channels were photosensitive to UV light in a state-dependent manner, excised membrane patches from oocytes expressing hERG in the presence of the orthogonal set and BZF were subjected to UV irradiation in the open state by applying a holding voltage of 0 mV. A family of current traces was recorded when hERG channels were irradiated at a 0 mV holding potential before UV irradiation (0 s, black traces) (Fig. 3 A) and after UV irradiation (15 s, blue traces) (Fig. 3 B). A G-V relationship was plotted for all currents recorded at the discrete UV irradiation intervals, and we detected an average V0.5 of −42 ± 3 mV before UV irradiation and −40 ± 3 mV after 15 s UV irradiation, indicating there was no significant change in the control hERG channel G-V relationship under these conditions (Fig. 3 C), and that control hERG channels were not measurably changed by UV light in the closed (Fig. 2, A–C and M) or open configuration (Fig. 3, A–C and M; Table 2; Fig. S1 A; Tables S1 and S2).
Figure 3.
Currents and steady-state activation of hERG and hERG-BZF channels before and after UV irradiation in the open state. Family of currents from (A and B) hERG, (D and E) hERG-G47BZF, (G and H) hERG-F48BZF, and (J and K) hERG-E50BZF channels, recorded before (0 s) UV irradiation (black traces) or after (blue traces) 15 s of UV irradiation. UV light was applied in the open state (0 mV) in all experiments. After UV irradiation at 0 mV, currents were elicited by a voltage protocol (red traces, inset) with 20-mV steps from a holding voltage of −100 mV. Scale bars are indicated for each family of currents. Conductance-voltage (G-V) plots for (C) hERG, (F) hERG-G47BZF, (I) hERG-F48BZF, and (L) hERG-E50BZF, before (0 s) or after UV irradiation for durations of 0, 1, 3, 7 or 15 s. A right-shift in the GV (black arrow) was measured for hERG-F48BZF. (M) Scatter plot of the midpoint of the G-V curves before (black symbols) or after (blue symbols) 15 s of UV irradiation. n = 3 for each experiment. ns denotes p > 0.05, ∗ denotes p ≤ 0.05.
Table 2.
Biophysical Properties of hERG and hERG-BZF Channels Before and After UV Irradiation in the Open State
| State: open | ||||||||
|---|---|---|---|---|---|---|---|---|
| Channel | hERG | hERG | hERG-G47BZF | hERG-G47BZF | hERG-F48BZF | hERG-F48BZF | hERG-E50BZF | hERG-E50BZF |
| UV irradiation time (sec) | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 |
| V0.5 (mV) | −42 ± 3 | −40 ± 3 | −48 ± 0.4 | −44 ± 2 | −49 ± 1 | −37 ± 1 | −39 ± 1 | −35 ± 3 |
| Tau fast (ms) | 45 ± 3 | 44 ± 1 | 38 ± 6 | 16 ± 1 | 35 ± 3 | 21 ± 1 | 37 ± 2 | 34 ± 2 |
| Time to half max (sec) | 0.59 ± 0.01 | 0.59 ± 0.01 | 0.55 ± 0.01 | 0.55 ± 0.01 | 0.51 ± 0.04 | 0.64 ± 0.03 | 0.58 ± 0.03 | 0.58 ± 0.03 |
Using the same experimental approach as for control hERG (Fig. 3, A–C), membrane patches were excised from oocytes expressing hERG-G47BZF channels, and the family of currents was recorded (Fig. 3, D and E). The average V 0.5 for hERG-G47BZF was −47 ± 0.4 mV before UV exposure and −44 ± 2 mV after UV exposure, showing that similar to control hERG, we observed no significant change in the G-V relationship after 15 s of UV irradiation (Fig. 3, F and M). In membrane patches with hERG-F48BZF channels, we recorded a family of currents before (Fig. 3 G) and after 15 s UV irradiation (Fig. 3 H). We measured a large and statistically significant rightward shift in the G-V relationship with UV irradiation for hERG-F48BZF (Fig. 3 I). The average V0.5 for hERG-F48BZF was −49 ± 1 mV before UV exposure and −37 ± 1 mV after UV exposure. However, for hERG-E50BZF (Fig. 3, J and K), we observed no significant shift in the conductance-voltage relationship after 15 s of UV light when irradiated in the open state (Fig. 3 L). For hERG-E50BZF, the average V0.5 was −39 ± 1 mV before UV exposure and −35 ± 3 mV after UV exposure. A plot of the changes in V0.5 at −100 mV or 0 mV holding potential and at 0 or 15 s of UV irradiation shows a state-dependent change in the V0.5 for hERG-E50BZF channels (Tables 1 and 2; Fig. S1 A; Tables S1 and S2), suggesting that the hERG PAS domain undergoes a rearrangement between open and closed states.
