Abstract
Tight junctions are cell-cell adhesion complexes that act as gatekeepers of the paracellular space. Formed by several transmembrane proteins, the claudin family performs the primary gate-keeping function. The claudin proteins form charge and size-selective diffusion barriers to maintain homeostasis across endothelial and epithelial tissue. Of the 27 known claudins in mammals, some are known to seal the paracellular space, while others provide selective permeability. The differences in permeability arise due to the varying expression levels of claudins in each tissue. The tight junctions are observed as strands in freeze-fracture electron monographs; however, at the molecular level, tight junction strands form when multiple claudin proteins assemble laterally (cis assembly) within a cell and head-on (trans assembly) with claudins of the adjacent cell in a zipper-like architecture, closing the gap between the neighboring cells. The disruption of tight junctions caused by changing claudin expression levels or mutations can lead to diseases. Therefore, knowledge of the molecular architecture of the tight junctions and how that is tied to tissue-specific function is critical for fighting diseases. Here, we review the current understanding of the tight junctions accrued over the last three decades from experimental and computational biophysics perspectives.
Significance
Tight junctions are multiprotein complexes that act as gatekeepers of the intercellular space in endothelial and epithelial cells. The critical determinants of the tight junction selectivity are the claudin family of proteins that self-assemble into strand-like architectures that form barriers and charge- and size-selective pores that regulate the passive diffusion of molecules and ions across the cell-cell interface. This mechanism is critical for maintaining compartmentalization and homeostasis in organs and tissues, such as the skin and blood-brain barriers. In this article, we present the milestone breakthroughs that have informed our current knowledge of the molecular architecture of tight junctions over the past three decades since the discovery of the first integral tight junction membrane protein.
Introduction
Cell junctions are multiprotein complexes that maintain contact or adhesion between neighboring epithelial or endothelial cells. These adhesion complexes include tight junctions, gap junctions, adherens junctions, and desmosomes (Fig. 1). Each junction serves a distinct function: paracellular diffusion (1,2,3), intercellular signaling (4,5), actin cytoskeleton regulation (6), and mechanical stress maintenance (7,8). The primary function of the tight junctions, located in the apical region of the cell, is to regulate the charge and size-selective permeability across this intercellular (or paracellular) space. The tight junctions have complex molecular architecture composed of many transmembrane proteins such as claudins, occludins, and junction adhesion molecules to stabilize the cell-cell adhesion and cytosolic scaffolding proteins such as zonula occludens (ZO-1, ZO-2, and ZO-3) that interact with the termini of the transmembrane proteins (9,10,11,12,13).
Figure 1.
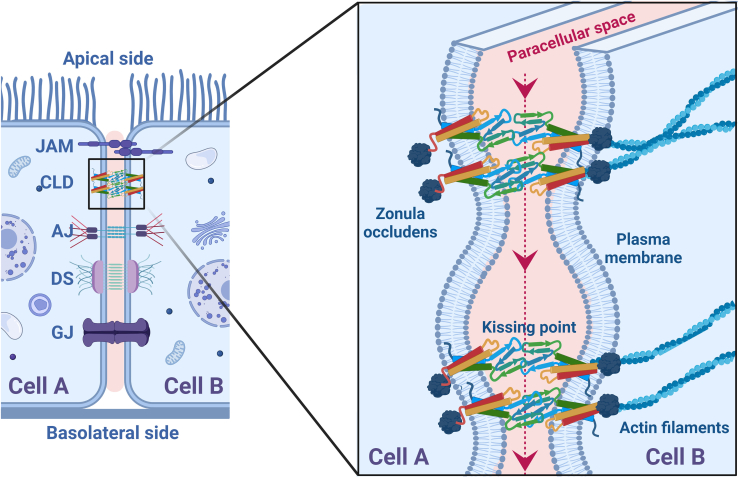
Epithelial cell-cell adhesion complex. The (left) schematic shows adjacent epithelial cells (cell A and cell B) in contact through junction adhesion molecules (JAM), claudins (CLD), adherens junctions (AJ), desmosomes (DS), and gap junctions (GJ). The (right) schematic shows the zoomed-in view of the tight junctions (TJ strands) located on the apical side of the cells. Tight junctions are formed when proteins such as claudins form cis (within a cell) and trans (across the cell) structures, causing the plasma membranes of the two cells to come closer to form kissing points and seal the paracellular space. The transport of water, ions, and small nutrients occurs in the direction perpendicular to the kissing points (red arrows). In the cytosol, the tight junction proteins interact with scaffolding proteins such as zonula occludens (ZO-1, ZO-2, and ZO-3). To see this figure in color, go online.
The permeability of the tight junctions is different in each tissue: in the skin, tight junctions form a barrier to the external environment (14); in the blood-testis barrier, the tight junctions protect the germ line cells from the body’s immune system (15); gut tight junctions allow the selective permeability of the nutrients and water (16); the blood-brain barrier tight junctions have highly selective ion and water transport to protect the central nervous system from blood-borne toxins (17); and the renal tubule tight junctions maintain the ion balance in the body through selective ion transport (18). These are a few examples that illustrate the role of tight junctions as paracellular gatekeepers to maintain tissue homeostasis via selective permeability.
The molecular architecture of the tight junctions consists of rows of claudin proteins from two adjacent cells that fasten the paracellular space like a zipper (Fig. 1). The epithelial cells, as in the intestinal lumen, are polarized in the apical and basolateral regions. The tight junctions in the apical region form a network of zippers, fastening the adjacent cells at multiple points, referred to as kissing points (9). The transport of ions and small nutrients occurs in the direction perpendicular to the kissing points (Fig. 1). The endothelial cells, as in blood capillaries, are not organized into distinct apical and basolateral domains but still show a network of interwoven claudin zippers (19,20). The micrographs of the apical view of the epithelial cells show tight junctions lining the cell borders, while in endothelial cells these appear as a meshwork of strands (19).
This review focusses on the discoveries and advances in the past three decades that have led to our current understanding of the molecular architecture and function of claudin proteins critical to tight junctions. Like other research fields, tight junction research has undergone technological advancements and has been a focus of multiple research groups worldwide.
The claudin family of transmembrane proteins
The claudins belong to the pfam00822 protein superfamily, recognized for their four-pass transmembrane regions. With a molecular weight in the 21–34 kDa range, the claudin proteins comprise 207–305 amino acids (21). Claudins have been reported to be present in vertebrates and predicted in several lower invertebrates (22). To date, 27 claudin genes have been found in mammals, among which at least 23 have been reported in humans. Claudin family members are classified into classic (claudins 1–10, 14, 15, 17, and 19) and nonclassic (claudins 11, 12, 13, 16, 18, and 20–24) based on their phylogenetic relationships (23). An in-depth genetic profile of the human claudins has been presented earlier (24).
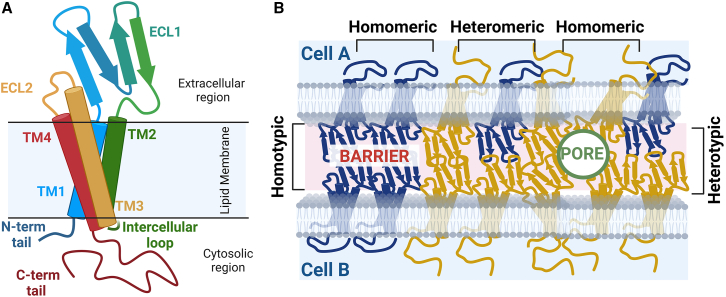
A claudin protein has three structural regions relative to the cell membrane—extracellular, transmembrane, and cytoplasmic (Fig. 2). The extracellular region has two domains (ECL1 and ECL2), four transmembrane (TM) helices (TM1–4), and three cytoplasmic domains composed of an intracellular loop, an N-terminal tail, and a C-terminal tail. The ECL domains form extracellular intermolecular interactions with neighboring claudins to facilitate cell-cell adhesion. Both ECL domains can vary in length and amino acid composition among members of the claudin family, which ultimately confers each claudin its unique charge and size-selective permeability (25,26,27,28). The four-helix TM bundle lies partially embedded in the lipid bilayer. The TMs are of unequal lengths, with TM3 being the longest (Fig. 2). It extends to the extracellular region and can interact intermolecularly with other claudin neighbors. The membrane-embedded portions of the TMs are hydrophobic and interact with lipids (29). Proximal to the cytosolic leaflet of the lipid membrane, most claudins have one or two pairs of cysteine residues that can undergo posttranslational lipid modifications, which have been shown to play a role in tight junction formation (30,31,32).
Figure 2.
Claudin structure and assembly. (A) Schematic of a claudin protein embedded in a lipid bilayer membrane. The extracellular region has two domains (ECL1 and ECL2), four transmembrane helices (TM1, TM2, TM3, and TM4), and three cytoplasmic domains composed of an intracellular loop, an N-terminal tail, and a C-terminal tail. (B) A cartoon depiction of cis and trans claudin interactions in homo or hetero combinations. The homo interactions may take place either in a cis (homomeric) or trans (homotypic) manner. Likewise, the hetero interactions can occur in cis (heteromeric) or trans (heterotypic) configurations. Together these interactions can form barrier or pore architectures that control paracellular permeabilities. To see this figure in color, go online.
The N- and C-terminal tails of claudins lie in the cell cytoplasm are of unequal lengths; the N-terminal tail is shorter (1–10 residues) compared with the C-terminal tail (26–114 residues), although both tails lack secondary structure. The unstructured C-terminal tails of claudins bind to a hydrophobic pocket of the PDZ (initials of three proteins—postsynaptic density protein, Drosophila disc large tumor suppressor, and ZO-1) domains of the scaffolding proteins (33). The rest of the PDZ domains bind to each other to form a multiprotein complex in the cytosol just below the base of the tight junction proteins. The ZO proteins have been shown to enable a cholesterol-rich environment in the plasma membrane to facilitate tight junction formation (34,35). Moreover, these cytosolic scaffolding proteins anchor the transmembrane proteins and bridge them with actin filaments (36). A rich literature on the interaction of claudins with cytoplasmic scaffolding protein has been presented in earlier reviews (37).
Although each structural component of claudin has a specific role, these proteins do not function in isolation. The molecular structure and self-assembly of claudins enable their paracellular permeability function. Claudins assemble laterally (cis) and head-on (trans) across the cell-cell interface (23). The cis assembly is facilitated by residues in the TM and ECL domains, while the trans assembly is primarily through the ECL domains (38,39,40). The cis- and trans-assembled claudins zip together to form contiguous strands that can be observed via freeze-fracture electron microscopy (FFEM), immunostaining of proteins, and other high-resolution microscopy techniques (19,36,41,42,43). Based on the relative orientations of cis/trans assembled claudins, the zippered strand can seal the paracellular or have subnanometer-sized pores (Fig. 2 B). The chemical nature of the pore-lining amino acid residues determines the pore’s size and charge selectivity (26,44,45).
Furthermore, cells express multiple members of the claudin family; as a result, claudin cis and trans assembly can occur between the same (homo) claudins or different (hetero) members of the claudin family (Fig. 2). The homo interactions can be cis (homomeric) or trans (homotypic). Similarly, the hetero interactions can be cis (heteromeric) or trans (heterotypic). Although a claudin strand can be created using multiple members of the family, the stability of homo versus hetero interactions can vary significantly among the claudin family. Ultimately, the coexpression level of the different claudins in a cell influences the paracellular permeability (40).
Techniques for visualizing and analyzing claudin strand meshworks in tight junctions
Claudin strand meshworks can be observed using techniques ranging from static high-resolution imaging to live-cell dynamics. FFEM has long been the gold standard for visualizing tight junction strands (41,42,46). This technique offers high-resolution, micron-scale images of the two-dimensional architecture of strands within the membrane plane, enabling detailed analysis of properties such as strand width, curvature, branch points, and density (46). Despite its advantages, FFEM is expensive, suited for specific cell lines, and does not provide time-resolved information on the dynamic behavior of strands in live cells (47). Confocal microscopy of fluorescently tagged claudins is a more accessible and cost-effective alternative to studying strand dynamics in live cells. However, it has a lower resolution than electron microscopy, making it challenging to visualize the fine details of tight junction strands at the nanometer scale.
Stimulated emission depletion (STED) microscopy, a more effective technique, has been successfully used in several studies to image tight junction strands in live cells (19,43). This technique, which offers higher resolution than confocal microscopy, provides real-time insights into how tight junction strands change. For instance, in a study by Sasaki et al., STED microscopy was used to observe the redistribution of claudins during tight junction remodeling (48). STED images enable precise quantitative analysis of tight junction properties, including strand density, distribution, and organization. In addition, STED microscopy can be combined with other imaging techniques and molecular biology methods to gain comprehensive insights into tight junction structure and function.
Fluorescence recovery after photobleaching (FRAP) is another valuable tool for evaluating tight junction strands. This technique involves tagging claudin proteins with fluorescent markers, photobleaching a specific region of the tight junction strand, and then monitoring the rate of recovery of fluorescence as unbleached proteins move into the area. The FRAP data of reconstituted claudin meshworks in fibroblasts revealed that individual claudins are immobile (36,48), with the meshwork growing through the incorporation of claudins at strand ends (36). Studies of strand dynamics in epithelial tight junctions confirmed that claudins within the strands have small mobile fractions, ranging from 20 to 35% (49,50), with FRAP recovery occurring through lateral diffusion within the membrane. In addition, FRAP has been used to study the mobility of other tight junction-associated proteins, which display different mobility characteristics compared with claudins.
To quantify the paracellular permeability, transepithelial/transendothelial electrical resistance (TEER) experiments are performed in vivo or in vitro on monolayer epithelial or endothelial cell culture models (51). Because TEER measurements are nondestructive to the cells, they provide an easy assay to evaluate the transport of ions and solutes for different claudins expressed in cell models. However, distinguishing between the permeabilities of individual claudins is difficult in cells coexpressing multiple claudins. There are two strategies to overcome this limitation. First, nonclaudin-expressing cells (such as fibroblasts) are used, and specific exogenous claudin is transfected to form tight junction strands and measure the decrease in permeability (higher TEER) (48,52,53,54). Second, the knockdown or knockout (KO) of specific claudin alters the paracellular permeability of cells with preexisting tight junctions (53). For example, if the preexisting tight junctions are formed by barrier-forming claudin; in that case, the knockdown of specific claudin should increase the permeability (lower TEER) and, conversely, the knockdown of a pore-forming claudin should decrease permeability (higher TEER). Based on a large set of experiments, it has been established that claudin-2, -10b, and -15 are cation pores (39,44,45,55,56,57,58,59,60,61,62); claudin-10a and -17 are anion pores (57,61,63,64); and claudin-1, -3, and -5 are barrier-forming (19,60,65,66,67,68,69,70,71,72).
Recent advances in computational hardware and software have significantly enhanced the visualization and analysis of claudin strand meshworks in tight junctions, covering scales from Angstroms to nanometers (19,73). Molecular dynamics (MD) simulations provide atomic-level insights into how claudin residues interact in both cis and trans conformations and with membrane lipids, ions, and other small molecules. An accurate starting structure typically solved using cryogenic electron microscopy or x-ray crystallography (74,75), is crucial for MD simulations. These studies have reported on conformational changes, stability, and interactions of claudin proteins within lipid bilayers, predicted tight junction pore models, ion permeability profiles, and the effects of specific mutations (28,29,32,38,76). In addition, MD simulations help study lipid-protein interactions and drug binding, offering valuable information for therapeutic design and optimization. Over the past decade, MD simulations have provided insights that complement experimental studies and are increasingly utilized in tight junction research (38,77,78,79,80,81,82).
Role of claudin proteins in cancer and disease pathophysiology
Aberrant expression of claudin proteins has been reported in malignant tumors. Claudin overexpression or downregulation in tumor cells has been implicated in uncontrolled growth and metastasis. The tumor cells have dysfunctional cell-cell contacts, causing the claudins to spread over the entire tumor cell surface instead of being limited to tight junctions in paracellular space. This surface presence, however, makes claudins suitable markers for cancer prognosis and therapeutic targets (83,84,85,86,87,88,89,90). In recent years, claudin-18.2 has been a target for diagnosing calcitrant metastatic gastric cancer. Claudin-18.2 is a lineage-specific marker observed in many gastric, pancreatic, and lung cancers (88). Claudin-1, -2, -3, -4, -7, and -18 are overexpressed in gastrointestinal tumors (90). In contrast, decreased expression of claudin protein results in the disruption of tight junction structures and the activation of downstream signaling pathways, leading to diseases such as claudin-low breast cancer, where reduced claudin results in poor maintenance of water and electrolyte balance in mammary glands (91).
Claudins are not only a factor in cancers but also in diseases such as hepatitis C (92,93,94), deafness (46,95,96,97), irritable bowel syndrome (98,99,100,101), and irritable bowel disease (89,98,99,101). The paracellular permeability of the gut tight junctions with healthy and diseased physiologies was reviewed in a recent publication (89). There is evidence that downregulation of claudin-1 is observed in human atopic dermatitis (102). A defect in claudin-16 and -19 expression is implicated in inherited human renal disorder familial hypomagnesemia with hypercalciuria and nephrocalcinosis (Fig. 3) (103,104). Similarly, mutations in claudin-14 cause autosomal recessive deafness (DFNB29) due to loss of compartmentalization in the organ of Corti located in the inner ear (Fig. 3) (46,96,105). In addition, a mutation in claudin-11 is implicated in the loss of fertility in mice due to disruption in the blood-testis barrier (106).
Figure 3.