Photochemical cross-linking speeds hERG-G47BZF and hERG-E50BZF channel deactivation in a UV- and state-dependent manner
We next investigated photochemical cross-linking effects on channel kinetics by measuring the time course of hERG-BZF channel closing (deactivation) before and after UV irradiation. We measured inward tail currents (Fig. 4, A–H) with a voltage step to −100 mV after a step to 40 mV (Fig. 4 inset, red trace), from which the time constant (tau fast) of deactivation was determined from an exponential fit to the tail current. Tail currents were from data sets in Figs. 2 and 3 but were expanded here to show the first 500 ms for clarity. To assess state dependence of cross-linking, before recording tail currents, channels were held in the closed state (at −100 mV) or in the open state (at 0 mV) and photoactivated by UV irradiation for 15 s. Tail currents were recorded either before (black traces) or after (blue traces) UV irradiation (Fig. 4, A–H).
Figure 4.
hERG and hERG-BZF channel tail currents report the time course of channel deactivation before and after UV irradiation. Normalized tail currents from (A and B) hERG, (C and D) hERG-G47BZF, (E and F) hERG-F48BZF, or (G and H) hERG-E50BZF before (black traces) or after 15 s of UV irradiation (blue traces). UV light was applied when channels were held in closed state at −100 mV or in the open state at 0 mV, as indicated. The scale bar represents 100 ms, and the dashed line indicates zero current level. Inset (red trace) depicts voltage pulse. Scatter plots of the time constant of deactivation (tau fast) before (black symbols) or after (blue symbols)15 s of UV irradiation applied at (I) −100 mV or (J) 0 mV. n = 3, ns denotes p > 0.05, ∗∗ denotes p ≤ 0.01, ∗∗∗ denotes p ≤ 0.005, ∗∗∗∗ denotes p ≤ 0.005.
In hERG channels held in the closed state (at −100 mV), before UV, the tail current (Fig. 4 A, black trace) had a tau for deactivation of 42 ± 1 ms (Fig. 4 I) and after UV the tail current (Fig. 4 A, blue trace) had a tau of deactivation of 44 ± 2 ms (Fig. 4 I). For hERG channels held in the open state (at 0 mV), before UV, the tail current (Fig. 4 B, black trace) had a tau of deactivation of 45 ± 3 ms (Fig. 4 J) and after UV the tail current (Fig. 4 B, blue trace) had a tau of 44 ± 1 ms (Fig. 4 J), indicating that UV irradiation had no measurable effect on hERG channel deactivation (Tables 1 and 2; Fig. S1 B; Tables S1 and S2).
In contrast to hERG channels, hERG-G47BZF channels had a state-dependent change in the time course of deactivation after photoactivation. For hERG G47BZF channels held in the closed state (at −100 mV), before UV, the tail currents (Fig. 4 C, black trace) had a tau of 29 ± 5 ms (Fig. 4 I) and after UV the tail currents (Fig. 4 C, blue trace) had a tau of 27 ± 1 ms, which were not significantly different (Fig. 4 I). But, for hERG-G47BZF channels held in the open state (at 0 mV), before UV, the tail current (Fig. 4 D, black trace) had a tau of 38 ± 6 ms (Fig. 4 I) and after UV the current (Fig. 4 D, blue trace) had a tau of 16 ± 1 ms (Fig. 4 J), indicating a speeding up of channel deactivation. Thus, after UV-dependent cross-linking, deactivation was sped up 2.3-fold in the open state, but not the closed state (Fig. 4, I and J; Tables 1 and 2; Fig. S1 B; Tables S1 and S2), indicating a state dependence to cross-linking for hERG-G47BZF.
Next, we found that hERG-F48BZF channels had a state-independent change in deactivation after photoactivation. For hERG-F48BZF channels held in the closed state (at −100 mV), the tail current (Fig. 4 E, black) had a tau before UV of 32 ± 2 ms (Fig. 4 I) and after UV the current (Fig. 4 E, blue) had a tau of 21 ± 2 ms (Fig. 4 I). When hERG-F48BZF channels were held in the open state (at 0 mV), the tail current (Fig. 4 F, black) had a tau of deactivation before UV of 35 ± 3 ms (Fig. 4 J), and after UV, the tau (Fig. 4 F, blue trace) was 21 ± 1 ms (Fig. 4 J). These results show that after UV-dependent cross-linking, deactivation was sped up 1.5-fold in both the closed and open states (Tables 1 and 2; Fig. S1 B; Tables S1 and S2).