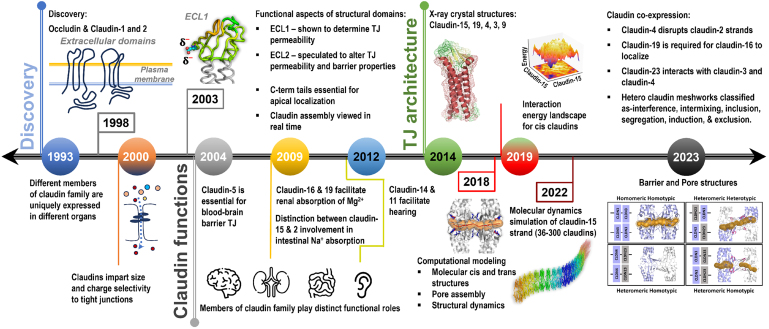
Key milestones in the discovery and functional roles of the claudin family of tight junction-forming proteins over the past 30 years. To see this figure in color, go online.
Several claudin family members do not have monoclonal antibodies capable of targeted binding to a specific claudin in the tight junction complex. However, the bacterium Clostridium perfringens enterotoxin (CPE) is known to bind to the extracellular domains of claudins to disrupt tight junction strands, especially claudin-3 and -4, inducing leaky pathways in the gut and causing general food poisoning (107,108,109). The extracellular CPE-claudin binding has been used as a claudin biomarker in tumor cells (109,110,111,112,113). For example, in pancreatic cancer, one of the most malignant human diseases, claudin-4 is upregulated and is a receptor for CPE. The CPE/claudin-4 binding is a promising therapeutic tool against pancreatic cancer (111).
These studies demonstrate that the claudin family is closely connected to the pathophysiology of various diseases. While disrupting the barrier can sometimes facilitate drug delivery, it can also inadvertently allow toxic molecules to pass through. The cases presented reveal that defects in claudins can significantly alter strand architecture, sometimes preventing their formation entirely, even due to changes in a single residue. Thus, understanding the specific roles of different claudins is critical and can provide valuable insights into potential therapeutic targets for treatment.
Discovery and functional diversity of claudins in tight junctions
The first tight junction protein discovered was ZO-1 in 1970 (41). However, the first tight junction transmembrane protein to be discovered was occludin in chicken liver cells by Furuse et al. in 1993 (10). Five years later, Furuse et al. found other peptide sequences from the original chicken liver cells that differed from occludin and later named them claudin-1 and -2 (Fig. 3). Derived from the Latin word “claudere” or “to close,” Furuse et al. characterized the first claudins to have occludin-like structures with four pass transmembrane helices and two extracellular loops. The hydropathy analysis of these proteins revealed the hydrophobic character of the first extracellular loop, implicating the loop for the barrier function of the tight junctions (114). Soon after, evidence of a few other claudin-like proteins emerged (114,115,116,117), and the investigations to understand the structure, function, and disease implications of claudins began (Fig. 3).
Members of the claudin family were found to have specific roles in the organ in which they are expressed. For example, claudin-5 is expressed in the blood capillaries in the brain (66), claudin-16 is expressed in the kidney nephrons (116,118), claudin-2 in the small intestines (119,120), and claudin-1 in the skin (65,67,121). Moreover, claudin-5 was believed to be a very specific endothelial claudin and a critical factor in conferring barrier properties at the blood-brain interface (66). In contrast, claudin-1 was thought to be a significant contributor to skin epithelial barrier function. Furuse et al. tested claudin-1’s barrier function by knocking out claudin-1 in rats to show that water was not retained in the skin, killing the rats soon after birth compared with their counterparts with intact claudin-1 gene (67). Experiments like this and others provided evidence for the role of claudin proteins in maintaining the barrier function in various tissues.
In contrast to sealing barrier function, claudins found in the kidney nephrons allowed Na+ and Mg2+ transport (66,122,123,124), demonstrating that claudin tight junctions were charge and size selective (Fig. 3), which was confirmed by additional experiments (1,55,116,118,120,122,123,125,126). Amasheh et al. found that claudin-2 expressed in kidney and gastrointestinal tissue is cation-selective (55). In parallel, Colegio et al. found that, in claudin-4, the pore-lining residue K65 has a substantial effect on the selectivity of ions passing through the paracellular space, presenting the first conclusive evidence that claudins directly influence the charge selectivity of the paracellular pathway (1). These discoveries helped recognize that the claudin family was diverse in individual function within the tight junctions.
Claudin-1, -3, and -5 tight junctions showed high resistance to permeability for all cations and anions (3,53,65,66,67,68,70,127,128,129,130,131,132,133,134,135). To further test this result, Nitta et al. created claudin-5-deficient mice and observed “loosened” tight junctions that allowed molecules <800 Da to diffuse through the blood-brain barrier tight junctions (53). Through these studies, claudin-1 and claudin-5 were classified as barrier-forming claudins. Van Itallie et al. showed that specific ECL1 residues influence paracellular permeability, like K65 in claudin-4 (56). The impact of ECL2 residues on permeability was observed, but the evidence is not as straightforward as for ECL1 residues. Later, Rossa et al. investigated TM3 and ECL2 residues for claudin-3 and -5 and found that specific charged and hydrophobic residues in TM3 helix and ECL2 impacted folding and assembly. This result demonstrated that ECL2 residues could contribute to dimer assemblies (136).
It was discovered that some tight junctions required the coexpression of two claudin family members for stable strand formation (103,104,137,138). For example, in the thick ascending limb of the nephron, hetero interactions between claudin-16 and -19 were found to be essential for forming tight junctions. Interestingly, claudin-16 functions as a cation pore, while claudin-19 functions as an anion barrier (137,139). When Hou et al. knocked down claudin-19, claudin-16 failed to form tight junctions, even though claudin-16’s expression level was unaltered (103). These results indicated that, despite the differing roles in paracellular permeability, maintaining a specific barrier can require more than a single claudin. The claudin-16/19 obligate hetero interactions are atypical compared with homo claudin-2 (25,55,56,58,59,140,141,142) or claudin-15 cation pores (1,3,56,143,144). Other examples of hetero interactions have been reported for anion pores formed by claudin-4 and -8 (138).
In contrast, claudin-11 and -14 did not form stable interaction partners (145). These coexpression rules can also be observed in heterotypic interactions. Daugherty et al. first investigated the significance of heterotypic interactions between claudin-1, -3, -4, and -5, finding that claudin-1 and -5 interact heterotypically with claudin-3 but not claudin-4. However, a single-point mutation, N44T in claudin-3, allowed heterotypic binding to claudin-4, showing that heterotypic binding can be highly residue specific (146). These observations demonstrated that the claudin family has coexpression rules, where specific hetero interactions are favored over others.
Moreover, there was a focus on examining the role of C-terminal tails and various posttranslationally modified claudins (2,30,128,147,148,149,150,151,152,153,154,155,156,157,158). Van Itallie et al. examined claudin-2 and -4 chimeras and found that replacing claudin-4’s shorter C-terminal tail with longer claudin-2’s tail promotes tight junction stability (148). Similarly, Rüffer and Gerke conducted an experiment that showed claudin-1 and -5 C-terminal tails were essential for apical localization (147). Furthermore, posttranslational modifications were examined, which included phosphorylation of claudin-1, -2, -3, -4, and -5 (128,149,151,152,153,154,155,156,158), palmitoylation of claudin-14 (30), and SUMOylation of claudin-2 (157). Later, Van Itallie and Henderson’s work demonstrated that claudins undergo posttranslational modifications such as palmitoylation and phosphorylation to correctly localize to the plasma membrane instead of sequestering in lysosomes (158).
These studies provided greater context on how the structure of claudin can affect its function in assembly and small-molecule transport. Because claudin is a multidomain protein, each domain showed an individual function. For instance, the extracellular loops greatly influence the selective permeability of the tight junctions, while the tails provide claudins with higher stability in the tight junction. It was also found that claudins were coexpressed in various tissues and dependent on other claudin family members to perform specific functions. Overall, these studies provided a deeper understanding of claudin structure, coexpression, and the introduction of live cell experiments.
Advances in claudin structure elucidation and their impact on understanding tight junctions
The elucidation of the x-ray crystal structures of some members of the claudin family has spurred a substantial increase in studies focused on tight junction proteins. These studies have collectively enhanced our understanding of the cell-cell adhesion complexes (Fig. 3). The first structure of a claudin family of proteins was reported in 2014. Suzuki et al. reported the structure of mouse claudin-15 (PDB: 4P79) at 2.4 Å resolution (74). In 2015, Saitoh et al. solved the structure for mouse claudin-19 (PDB: 3X29) at a resolution of 3.7 Å, incorporating the CPE with an S313A mutation (159). CPE was included to demonstrate precisely how enterotoxin can disrupt the epithelial barrier by binding to both extracellular domains of the claudin. It is worth noting that three membrane-proximal cysteines, responsible for palmitoylation, were replaced with alanine to facilitate crystallization. The mouse claudin-19 and -15 are structurally similar, except for the orientation of the extracellular domains and the longer length of β sheet 5 in mouse claudin-19 (159). Next, in 2016, Shinoda et al. solved the structure of human claudin-4 (PDB: 5B2G) at 3.5 Å, including CPE (160). In early 2019, mouse claudin-3 (PDB: 6AKE, 6AKF, and 6AKG) was solved at 3.6 Å by Nakamura et al. using CPE with a S313A mutant (161). This claudin is known to bind to CPE endogenously, although the mutation was included for thermostability (162). It was observed that mouse claudin-3, compared with other claudins, had a unique bend due to the presence of P134 in the TM3 helix. Point mutations, P134G and P134A, reduced or eliminated the bend, confirming the role of the proline residue in wild-type claudin-3. Because TM3 is usually the largest of the TMs, it is assumed that this bend can affect the cis and trans interactions. Nakamura speculated that this TM3 bend was significant in strand formation (161). Most recently, in 2019, Vecchio et al. solved the human claudin-9 crystal structure (PDB: 6OV2 and 6OV3) at 3.2 Å resolution, also with CPE (75).
In silico computational advances in studying claudins provide nanoscopic detail of claudin assembly and ion transport
The availability of molecular structures has enabled in silico modeling of a subset of claudins 1–5, 9, 10a, 10b, 15, 17, and 19 (28,32,38,39,44,46,62,64,72,73,74,75,76,77,79,80,81,159,160,161,163,164,165,166,167,168,169,170,171,172,173,174,175) and, more recently, nonclassic claudin-23 (40). Broadly, these studies have focused on conformational analysis of the homomeric assembly of claudin dimers and homotypic pore structures (28,38,40,44,46,64,72,73,77,79,80,81,160,163,164,165,166,167,168,169,170,171,173), ion permeability across the pores (28,38,39,40,44,62,64,72,76,77,80,163,166,174,175), and tight junction strand formation (28,38,40,46,72,79,80,81,159,163,164,165,166,167,168,169,170,171,173). The effect of lipid composition (73,81,167), posttranslational modifications (73), and point mutations on claudin assemblies have also been reported. The computational studies are based on static x-ray crystal structure of homology-modeled structures but offer insights into the dynamic nature of the claudins in the tight junction architecture. The simulations have been performed at all-atom, coarse-grain, hybrid resolution, nanometer-length scales, and microsecond timescales. Some of the computational advances have been summarized in earlier review articles (73,79,163).
Claudin-15 computational insights lead to initial pore channel predictions and strand assembly mechanisms
To date, mouse claudin-15 is computationally the most well-studied tight junction protein (Fig. 4). The mouse claudin-15 crystal structure (74) led to a pore model (166), referred to as the Suzuki model or pore I. To develop the model, Suzuki et al. utilized FFEM micrographs to determine the dimensions of the claudin-15 strand that had a width consistent with a claudin dimer. Then, using crystal structure conformation, Suzuki et al. proposed that claudins form an antiparallel double-row arrangement to create a pore channel through the claudin “wall.” Each pore is composed of homomeric-homotypic interaction of claudin-15 tetramers. Since then, the Suzuki model has been used as a general guideline for molecular simulations (40,64,76). Suzuki et al. hypothesized that claudin strand formation could be driven by residues found in the β sheet domains due to favorable hydrogen bonding, especially by incorporating the claudin’s extracellular helix found in ECL1. Alberini et al. utilized MD simulations to later characterize the size of the pore to be between 5 and 6 Å in diameter (77,78). This result was in agreement with a previous experimental study conducted on claudin-2, showing a 6.5 Å diameter at the narrowest point (176). Samanta et al. further investigated the claudin-15 pore using in silico mutational analysis. They identified the four aspartic acid residues (D55), located in the center of the pore, for facilitating the cation permeability (80). Fuladi et al. used MD simulations to investigate the dynamics of claudin-15 strands composed of 36–300 monomers and with length ranging from 30 to 225 nm over microsecond timescales (Fig. 3). They reported a persistence length of 137 nm, consistent with experimental studies (81).
Figure 4.
Molecular structures of selected members of the mouse and human claudin families. Side-view (top row) and zoomed-in views of extracellular loops (bottom row) of mouse claudin-3 (mClaudin-3), human claudin-4 (hClaudin-4), human claudin-9 (hClaudin-9), and mouse claudin-15 in ribbon representation, colored from N- (blue) to C-terminal (red). Stick representations show key residues involved in claudin-claudin assembly identified via biochemical studies or computational modeling. The color of the labels indicates the residue’s involvement in cis-only (blue), trans-only (yellow), and cis and trans (green) assembly. The pore-lining residues (magenta) and other residues with less defined involvement (black) are also shown. To see this figure in color, go online.
Claudin-5 computational insights develop a second pore model
A series of computational studies have investigated the role of claudin-5 tight junctions in selective permeability across the human blood-brain barrier. In 2016, Irudayanathan et al. reported the first homology-modeled structure of human claudin-5 built using mouse claudin-15 (167). This work demonstrated the dynamic assembly of claudin-5 into homomeric oligomers in lipid membranes in 10 μs simulations. This study reported five key dimer conformations that were repeatedly observed in the self-assembly simulations. The study also stipulated that the lipid membrane composition can influence strand formation. These simulations demonstrated that multiple dimer conformations are required for tight junction strand formation, not just the ones directly assembled using the static Suzuki model (167).
In a follow-up article, Irudayanathan et al. used computationally determined claudin-5 dimer conformations to characterize two pore models—pore I and II (76). As in the Suzuki model, claudin-5 pore I had ECL domains pointed toward each other in the pore’s center, resulting in a 1.0 nm diameter, whereas pore II had the ECL loops pointing outside the pore complex with a 0.8 nm diameter. This study established a second possible pore orientation for claudins, showing that barrier-forming claudin-3 and -5 can form pores (76). This study reaffirmed residues that contributed to the pore channel function. A follow-up by Rajagopal et al. looked at how pore I and II structures were unique to specific claudins (28). Rajagopal et al. also commented that TMs would be involved in assembly but not in selectivity concerning the pore channel. In 2022, Berselli et al. showed that both pores were water-permeable but disputed whether the pore II model could allow the transport of ions (72,174).
Each member of the claudin family has unique biophysical properties
A large body of in vitro, in vivo, and in silico studies have shown that each member of the claudin family has unique biophysical properties. For example, consider the tale of two claudins, 2 and 15, expressed in the intestinal tract to regulate paracellular ion flow (26,45,60,144,177,178,179,180,181,182,183,184). However, Ong et al. found that, in intestinal epithelial tissue, the expression levels of claudin-2 and -15 vary with age; young mice have higher claudin-2 expression than claudin-15, which reverses in adult mice (182). In 2017, Rosenthal et al. investigated claudin-2’s ability to transport cations and water concurrently through the same pore channel (26). Irudayanathan et al. also characterized claudin-2’s pore and showed that the pore II model has a 7.4 ± 1.1 Å diameter, wide enough to facilitate simultaneous paracellular cation and water flux (38). In 2020, Rosenthal et al. showed that, in contrast to claudin-2, the claudin-15 pore lacks the tandem movement of ion and water; the pore can permit either the cation’s or water’s transport at a time (45). These studies show that functionally similar claudins, localized in the same tissue, have different ion- and water-permeability mechanisms.
In 2016, Conrad et al. used homology-modeled structure and experiments to investigate residues that contribute to claudin-17’s anion selectivity. They showed that, as in claudin-2, the pore-lining residue in the 65th position, K65 in claudin-17 is critical to the channel function. Other residues—E44, E48, and R31 in ECL1—also contributed to claudin-17’s pore function. In addition, ECL2’s H154 showed involvement in anion permeability but not in forming homotypic interactions, dispelling the perception that ECL2 contributes only to homotypic interactions (64).
In another study, Irudayanathan et al. performed in silico strand assemblies of homomeric claudin-1, -2, -15, and -19, and found that the prevalent dimer conformations in the homomeric strand were different in each claudin (38). A similar conclusion was reported by Zhao et al. from a combined in vitro and in silico study of claudin-15 and -14. They showed that multiple homomeric interfaces are required for claudins to form flexible strands (46).
The concept of multiple homomeric interfaces was confirmed by Rajagopal et al. by developing a computational algorithm called PANEL (protein association energy landscape) (171). PANEL takes two claudin monomers (homo or hetero combination) embedded in a patch of lipid membrane as input to sample the 360° × 360° rotational space of the proteins to identify stable dimeric conformations that can potentially form tight junction strands (Fig. 3). This method requires an order of magnitude smaller computational cost or wall clock time compared with large self-assembly simulations. Initially used for claudin-5 (171), PANEL was later used to investigate claudin-2, -4, and -15 dimer conformation (44). In each homomeric system, different stable conformations emerged, demonstrating the structural basis of diversity in the claudin family. Rajagopal et al. further developed the concept of unique dimers by directly comparing claudin-5 and -15. They utilized these key dimers to create different double-row strand structures in silico. This study provided context to different strand behaviors among the claudin family members (28).