In hERG-E50BZF channels, we found a state-dependent change in deactivation with photoactivation but with the closed state being sensitive to photoactivation instead of the open state, which was opposite to that for hERG-G47BZF. For hERG-E50BZF channels held in the closed state (at −100 mV), before UV, the tail current (Fig. 4 G, black) had a tau of 36 ± 4 ms and after UV the current (Fig. 4 G, blue trace) had a tau of 21 ± 2 ms (Fig. 4 I), but when channels were held in the open state (0 mV), before UV, hERG-E50BZF channel currents (Fig. 4 H, black) had a tau of 37 ± 2 ms (Fig. 4 J) and after UV the currents (Fig. 4 H, blue) had a tau of 34 ± 2 ms (Fig. 4 J). Thus, hERG-E50 BZF channels had a state-dependent, 1.7-fold change in deactivation in the closed state but not the open state (Fig. 4, I and J; Tables 1 and 2; Fig. S1 B; Tables S1 and S2), indicating a closed-state dependence to UV cross-linking.
Photochemical cross-linking slows channel activation in a UV- and state-dependent manner
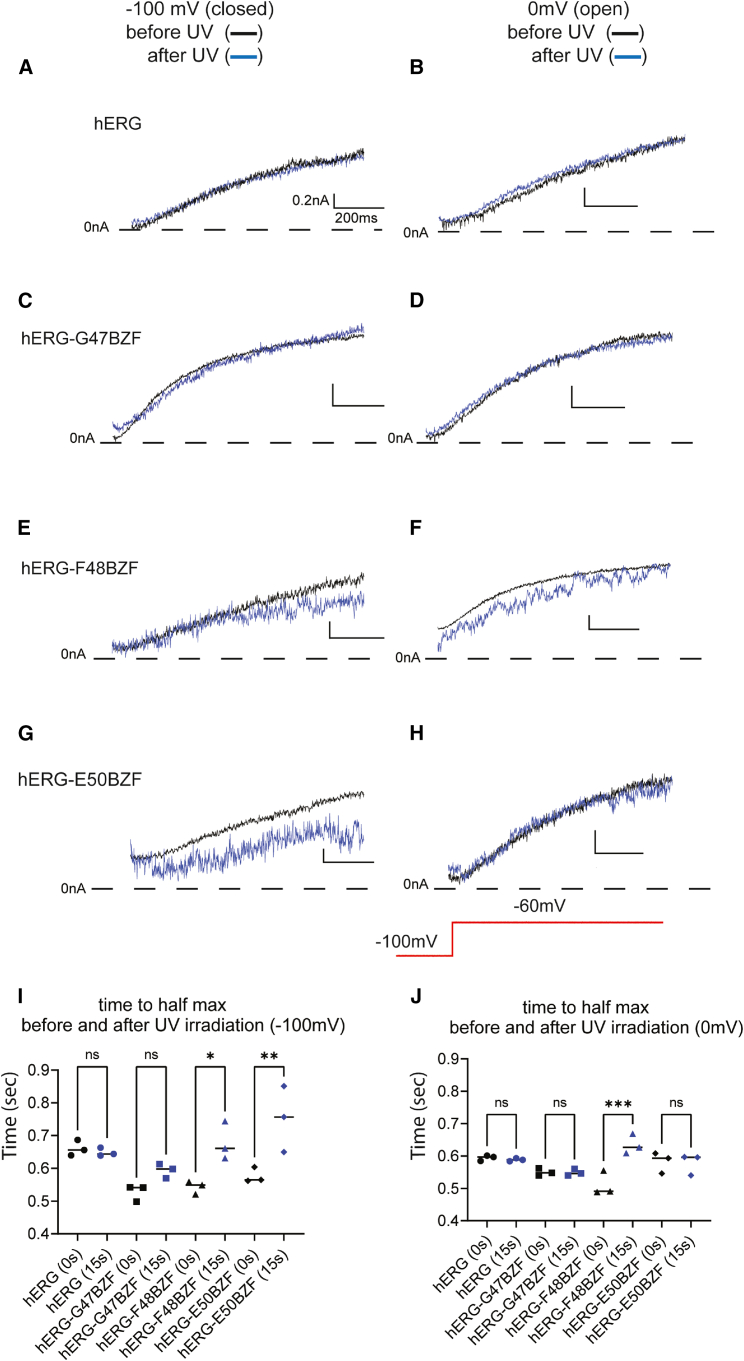
We examined channel activation by measuring the time to half-maximum current as in our previous studies (24,26,28) for hERG and hERG-BZF channels, before and after UV irradiation (Fig. 5). The initial observable current trace when hERG channels activate occurs at a step to −60 mV from a holding potential of −100 mV (Fig. 5, inset). For hERG, the trace before UV irradiation (Fig. 5 A, black trace) was normalized to the largest outward current value and overlaid with the normalized trace after UV irradiation (Fig. 5 A, blue trace) from when the channels were held at either −100 mV (Fig. 5 A) or 0 mV (Fig. 5 B) during UV irradiation.
Figure 5.