Tight junction meshwork morphologies and function depend on claudin coexpression
In the past 3 years, there has been increased focus on understanding claudin coexpression in different cells (19,37,40,43,183,185,186,187,188,189). To facilitate in vitro coexpression studies in epithelial cells, specialized Madin-Darby canine kidney (MDCK) I and II cell lines have been established with KOs of specific claudin genes (26,45,55,56,58,63,65,133,134,140,158,175,188,190,191). For example, claudin-4 KO MDCK I (C4KO) (43) or the quintuple knockout (MDCK) II (quinKO) cell line devoid of claudin-1, -2, -3, -4, and -7 (19,188). C4KO and QuinKO cell lines have enabled morphological and functional analysis of individual claudins and coexpression through exogenous reconstitution.
In a recent study, Shashikanth et al. examined claudin-4 coexpression with claudin-2, -7, -15, and -19 in live C4KO cells. The results showed that claudin-4 caused “interclaudin interference” in claudin-2, -7, -15, and -19 by disrupting the strand meshwork over time and increasing their mobile fractions at the tight junctions. In claudin-2, the interference of claudin-4 also reduced the cation permeability across the tight junction channels (43). In another study, Gonschior et al. examined the nanoscale architecture of tight junction strands formed by 26 mammalian claudins in live and fixed cells, including quinKO (19). The resulting tight junction strands were imaged using STED microscopy with a 20–200 nm spatial resolution (Fig. 3). Interestingly, only a small set of claudin family members could form their homotypic meshwork; others failed to self-assemble into detectable organized structures. More importantly, pairwise coexpression of claudins resulted in combinations with morphologically mixed or unmixed tight junction strand networks. The mixed strands showed colocalization of claudin pairs subdivided into intermixing, integration, and induction. In contrast, the lack of colocalization of claudin pairs resulted in unmixed strands, categorized as segregation and exclusion. For example, claudin-1 and -3 colocalized to create a peppered pattern in the strand meshwork, while claudin-11 and -3 avoided colocalization to form exclusive strand networks (19).
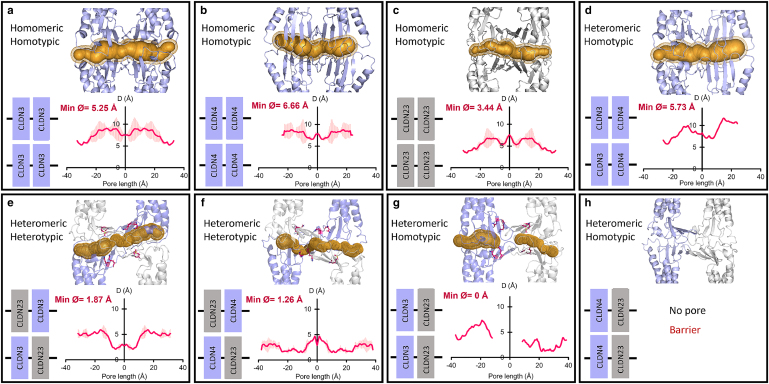
In another coexpression study, Raya-Sandino et al. examined the role of claudin-23, a nonclassic claudin with low sequence homology, with classic claudin-3 and -4 coexpressed in model human intestinal epithelial cells (SKCO15 and T84) (40). This work used a collaborative experimental and computational approach to examine these three claudins' epithelial barrier function contributions and provided molecular-level model pore architectures (Fig. 5). They showed that homomeric and homotypic claudin-3, -4, and -23 made pores of diameters 3.5–6.6 Å, while heterotypic interactions of claudin-23 with claudin-3 and -4 resulted in pore diameters less than 2 Å. Interestingly, pore formation was not observed in two heteromeric-homotypic cases—claudin-23/3 and claudin-23/4. This work showed that claudin-23 significantly alters gut permeability through interactions with other claudins coexpressed in gut epithelia (40).
Figure 5.
Pore diameters and structures of claudin-3, -4, and -23. (a–c) Homomeric-homotypic claudin-3/3, -4/4, and -23/23, (d) heteromeric-homotypic of claudin-3/4, and (e and f) heteromeric-heterotypic claudin-23/3 and claudin-23/4. (g and h) Claudin-23 does not form stable heteromeric-homotypic interactions with claudin-3 and -4. The secondary structure of claudins is shown in ribbon representation. The pore profile (gold) represents the pore diameter along the length of the pore. To see this figure in color, go online.
Summary and future directions
The last three decades have witnessed significant advances in understanding tight junction proteins and the molecular architecture that facilitates their function. We have discovered that no two claudins are the same on the structural and functional level. These differences can be attributed to the number and composition of each claudin’s primary amino acid sequence, which in turn affects the secondary and tertiary structures, ultimately leading to significant biophysical differences. For example, strand forming versus nonstrand forming, barrier versus pore-forming, cation versus anion permeable pores, concurrently versus sequential water and ion permeating, and homotypic coexpressing versus homomeric coexpressing. It is evident that tight junctions are critical in maintaining organ homeostasis, restricting toxins from crossing tissue barriers, and preventing cancer from metastasizing. Without regulated permeability, cells do not retain their correct function, which leads to various pathological conditions.
The next decade will rely heavily on computational modeling as the hardware cost continues to decline and the development of machine learning- and artificial intelligence-based algorithms accelerates. These advances will position simulations to match the length and timescales of in vitro and in vivo data. Continued advancements in imaging techniques, such as superresolution microscopy and live-cell imaging, will aid in studying tight junction dynamics in real time, enhancing our understanding of the spatiotemporal regulation of tight junctions. Overall, the key to understanding the complexity of the tight junction architecture lies in adopting synergistic experimental-computational approaches. The structural and functional insights from investigating claudin proteins will aid in engineering strategies to fight cancer and other debilitating diseases affecting many organ systems, including the kidney, intestines, ears, lungs, and brain.
Author contributions
P.M., N.R., and S.N. wrote the manuscript.
Acknowledgments
The National Science Foundation (NSF) CAREER CBET-1453312 and NSF-MCB-2221796 supported some of the computational work reviewed in this article. Computational support on Anton 2 was provided by the Pittsburgh Supercomputing Center (PSC) through grant R01GM116961 from the National Institutes of Health.
Declaration of interests
The authors declared no competing interests.
Editor: Meyer Jackson.
References
- 1.Colegio O.R., Van Itallie C.M., et al. Anderson J.M. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 2.Van Itallie C.M., Anderson J.M. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 3.Takehara M., Nishimura T., et al. Mizushima T. Effect of Claudin Expression on Paracellular Permeability, Migration and Invasion of Colonic Cancer Cells. Biol. Pharm. Bull. 2009;32:825–831. doi: 10.1248/bpb.32.825. [DOI] [PubMed] [Google Scholar]
- 4.Delva E., Tucker D.K., Kowalczyk A.P. The desmosome. Cold Spring Harbor Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meşe G., Richard G., White T.W. Gap Junctions: Basic Structure and Function. J. Invest. Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 6.Hartsock A., Nelson W.J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citi S. The mechanobiology of tight junctions. Biophys. Rev. 2019;11:783–793. doi: 10.1007/s12551-019-00582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen T.P., Otani T., et al. Furuse M. Tight junction membrane proteins regulate the mechanical resistance of the apical junctional complex. J. Cell Biol. 2024;223 doi: 10.1083/jcb.202307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farquhar M.G., Palade G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuse M., Hirase T., et al. Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuse M., Sasaki H., Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J. Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapierre L.A. The molecular structure of the tight junction. Adv. Drug Deliv. Rev. 2000;41:255–264. doi: 10.1016/s0169-409x(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 13.Anderson J.M. Molecular structure of tight junctions and their role in epithelial transport. News Physiol. Sci. 2001;16:126–130. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- 14.Niessen C.M. Tight junctions/adherens junctions: Basic structure and function. J. Invest. Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 15.Mruk D.D., Cheng C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015;36:564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 17.Pardridge W.M. Drug transport across the blood-brain barrier. J. Cerebr. Blood Flow Metabol. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu A.S.L. Claudins and the Kidney. J. Am. Soc. Nephrol. 2015;26:11–19. doi: 10.1681/ASN.2014030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonschior H., Schmied C., et al. Lehmann M. Nanoscale segregation of channel and barrier claudins enables paracellular ion flux. Nat. Commun. 2022;13:4985. doi: 10.1038/s41467-022-32533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claesson-Welsh L., Dejana E., McDonald D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021;27:314–331. doi: 10.1016/j.molmed.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Günzel D., Yu A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukendi C., Dean N., et al. Nikitina N.V. Evolution of the vertebrate claudin gene family: insights from a basal vertebrate, the sea lamprey. Int. J. Dev. Biol. 2016;60:39–51. doi: 10.1387/ijdb.150364nn. [DOI] [PubMed] [Google Scholar]