Current traces indicate time course of activation before and after UV irradiation. Normalized currents recorded from (A and B) hERG, (C and D) hERG-G47BZF, (E and F) hERG- F48BZF, and (G and H) hERG-E50BZF either before (black traces) or after 15 s of UV irradiation (blue traces). UV light was applied when channels were held in closed state at −100 mV or in the open state at 0 mV, as indicated. Scatter plot depicting time to half-maximum current before (black symbols, 0 s) and after UV irradiation (blue symbols, 15 s) after UV irradiation at a voltage of (I) −100 mV or (J) 0 mV. Scale bar represents 0.2 nA and 200 ms. n = 3, ns denotes p > 0.05, ∗ denotes p ≤ 0.05, ∗∗ denotes p ≤ 0.01, ∗∗∗ denotes p ≤ 0.001.
For hERG channels, we did not observe a measurable change in time to half-maximum current regardless of whether the channels were irradiated in the closed state (at −100 mV) (Fig. 5 A) or in the open state (at 0 mV) (Fig. 5 B) as the time to half-maximum current for hERG before irradiation in the closed state was 0.66 ± 0.02 s and after UV was 0.65 ± 0.01 s (Fig. 5 I), and the time to half-maximum current in the open state before irradiation was 0.59 ± 0.01 s and after UV irradiation was 0.59 ± 0.01 s (Fig. 5 J), indicating that UV light had no measurable effects on control hERG channel activation (Tables 1 and 2; Fig. S1 C; Tables S1 and S2).
Like for hERG channels, we observed no measurable changes for hERG-G47BZF channels by photoactivation with UV light (Fig. 5, A, B, I, and J). We observed no change in time to half-maximum current regardless of whether the channels were irradiated at −100 mV (Fig. 5 C) or 0 mV (Fig. 5 D). The time to half-maximum for hERG-G47BZF before irradiation in the closed state was 0.53 ± 0.03 s and after UV irradiation was 0.59 ± 0.02 s (Fig. 5, C and I), and the time to half-maximum for hERG-G47BZF before irradiation in the open state was 0.55 ± 0.01 s and after 15 s UV irradiation was 0.55 ± 0.01 s (Fig. 5, D and J), indicating no measurable changes in activation (Tables 1 and 2; Fig. S1 C; Tables S1 and S2).
In contrast, hERG-F48BZF channels showed a state-independent increase in the time to half-maximum current at −60 mV, which we interpret as a slowing of activation with UV cross-linking (Fig. 5, E, F, I and J). The time to half-maximum activation for hERG-F48BZF held in the closed state (−100 mV) before UV was 0.54 ± 0.02 s and after UV was 0.67 ± 0.05 s (Fig. 5 I), and the time to half-maximum current in the open state before irradiation was 0.51 ± 0.04 s and after UV was 0.64 ± 0.03 s (Fig. 5 J), indicating a state-independent change in activation (Tables 1 and 2; Fig. S1 C; Tables S1 and S2).
Distinct from hERG-F48BZF, hERG-E50BZF channels showed a state-dependent increase in the time to half-maximum current, indicating slower channel activation at −60 mV. When hERG-E50BZF channels were irradiated in the closed state (−100 mV), they opened more slowly after UV exposure (Fig. 5 G). But no change in time to half-maximum current was observed when the channels were irradiated in the open state (Fig. 5 H). The time to half-maximum before irradiation in the closed state was 0.58 ± 0.02 s and after UV was 0.75 ± 0.1 s (Fig. 5 I), whereas the time to half-maximum in the open state before irradiation was 0.58 ± 0.03 s and after UV was 0.58 ± 0.1 s (Fig. 5 J), revealing a 1.3-fold change in activation in the closed state but not the open state (Tables 1 and 2; Fig. S1 C; Tables S1 and S2).
Discussion
We show that in our experimental conditions, control hERG channels were insensitive to UV light. Neither the steady-state voltage dependence of activation nor the kinetics of activation or deactivation were markedly changed after exposure to UV light (Figs. 2, 3, 4, and 5; Tables 1 and 2; Fig. S1; Tables S1 and S2), which ruled out measurable sensitivity of control hERG channels to UV light, which is nontrivial as CNG channels are modified by UV light (36). Additionally, in control experiments with hERG, the orthogonal set and BZF were included as a negative control (see Fig. 1 G), and the lack of measurable UV-dependent changes further indicates that BZF was not incorporated nonspecifically into hERG, and any unincorporated BZF did not contribute to photoactivation. We observed robust currents only when both the orthogonal set and BZF were included with hERG amber stop codon mutant mRNAs, indicating that BZF incorporation was specific and that the observed changes after UV irradiation we report here were associated with formation of photochemical cross-links in hERG-BZF channels. Introduction of a bulky BZF amino acid had only minor effects on baseline channel gating, and thus, we were able to compare changes in steady-state and kinetic properties of hERG-BZF channels to that of control hERG and explicitly attribute UV-dependent changes to photochemical cross-linking.