- 23.Krause G., Winkler L., et al. Blasig I.E. Structure and function of claudins. Biochim. Biophys. Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Liu F., Koval M., et al. Baker M.S. Systems Proteomics View of the Endogenous Human Claudin Protein Family. J. Proteome Res. 2016;15:339–359. doi: 10.1021/acs.jproteome.5b00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou J., Rajagopal M., Yu A.S. Claudins and the Kidney. Annu. Rev. Physiol. 2013;75:479–501. doi: 10.1146/annurev-physiol-030212-183705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenthal R., Günzel D., et al. Yu A.S.L. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2017;219:521–536. doi: 10.1111/apha.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meoli L., Günzel D. Channel functions of claudins in the organization of biological systems. Biochim. Biophys. Acta Biomembr. 2020;1862:183344. doi: 10.1016/j.bbamem.2020.183344. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopal N., Nangia S. Unique structural features of claudin-5 and claudin-15 lead to functionally distinct tight junction strand architecture. Ann. N. Y. Acad. Sci. 2022;1517:225–233. doi: 10.1111/nyas.14891. [DOI] [PubMed] [Google Scholar]
- 29.Ji J., Carpentier B., et al. Nangia S. An Affordable Topography-Based Protocol for Assigning a Residue’s Character on a Hydropathy (PARCH) Scale. J. Chem. Theor. Comput. 2023 doi: 10.1021/acs.jctc.3c00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Itallie C.M., Gambling T.M., et al. Anderson J.M. Palmitoylation of claudins is required for efficient tight-junction localization. J. Cell Sci. 2005;118:1427–1436. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 31.Heiler S., Mu W., et al. Thuma F. The importance of claudin-7 palmitoylation on membrane subdomain localization and metastasis-promoting activities. Cell Commun. Signal. 2015;13:29. doi: 10.1186/s12964-015-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajagopal N., Irudayanathan F.J., Nangia S. Palmitoylation of Claudin-5 Proteins Influences Their Lipid Domain Affinity and Tight Junction Assembly at the Blood–Brain Barrier Interface. J. Phys. Chem. B. 2019;123:983–993. doi: 10.1021/acs.jpcb.8b09535. [DOI] [PubMed] [Google Scholar]
- 33.Nomme J., Antanasijevic A., et al. Lavie A. Structural Basis of a Key Factor Regulating the Affinity between the Zonula Occludens First PDZ Domain and Claudins. J. Biol. Chem. 2015;290:16595–16606. doi: 10.1074/jbc.M115.646695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shigetomi K., Ono Y., et al. Ikenouchi J. Cholesterol-rich domain formation mediated by ZO proteins is essential for tight junction formation. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2217561120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigetomi K., Ono Y., et al. Ikenouchi J. Adherens junctions influence tight junction formation via changes in membrane lipid composition. J. Cell Biol. 2018;217:2373–2381. doi: 10.1083/jcb.201711042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Itallie C.M., Tietgens A.J., Anderson J.M. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol. Biol. Cell. 2017;28:524–534. doi: 10.1091/mbc.E16-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suarez-Artiles L., Breiderhoff T., et al. Dittmar G. Pan-claudin family interactome analysis reveals shared and specific interactions. Cell Rep. 2022;41:111588. doi: 10.1016/j.celrep.2022.111588. [DOI] [PubMed] [Google Scholar]
- 38.Irudayanathan F.J., Wang X., et al. Nangia S. Self-Assembly Simulations of Classic Claudins—Insights into the Pore Structure, Selectivity, and Higher Order Complexes. J. Phys. Chem. B. 2018;122:7463–7474. doi: 10.1021/acs.jpcb.8b03842. [DOI] [PubMed] [Google Scholar]
- 39.Irudayanathan F.J., Nangia S. Paracellular Gatekeeping: What Does It Take for an Ion to Pass Through a Tight Junction Pore? Langmuir. 2020;36:6757–6764. doi: 10.1021/acs.langmuir.0c00877. [DOI] [PubMed] [Google Scholar]
- 40.Raya-Sandino A., Lozada-Soto K.M., et al. Nusrat A. Claudin-23 reshapes epithelial tight junction architecture to regulate barrier function. Nat. Commun. 2023;14:6214. doi: 10.1038/s41467-023-41999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalcroft J.P., Bullivant S. An Interpretation of Liver Cell Membrane and Junction Structure Based on Observation of Freeze-Fracture Replicas of both Sides of the Fracture. J. Cell Biol. 1970;47:49–60. doi: 10.1083/jcb.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claude P., Goodenough D.A. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J. Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shashikanth N., France M.M., et al. Turner J.R. Tight junction channel regulation by interclaudin interference. Nat. Commun. 2022;13:3780. doi: 10.1038/s41467-022-31587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajagopal N., Durand A.J., Nangia S. Predicting selectivity of paracellular pores for biomimetic applications. Mol. Syst. Des. Eng. 2020;5:686–696. [Google Scholar]
- 45.Rosenthal R., Günzel D., et al. Fromm M. Claudin-15 forms a water channel through the tight junction with distinct function compared to claudin-2. Acta Physiol. 2020;228 doi: 10.1111/apha.13334. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J., Krystofiak E.S., et al. Kachar B. Multiple claudin–claudin cis interfaces are required for tight junction strand formation and inherent flexibility. Commun. Biol. 2018;1:50. doi: 10.1038/s42003-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartle E.I., Rao T.C., et al. Mattheyses A.L. Bridging the gap: Super-resolution microscopy of epithelial cell junctions. Tissue Barriers. 2018;6 doi: 10.1080/21688370.2017.1404189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki H., Matsui C., et al. Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc. Natl. Acad. Sci. USA. 2003;100:3971–3976. doi: 10.1073/pnas.0630649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen L., Weber C.R., Turner J.R. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capaldo C.T., Farkas A.E., et al. Nusrat A. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol. Biol. Cell. 2014;25:2710–2719. doi: 10.1091/mbc.E14-02-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasan B., Kolli A.R., et al. Hickman J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. Jala. 2015;20:107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furuse M., Sasaki H., et al. Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nitta T., Hata M., et al. Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coyne C.B., Gambling T.M., et al. Johnson L.G. Role of claudin interactions in airway tight junctional permeability. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;285:L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 55.Amasheh S., Meiri N., et al. Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 56.Van Itallie C.M., Fanning A.S., Anderson J.M. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am. J. Physiol. Ren. Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 57.Van Itallie C.M., Rogan S., et al. Anderson J.M. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Ren. Physiol. 2006;291:F1288–F1299. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 58.Rosenthal R., Milatz S., et al. Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 59.Muto S., Furuse M., Kusano E. Claudins and renal salt transport. Clin. Exp. Nephrol. 2012;16:61–67. doi: 10.1007/s10157-011-0491-4. [DOI] [PubMed] [Google Scholar]
- 60.Rosenthal R., Günzel D., et al. Fromm M. Water channels and barriers formed by claudins. Ann. N. Y. Acad. Sci. 2017;1397:100–109. doi: 10.1111/nyas.13383. [DOI] [PubMed] [Google Scholar]
- 61.Milatz S. A Novel Claudinopathy Based on Claudin-10 Mutations. Int. J. Mol. Sci. 2019;20:5396. doi: 10.3390/ijms20215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagarajan S.K., Klein S., et al. Piontek J. Claudin-10b cation channels in tight junction strands: Octameric-interlocked pore barrels constitute paracellular channels with low water permeability. Comput. Struct. Biotechnol. J. 2023;21:1711–1727. doi: 10.1016/j.csbj.2023.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krug S.M., Günzel D., et al. Fromm M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell. Mol. Life Sci. 2012;69:2765–2778. doi: 10.1007/s00018-012-0949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conrad M.P., Piontek J., et al. Krug S.M. Molecular basis of claudin-17 anion selectivity. Cell. Mol. Life Sci. 2016;73:185–200. doi: 10.1007/s00018-015-1987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inai T., Kobayashi J., Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur. J. Cell Biol. 1999;78:849–855. doi: 10.1016/S0171-9335(99)80086-7. [DOI] [PubMed] [Google Scholar]
- 66.Morita K., Sasaki H., et al. Tsukita S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furuse M., Hata M., et al. Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amasheh S., Schmidt T., et al. Fromm M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 69.Mrsny R.J., Brown G.T., et al. Nusrat A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am. J. Pathol. 2008;172:905–915. doi: 10.2353/ajpath.2008.070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirschner N., Houdek P., et al. Brandner J.M. Tight junctions form a barrier in human epidermis. Eur. J. Cell Biol. 2010;89:839–842. doi: 10.1016/j.ejcb.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 71.Bardet C., Ribes S., et al. Chaussain C. Claudin Loss-of-Function Disrupts Tight Junctions and Impairs Amelogenesis. Front. Physiol. 2017;8:326. doi: 10.3389/fphys.2017.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berselli A., Alberini G., et al. Maragliano L. Computational Assessment of Different Structural Models for Claudin-5 Complexes in Blood-Brain Barrier Tight Junctions. ACS Chem. Neurosci. 2022;13:2140–2153. doi: 10.1021/acschemneuro.2c00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajagopal N., Irudayanathan F.J., Nangia S. Computational Nanoscopy of Tight Junctions at the Blood-Brain Barrier Interface. Int. J. Mol. Sci. 2019;20:5583. doi: 10.3390/ijms20225583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suzuki H., Nishizawa T., et al. Fujiyoshi Y. Crystal Structure of a Claudin Provides Insight into the Architecture of Tight Junctions. Science. 2014;344:304–307. doi: 10.1126/science.1248571. [DOI] [PubMed] [Google Scholar]
- 75.Vecchio A.J., Stroud R.M. Claudin-9 structures reveal mechanism for toxin-induced gut barrier breakdown. Proc. Natl. Acad. Sci. USA. 2019;116:17817–17824. doi: 10.1073/pnas.1908929116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irudayanathan F.J., Wang N., et al. Nangia S. Architecture of the paracellular channels formed by claudins of the blood–brain barrier tight junctions. Ann. N. Y. Acad. Sci. 2017;1405:131–146. doi: 10.1111/nyas.13378. [DOI] [PubMed] [Google Scholar]
- 77.Alberini G., Benfenati F., Maragliano L. A refined model of claudin-15 tight junction paracellular architecture by molecular dynamics simulations. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alberini G., Benfenati F., Maragliano L. Molecular Dynamics Simulations of Ion Selectivity in a Claudin-15 Paracellular Channel. J. Phys. Chem. B. 2018;122:10783–10792. doi: 10.1021/acs.jpcb.8b06484. [DOI] [PubMed] [Google Scholar]
- 79.Berselli A., Benfenati F., et al. Alberini G. Multiscale modelling of claudin-based assemblies: A magnifying glass for novel structures of biological interfaces. Comput. Struct. Biotechnol. J. 2022;20:5984–6010. doi: 10.1016/j.csbj.2022.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samanta P., Wang Y., et al. Khalili-Araghi F. Molecular determination of claudin-15 organization and channel selectivity. J. Gen. Physiol. 2018;150:949–968. doi: 10.1085/jgp.201711868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuladi S., McGuinness S., et al. Khalili-Araghi F. Molecular mechanism of claudin-15 strand flexibility: A computational study. J. Gen. Physiol. 2022;154 doi: 10.1085/jgp.202213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McGuinness S., Sajjadi S., et al. Khalili-Araghi F. Computational Models of Claudin Assembly in Tight Junctions and Strand Properties. Int. J. Mol. Sci. 2024;25:3364. doi: 10.3390/ijms25063364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morin P.J. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 84.Swisshelm K., Macek R., Kubbies M. Role of claudins in tumorigenesis. Adv. Drug Deliv. Rev. 2005;57:919–928. doi: 10.1016/j.addr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Lal-Nag M., Morin P.J. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ouban A., Ahmed A.A. Claudins in human cancer: A review. Histol. Histopathol. 2010;25:83–90. doi: 10.14670/HH-25.83. [DOI] [PubMed] [Google Scholar]
- 87.Wang D.W., Zhang W.H., et al. Hu J.K. The role and mechanism of claudins in cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1051497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mullard A. Claudin-18.2 attracts the cancer crowd. Nat. Rev. Drug Discov. 2023;22:683–686. doi: 10.1038/d41573-023-00120-x. [DOI] [PubMed] [Google Scholar]
- 89.Horowitz A., Chanez-Paredes S.D., et al. Turner J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023;20:417–432. doi: 10.1038/s41575-023-00766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ouban A., Arabi T.Z. Expression of Claudins in Preneoplastic Conditions of the Gastrointestinal Tract: A Review. Cancers. 2023;15:4095. doi: 10.3390/cancers15164095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan C., Xu A., et al. Chen C. Research progress of Claudin-low breast cancer. Front. Oncol. 2023;13:1226118. doi: 10.3389/fonc.2023.1226118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng A., Yuan F., et al. Deng H. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J. Virol. 2007;81:12465–12471. doi: 10.1128/JVI.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartosch B., Dubuisson J. Recent Advances in Hepatitis C Virus Cell Entry. Viruses. 2010;2:692–709. doi: 10.3390/v2030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torres-Flores J.M., Arias C.F. Tight Junctions Go Viral. Viruses. 2015;7:5145–5154. doi: 10.3390/v7092865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gow A., Davies C., et al. Kachar B. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J. Neurosci. 2004;24:7051–7062. doi: 10.1523/JNEUROSCI.1640-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hou J.H. In: Annals of the New York Academy of Sciences, Barriers and Channels Formed by Tight Junction Proteins Ii. Fromm M., Schulzke J.D., editors. 2012. The yin and yang of claudin-14 function in human diseases; pp. 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sineni C.J., Yildirim-Baylan M., et al. Tekin M. A truncating CLDN9 variant is associated with autosomal recessive nonsyndromic hearing loss. Hum. Genet. 2019;138:1071–1075. doi: 10.1007/s00439-019-02037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bertiaux-Vandaële N., Youmba S.B., et al. Coëffier M. The Expression and the Cellular Distribution of the Tight Junction Proteins Are Altered in Irritable Bowel Syndrome Patients With Differences According to the Disease Subtype. Am. J. Gastroenterol. 2011;106:2165–2173. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 99.Das P., Goswami P., et al. Makharia G.K. Comparative tight junction protein expressions in colonic Crohn's disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch. 2012;460:261–270. doi: 10.1007/s00428-012-1195-1. [DOI] [PubMed] [Google Scholar]
- 100.Vazquez-Roque M.I., Camilleri M., et al. Zinsmeister A.R. A Controlled Trial of Gluten-Free Diet in Patients With Irritable Bowel Syndrome-Diarrhea: Effects on Bowel Frequency and Intestinal Function. Gastroenterology. 2013;144:903–911.e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barmeyer C., Schulzke J.D., Fromm M. Claudin-related intestinal diseases. Semin. Cell Dev. Biol. 2015;42:30–38. doi: 10.1016/j.semcdb.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 102.De Benedetto A., Rafaels N.M., et al. Beck L.A. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011;127:773–786.e867. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hou J., Renigunta A., et al. Goodenough D.A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. USA. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hou J.H., Konrad M. In: Current Topics in Membranes. Yu A.S.L., editor. Claudins; 2010. Claudins and Renal Magnesium Handling; pp. 151–176. [Google Scholar]
- 105.Ben-Yosef T., Belyantseva I.A., et al. Friedman T.B. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 106.Mazaud-Guittot S., Meugnier E., et al. Le Magueresse-Battistoni B. Claudin 11 Deficiency in Mice Results in Loss of the Sertoli Cell Epithelial Phenotype in the Testis. Biol. Reprod. 2010;82:202–213. doi: 10.1095/biolreprod.109.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mitchell L.A., Koval M. Specificity of Interaction between Clostridium perfringens Enterotoxin and Claudin-Family Tight Junction Proteins. Toxins. 2010;2:1595–1611. doi: 10.3390/toxins2071595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veshnyakova A., Protze J., et al. Piontek J. On the Interaction of Clostridium perfringens Enterotoxin with Claudins. Toxins. 2010;2:1336–1356. doi: 10.3390/toxins2061336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freedman J.C., Shrestha A., McClane B.A. Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxins. 2016;8:73. doi: 10.3390/toxins8030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuan X., Lin X., et al. Howell S.B. Recombinant CPE fused to tumor necrosis factor targets human ovarian cancer cells expressing the claudin-3 and claudin-4 receptors. Mol. Cancer Therapeut. 2009;8:1906–1915. doi: 10.1158/1535-7163.MCT-09-0106. [DOI] [PubMed] [Google Scholar]
- 111.Neesse A., Griesmann H., et al. Michl P. Claudin-4 as therapeutic target in cancer. Arch. Biochem. Biophys. 2012;524:64–70. doi: 10.1016/j.abb.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 112.Shrestha A., Uzal F.A., McClane B.A. The interaction of Clostridium perfringens enterotoxin with receptor claudins. Anaerobe. 2016;41:18–26. doi: 10.1016/j.anaerobe.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Safaei S., Imani M. Computational design ofa chimeric toxin against Claudin-4-expressing cancer cells: molecular modeling, docking and molecular dynamics simulation analysis. Vet. Res. Forum. 2023;14:259–265. doi: 10.30466/vrf.2022.548415.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Furuse M., Fujita K., et al. Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morita K., Furuse M., et al. Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simon D.B., Lu Y., et al. Lifton R.P. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 117.Niimi T., Nagashima K., et al. Kimura S. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol. Cell Biol. 2001;21:7380–7390. doi: 10.1128/MCB.21.21.7380-7390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mitic L.L., van Itallie C.M., Anderson J.M. Molecular Physiology and Pathophysiology of Tight Junctions - I. Tight junction structure and function: lessons from mutant animals and proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 119.Kinugasa T., Sakaguchi T., et al. Reinecker H.C. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 120.Rahner C., Mitic L.L., Anderson J.M. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 121.Brandner J.M., Kief S., et al. Moll I. Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur. J. Cell Biol. 2002;81:253–263. doi: 10.1078/0171-9335-00244. [DOI] [PubMed] [Google Scholar]