We observed that UV photo-cross-linking altered each hERG-BZF channel in distinctive ways. First, hERG-G47BZF channels that were cross-linked in the open state had faster deactivation. Second, hERG-F48BZF channels that were cross-linked in either the closed or open state had right-shifted voltage dependence of activation, faster deactivation, and slower activation. Third, hERG-E50BZF channels that were cross-linked in the closed state had a right-shifted voltage dependence of activation, faster deactivation, and slower activation. Since we detected a state dependence to cross-linking for hERG-G47BZF and hERG-E50BZF channels, we interpret this to mean that the PAS domain was in a different conformation relative to other channel domains in the closed versus open states, indicating a relative movement of PAS in the closed versus open states. hERG-G47BZF had open-state dependence, and hERG-E50BZF had closed state-dependence, which was also consistent with relative motion of the PAS during gating. Since G47, F48, and E50 are nearby one another in the PAS alpha A helix, but we observed open state-dependent, state-independent, and closed state-dependent cross-linking, respectively, at these sites, we propose that some conformational changes in PAS are local.
Our results suggest that relative motions in the PAS domain that facilitate channel activation were inhibited by photochemical cross-linking and that relative motions that facilitate channel deactivation were promoted by photochemical cross-linking. For instance, UV irradiation sped deactivation in hERG-G47BZF channels and right-shifted steady-state activation, sped up deactivation, and slowed activation in hERG-F48BZF and hERG-E50BZF, consistent with cross-linking stabilizing a closed state (relative to the open state). Interestingly, UV irradiation of BZF introduced to glutamate receptors also resulted in photoinhibition, due to state-dependent conformational changes in the ligand receptor binding domain (37).
How might cross-linking the PAS domain alter the molecular mechanism for hERG deactivation gating? To explain this, we first present a working model for hERG deactivation gating (Fig. 6). In the open state, the S4 domains of hERG are “up,” the S6 gates are open, and the PAS-Cap points upward toward the S4, S4-S5 linker, and C-linker as based on the CryoEM structure of the open state of hERG (2). The interaction of the PAS and the CNBHD, and the location of the PAS-Cap, stabilize the gating machinery (Fig. 6 A). In the closed state, the S4s are downward and bent, and the S6 gates are closed due to steric effects, as based on the down-state CryoEM structure of EAG channels (38). The interaction between the PAS and CNBHD is altered compared with the open state, with the PAS rearranged to interact in a distinct “closed configuration” and to be further from the IL motif (23,39,40), with the consequence that the PAS-Cap is located further away from the S4, S4-S5 linker, and C-linker, stabilizing the closed state (Fig. 6 B). Our results in this study suggest that the PAS alpha A helix is involved in the mechanism of deactivation gating and is dynamic, as shown by different positions for the PAS alpha A helix in the open versus the closed state (Fig. 6 A and B).
Figure 6.
Schematic of the effect UV irradiation on hERG-BZF channels. Schematic of hERG-BZF channels (A) in the open state and (B) in the closed state before UV irradiation. Black arrows depict transitions between open and closed conformations due to change in membrane voltage. Schematic of hERG-BZF channels after UV irradiation and cross-linking (C) in the open state and (D) in the closed state. Cross-links (red dashed lines) depict the PAS domain alpha A helix cross-linked to either the PAS domain or to the CNBHD. Robust black arrow depicts faster closing, and faint black arrow depicts slower opening.
To explain the direct effects of cross-linking in this study, we propose a working model for cross-linked hERG channels (Fig. 6 C and D). In the open state, we propose that the S4 domains are “up,” and the S6 gate is open, but that the overall PAS and CNBHD interaction is locked in a “closed configuration” similar to that in closed channels (as in Fig. 6 B), and dynamic motions within the PAS or between the PAS and CNBHD are limited. We propose that cross-links (red dashed lines) occur from the PAS alpha A helix to the CNBHD at E50 or between the PAS alpha A helix and the rest of the PAS at G47, F48, or E50 (and see Fig. S2). With the alpha A helix cross-linked, the PAS is less able to dynamically regulate channel gating, favors the closed state, and promotes more rapid channel deactivation (large horizontal black arrow from C to D). The closed, cross-linked state (Fig. 6 D) is similar to the closed state of the channel (Fig. 6 B), with the exception that transitions to the open state are slower (faint black arrow from D to C) since cross-linking stabilizes the closed state. We propose that since hERG-F48BZF had similar cross-linking in the closed and open state, but G47BZF was open-state dependent and E50BZF was closed-state dependent, F48 may serve as a pivot point in the alpha A helix (Fig. 6 A and B).
Where might the PAS domain residues 47, 48, and 50 cross-link? Our results presented here do not directly address this, but cross-linking of UV-activated BZF has a geometric component in which C-H bonds within 3.1 Å and in a coplanar arrangement are sites of attack for the double-bonded oxygen of BZF (41). Inspection of a model of the PAS domain in which we substituted BZF at residues 47, 48, or 50 indicates that the nearest coplanar C–H bond (as shown with a cyan dashed line) for G47BZF is PAS domain residue S26, for F48BZF is PAS domain residue L69, and for E50BZF are PAS domain residue D46 and CNBHD residue K741 (Fig. S2). We propose that C–H bonds from these residues are plausible candidate sites for cross-linking to BZF.
How can we explain the state dependence of cross-linking in the context of a mechanism for deactivation? For hERG-E50BZF, we detected UV-dependent effects only in the closed state, suggesting that BZF cross-linking (to either K741 or D46) stabilized the closed state and a “closed configuration” of the PAS-CNBHD as the most parsimonious explanation (Fig. 6 D). For hERG-F48BZF, UV-dependent effects occurred in either the closed or open state, suggesting a state-independent cross-link (with L69) that stabilized a closed state and “closed configuration” of the PAS and CNBHD (Fig. 6 D). hERG-G47BZF had UV-dependent effects on channel gating only in the open state. Although we initially anticipated that open-state-dependent effects would stabilize the open state and would be apparent as a slowing of the deactivation kinetics or left-shifting the G-V curve, here, we instead detected a speeding up of deactivation kinetics and a right-shift of the G-V curve (Figs. 2, 3, and 4). We propose a few ways that this might occur. We consider that cross-linking hERG-G47BZF may be functionally similar to introduction of a deleterious point mutation that disrupts PAS domain function and speeds up deactivation, but that occurs here in real time with UV light irradiation, or that there were no accessible cross-link partners for hERG-G47BZF in the “closed configuration.” But we cannot rule out that UV irradiation of hERG-G47BZF cross-linked an “open configuration” of the PAS-CNBHD (as in Fig. 6 A), but channels closed due to closure of a pore gate, which is consistent with PAS also speeding C-type inactivation (21,28) that is localized to the pore (42,43) or that the S6 gates closed due to a desensitization to voltage mechanism as in HCN channels (44).
Our results fit with previous work on mechanisms of PAS domain regulation in hERG channels. For instance, deletion of the PAS domain causes a rightward shift in the voltage dependence of activation and speeds up deactivation in hERG (24,26,28), which is functionally similar to the rightward shift in voltage dependence and speeding up of deactivation for UV-dependent cross-linking in hERG-E50BZF and hERG-F48BZF channels. The motions we propose that affect gating may be small, since it was shown that movements of a few angstroms in the PAS-CNBHD of ELK channels occurred during voltage-dependent potentiation of gating (39), and that in hERG, a repositioning of the PAS-Cap away from the transmembrane domains by a few angstroms speeds up deactivation (19,45). Evidence for the PAS and CNBHD communication with the VSDs is supported by results in which the off-gating charge was modified by deletion of the PAS or mutations in the PAS-Cap (46). As some disease-causing mutations in the PAS speed up deactivation (47,48), the putative mechanism we present here may also be disrupted in disease states.
Author contributions
S.J.C. and M.C.T. designed research, S.J.C. performed research and analyzed data, and S.J.C. and M.C.T. wrote the paper.
Acknowledgments
We thank Drs. Andrew Plested, Ivy Dick, Ashley A. Johnson, Moradeke Bamgboye, and Gail A. Robertson for their helpful discussions. This work was supported by NIH/NIGMS R01GM130701, R01GM12752301-A1, and NIH/NINDS R01NS081320 (M.C.T.) and NIH/NIGMS K99GM144684 (S.J.C.).
Declaration of interests
The authors declare no competing interests.
Editor: Brad Rothberg.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2024.05.024.
Supporting Material
References
- 1.Codding S.J., Trudeau M.C. In: Vol 2. Textbook of Ion Channels. Zheng J., Trudeau M.C., editors. Taylor and Francis/CRC Press; London: 2023. hERG Potassium Channels. [Google Scholar]
- 2.Wang W., MacKinnon R. Cryo-EM Structure of the Open Human Ether-a-go-go-Related K(+) Channel hERG. Cell. 2017;169:422–430.e410. doi: 10.1016/j.cell.2017.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warmke J.W., Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc. Natl. Acad. Sci. USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. http://www.ncbi.nlm.nih.gov/pubmed/8159766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy H.R., Durell S.R., et al. Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science. 1991;254:730. doi: 10.1126/science.1658932. http://www.ncbi.nlm.nih.gov/pubmed/1658932 [DOI] [PubMed] [Google Scholar]
- 5.James Z.M., Zagotta W.N. Structural insights into the mechanisms of CNBD channel function. J. Gen. Physiol. 2018;150:225–244. doi: 10.1085/jgp.201711898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson G.A., Warmke J.M., Ganetzky B. Potassium currents expressed from Drosophila and mouse eag cDNAs in Xenopus oocytes. Neuropharmacology. 1996;35:841–850. doi: 10.1016/0028-3908(96)00113-x. [DOI] [PubMed] [Google Scholar]
- 7.Brelidze T.I., Carlson A.E., Zagotta W.N. Absence of direct cyclic nucleotide modulation of mEAG1 and hERG1 channels revealed with fluorescence and electrophysiological methods. J. Biol. Chem. 2009;284:27989–27997. doi: 10.1074/jbc.M109.016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brelidze T.I., Carlson A.E., et al. Zagotta W.N. Structure of the carboxy-terminal region of a KCNH channel. Nature. 2012;481:530–533. doi: 10.1038/nature10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brelidze T.I., Gianulis E.C., et al. Zagotta W.N. Structure of the C-terminal region of an ERG channel and functional implications. Proc. Natl. Acad. Sci. USA. 2013;110:11648–11653. doi: 10.1073/pnas.1306887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marques-Carvalho M.J., Sahoo N., et al. Morais-Cabral J.H. Structural, biochemical, and functional characterization of the cyclic nucleotide binding homology domain from the mouse EAG1 potassium channel. J. Mol. Biol. 2012;423:34–46. doi: 10.1016/j.jmb.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Sanguinetti M.C., Jiang C., et al. Keating M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 12.Sanguinetti M.C., Jurkiewicz N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. http://www.ncbi.nlm.nih.gov/pubmed/2170562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trudeau M.C., Warmke J.W., et al. Robertson G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. http://www.ncbi.nlm.nih.gov/pubmed/7604285 [DOI] [PubMed] [Google Scholar]
- 14.Curran M.E., Splawski I., et al. Keating M.T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. http://www.ncbi.nlm.nih.gov/pubmed/7889573 [DOI] [PubMed] [Google Scholar]
- 15.Roden D.M. Drug-induced prolongation of the QT interval. N. Engl. J. Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 16.Morais Cabral J.H., Lee A., et al. Mackinnon R. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell. 1998;95:649–655. doi: 10.1016/s0092-8674(00)81635-9. http://www.ncbi.nlm.nih.gov/pubmed/9845367 [DOI] [PubMed] [Google Scholar]
- 17.Ng C.A., Hunter M.J., et al. Vandenberg J.I. The N-terminal tail of hERG contains an amphipathic alpha-helix that regulates channel deactivation. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector P.S., Curran M.E., et al. Sanguinetti M.C. Fast inactivation causes rectification of the IKr channel. J. Gen. Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. http://www.ncbi.nlm.nih.gov/pubmed/8740374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens-Sostre W.A., Flores-Aldama L., et al. Robertson G.A. An intracellular hydrophobic nexus critical for hERG1 channel slow deactivation. Biophys. J. 2024 doi: 10.1016/j.bpj.2024.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Myers C.D., Robertson G.A. Dynamic control of deactivation gating by a soluble amino-terminal domain in HERG K(+) channels. J. Gen. Physiol. 2000;115:749–758. doi: 10.1085/jgp.115.6.749. http://www.ncbi.nlm.nih.gov/pubmed/10828248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Trudeau M.C., et al. Robertson G.A. Regulation of deactivation by an amino terminal domain in human ether-a- go-go-related gene potassium channels [published erratum appears in J Gen Physiol 1999 Feb;113(2):359] J. Gen. Physiol. 1998;112:637–647. doi: 10.1085/jgp.112.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Codding S.J., Johnson A.A., Trudeau M.C. Gating and regulation of KCNH (ERG, EAG, and ELK) channels by intracellular domains. Channels. 2020;14:294–309. doi: 10.1080/19336950.2020.1816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Codding S.J., Trudeau M.C. The hERG potassium channel intrinsic ligand regulates N- and C-terminal interactions and channel closure. J. Gen. Physiol. 2019;151:478–488. doi: 10.1085/jgp.201812129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianulis E.C., Liu Q., Trudeau M.C. Direct interaction of eag domains and cyclic nucleotide-binding homology domains regulate deactivation gating in hERG channels. J. Gen. Physiol. 2013;142:351–366. doi: 10.1085/jgp.201310995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustina A.S., Trudeau M.C. A recombinant N-terminal domain fully restores deactivation gating in N-truncated and long QT syndrome mutant hERG potassium channels. Proc. Natl. Acad. Sci. USA. 2009;106:13082–13087. doi: 10.1073/pnas.0900180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustina A.S., Trudeau M.C. hERG potassium channel gating is mediated by N- and C-terminal region interactions. J. Gen. Physiol. 2011;137:315–325. doi: 10.1085/jgp.201010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustina A.S., Trudeau M.C. HERG potassium channel regulation by the N-terminal eag domain. Cell. Signal. 2012;24:1592–1598. doi: 10.1016/j.cellsig.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustina A.S., Trudeau M.C. The eag domain regulates hERG channel inactivation gating via a direct interaction. J. Gen. Physiol. 2013;141:229–241. doi: 10.1085/jgp.201210870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmgren M., Shin K.S., Yellen G. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 1998;21:617–621. doi: 10.1016/s0896-6273(00)80571-1. http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.neuron.org/cgi/content/full/21/3/617 [DOI] [PubMed] [Google Scholar]
- 30.Preston G.W., Wilson A.J. Photo-induced covalent cross-linking for the analysis of biomolecular interactions. Chem. Soc. Rev. 2013;42:3289–3301. doi: 10.1039/c3cs35459h. [DOI] [PubMed] [Google Scholar]
- 31.Wang L., Brock A., et al. Schultz P.G. Expanding the genetic code of Escherichia coli. Science. 2001;292:498–500. doi: 10.1126/science.1060077. [DOI] [PubMed] [Google Scholar]
- 32.Beene D.L., Dougherty D.A., Lester H.A. Unnatural amino acid mutagenesis in mapping ion channel function. Curr. Opin. Neurobiol. 2003;13:264–270. doi: 10.1016/s0959-4388(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 33.Liu W., Brock A., et al. Schultz P.G. Genetic incorporation of unnatural amino acids into proteins in mammalian cells. Nat. Methods. 2007;4:239–244. doi: 10.1038/nmeth1016. [DOI] [PubMed] [Google Scholar]
- 34.Ye S., Riou M., et al. Paoletti P. Expanding the genetic code in Xenopus laevis oocytes. Chembiochem. 2013;14:230–235. doi: 10.1002/cbic.201200515. [DOI] [PubMed] [Google Scholar]
- 35.Plested A.J.R., Poulsen M.H. Crosslinking glutamate receptor ion channels. Methods Enzymol. 2021;652:161–192. doi: 10.1016/bs.mie.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Middendorf T.R., Aldrich R.W. Effects of ultraviolet modification on the gating energetics of cyclic nucleotide-gated channels. J. Gen. Physiol. 2000;116:253–282. doi: 10.1085/jgp.116.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klippenstein V., Ghisi V., et al. Plested A.J.R. Photoinactivation of glutamate receptors by genetically encoded unnatural amino acids. J. Neurosci. 2014;34:980–991. doi: 10.1523/JNEUROSCI.3725-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandala V.S., MacKinnon R. Voltage-sensor movements in the Eag Kv channel under an applied electric field. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2214151119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai G., James Z.M., Zagotta W.N. Dynamic rearrangement of the intrinsic ligand regulates KCNH potassium channels. J. Gen. Physiol. 2018;150:625–635. doi: 10.1085/jgp.201711989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai G., Zagotta W.N. Molecular mechanism of voltage-dependent potentiation of KCNH potassium channels. Elife. 2017;6 doi: 10.7554/eLife.26355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorman G., Prestwich G.D. Benzophenone photophores in biochemistry. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 42.Herzberg I.M., Trudeau M.C., Robertson G.A. Transfer of rapid inactivation and sensitivity to the class III antiarrhythmic drug E-4031 from HERG to M-eag channels. J. Physiol. 1998;511:3–14. doi: 10.1111/j.1469-7793.1998.003bi.x. http://www.ncbi.nlm.nih.gov/pubmed/9679158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith P.L., Baukrowitz T., Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 44.Shin K.S., Maertens C., et al. Yellen G. Inactivation in HCN channels results from reclosure of the activation gate: desensitization to voltage. Neuron. 2004;41:737–744. doi: 10.1016/s0896-6273(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 45.Trudeau M.C. A hydrophobic nexus at the heart of hERG K channel gating. Biophys. J. 2024 doi: 10.1016/j.bpj.2024.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodchild S.J., Macdonald L.C., Fedida D. Sequence of gating charge movement and pore gating in HERG activation and deactivation pathways. Biophys. J. 2015;108:1435–1447. doi: 10.1016/j.bpj.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gianulis E.C., Trudeau M.C. Rescue of aberrant gating by a genetically encoded PAS (Per-Arnt-Sim) domain in several long QT syndrome mutant human ether-á-go-go-related gene potassium channels. J. Biol. Chem. 2011;286:22160–22169. doi: 10.1074/jbc.M110.205948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J., Zou A., et al. Sanguinetti M.C. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J. Biol. Chem. 1999;274:10113–10118. doi: 10.1074/jbc.274.15.10113. http://www.ncbi.nlm.nih.gov/pubmed/10187793 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.