- 122.Enck A.H., Berger U.V., Yu A.S. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am. J. Physiol. Ren. Physiol. 2001;281:F966–F974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 123.Kollmar R., Nakamura S.K., et al. Hudspeth A.J. Expression and phylogeny of claudins in vertebrate primordia. Proc. Natl. Acad. Sci. USA. 2001;98:10196–10201. doi: 10.1073/pnas.171325898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kiuchi-Saishin Y., Gotoh S., et al. Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J. Am. Soc. Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 125.Furuse M., Furuse K., et al. Tsukita S. Conversion of Zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Itallie C., Rahner C., Anderson J.M. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J. Clin. Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sonoda N., Furuse M., et al. Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands:: Evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soma T., Chiba H., et al. Sawada N. Thr207 of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp. Cell Res. 2004;300:202–212. doi: 10.1016/j.yexcr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 129.Wen H., Watry D.D., et al. Fox H.S. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol. Cell Biol. 2004;24:8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neuhaus W., Wirth M., et al. Noe C.R. Expression of Claudin-1, Claudin-3 and Claudin-5 in human blood-brain barrier mimicking cell line ECV304 is inducible by glioma-conditioned media. Neurosci. Lett. 2008;446:59–64. doi: 10.1016/j.neulet.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 131.Malina K.C.K., Cooper I., Teichberg V.I. Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Res. 2009;1284:12–21. doi: 10.1016/j.brainres.2009.05.072. [DOI] [PubMed] [Google Scholar]
- 132.Burek M., Arias-Loza P.A., et al. Förster C.Y. Claudin-5 as a Novel Estrogen Target in Vascular Endothelium. Arterioscler. Thromb. Vasc. Biol. 2010;30:298–304. doi: 10.1161/ATVBAHA.109.197582. [DOI] [PubMed] [Google Scholar]
- 133.Milatz S., Krug S.M., et al. Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim. Biophys. Acta. 2010;1798:2048–2057. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 134.Piehl C., Piontek J., et al. Blasig I.E. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell. Mol. Life Sci. 2010;67:2131–2140. doi: 10.1007/s00018-010-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fan L., Liu Y., et al. Zhang H. Increasing of Blood-tumor Barrier Permeability through Paracellular Pathway by Low-frequency Ultrasound Irradiation In Vitro. J. Mol. Neurosci. 2011;43:541–548. doi: 10.1007/s12031-010-9479-x. [DOI] [PubMed] [Google Scholar]
- 136.Rossa J., Ploeger C., et al. Piontek J. Claudin-3 and Claudin-5 Protein Folding and Assembly into the Tight Junction Are Controlled by Non-conserved Residues in the Transmembrane 3 (TM3) and Extracellular Loop 2 (ECL2) Segments. J. Biol. Chem. 2014;289:7641–7653. doi: 10.1074/jbc.M113.531012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hou J., Renigunta A., et al. Goodenough D.A. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hou J., Renigunta A., et al. Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl. Acad. Sci. USA. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hou J., Paul D.L., Goodenough D.A. Paracellin-1 and the modulation of ion selectivity of tight junctions. J. Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 140.Van Itallie C.M., Mitic L.L., Anderson J.M. Claudin-2 Forms Homodimers and Is a Component of a High Molecular Weight Protein Complex. J. Biol. Chem. 2011;286:3442–3450. doi: 10.1074/jbc.M110.195578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weber C.R., Nalle S.C., et al. Turner J.R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Invest. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li J., Angelow S., et al. Yu A.S.L. Claudin-2 pore function requires an intramolecular disulfide bond between two conserved extracellular cysteines. Am. J. Physiol. Cell Physiol. 2013;305:C190–C196. doi: 10.1152/ajpcell.00074.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sengoku A., Inai T., Shibata Y. Formation of aberrant TJ strands by overexpression of claudin-15 in MDCK II cells. Histochem. Cell Biol. 2008;129:211–222. doi: 10.1007/s00418-007-0354-y. [DOI] [PubMed] [Google Scholar]
- 144.Tamura A., Hayashi H., et al. Tsukita S. Loss of Claudin-15, but Not Claudin-2, Causes Na+ Deficiency and Glucose Malabsorption in Mouse Small Intestine. Gastroenterology. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 145.Elkouby-Naor L., Abassi Z., et al. Ben-Yosef T. Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res. 2008;333:427–438. doi: 10.1007/s00441-008-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Daugherty B.L., Ward C., et al. Koval M. Regulation of heterotypic claudin compatibility. J. Biol. Chem. 2007;282:30005–30013. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 147.Rüffer C., Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur. J. Cell Biol. 2004;83:135–144. doi: 10.1078/0171-9335-00366. [DOI] [PubMed] [Google Scholar]
- 148.Van Itallie C.M., Colegio O.R., Anderson J.M. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J. Membr. Biol. 2004;199:29–38. doi: 10.1007/s00232-004-0673-z. [DOI] [PubMed] [Google Scholar]
- 149.D'Souza T., Agarwal R., Morin P.J. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J. Biol. Chem. 2005;280:26233–26240. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 150.Arabzadeh A., Troy T.C., Turksen K. Role of the Cldn6 cytoplasmic tail domain in membrane targeting and epidermal differentiation in vivo. Mol. Cell Biol. 2006;26:5876–5887. doi: 10.1128/MCB.02342-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aono S., Hirai Y. Phosphorylation of claudin-4 is required for tight junction formation in a human keratinocyte cell line. Exp. Cell Res. 2008;314:3326–3339. doi: 10.1016/j.yexcr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 152.Ikari A., Ito M., et al. Miwa M. Claudin-16 is directly phosphorylated by protein kinase A independently of a vasodilator-stimulated phosphoprotein-mediated pathway. J. Cell. Physiol. 2008;214:221–229. doi: 10.1002/jcp.21178. [DOI] [PubMed] [Google Scholar]
- 153.Yamamoto M., Ramirez S.H., et al. Ikezu T. Phosphorylation of claudin-5 and occludin by Rho kinase in brain endothelial cells. Am. J. Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ikari A., Kinjo K., et al. Sugatani J. Extracellular Mg2+ regulates the tight junctional localization of claudin-16 mediated by ERK-dependent phosphorylation. Biochim. Biophys. Acta. 2010;1798:415–421. doi: 10.1016/j.bbamem.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 155.Ahmad W., Shabbiri K., et al. Hassan S. Claudin-1 required for HCV virus entry has high potential for phosphorylation and O-glycosylation. Virol. J. 2011;8:229. doi: 10.1186/1743-422X-8-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Butt A.M., Khan I.B., et al. Tong Y. Role of post translational modifications and novel crosstalk between phosphorylation and O-beta-GlcNAc modifications in human claudin-1,-3 and-4. Mol. Biol. Rep. 2012;39:1359–1369. doi: 10.1007/s11033-011-0870-7. [DOI] [PubMed] [Google Scholar]
- 157.Van Itallie C.M., Mitic L.L., Anderson J.M. In: Annals of the New York Academy of Sciences, Barriers and Channels Formed by Tight Junction Proteins Ii. Fromm M., Schulzke J.D., editors. 2012. SUMOylation of claudin-2; pp. 60–64. [DOI] [PubMed] [Google Scholar]
- 158.Van Itallie C.M., Tietgens A.J., et al. Anderson J.M. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. J. Cell Sci. 2012;125:4902–4912. doi: 10.1242/jcs.111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saitoh Y., Suzuki H., et al. Fujiyoshi Y. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science. 2015;347:775–778. doi: 10.1126/science.1261833. [DOI] [PubMed] [Google Scholar]
- 160.Shinoda T., Shinya N., et al. Shirouzu M. Structural basis for disruption of claudin assembly in tight junctions by an enterotoxin. Sci. Rep. 2016;6:33632. doi: 10.1038/srep33632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakamura S., Irie K., et al. Fujiyoshi Y. Morphologic determinant of tight junctions revealed by claudin-3 structures. Nat. Commun. 2019;10:816. doi: 10.1038/s41467-019-08760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakamura S., Fujiyoshi Y., Irie K. Enhancement of the thermostability of mouse claudin-3 on complex formation with the carboxyl-terminal region of Clostridium perfringens enterotoxin improves crystal quality. Acta Crystallogr. F Struct. Biol. Commun. 2018;74:150–155. doi: 10.1107/S2053230X18002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krause G., Protze J., Piontek J. Assembly and function of claudins: Structure-function relationships based on homology models and crystal structures. Semin. Cell Dev. Biol. 2015;42:3–12. doi: 10.1016/j.semcdb.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 164.Milatz S., Piontek J., et al. Günzel D. Probing the cis-arrangement of prototype tight junction proteins claudin-1 and claudin-3. Biochem. J. 2015;468:449–458. doi: 10.1042/BJ20150148. [DOI] [PubMed] [Google Scholar]
- 165.Protze J., Eichner M., et al. Krause G. Directed structural modification of Clostridium perfringens enterotoxin to enhance binding to claudin-5. Cell. Mol. Life Sci. 2015;72:1417–1432. doi: 10.1007/s00018-014-1761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Suzuki H., Tani K., et al. Fujiyoshi Y. Model for the Architecture of Claudin-Based Paracellular Ion Channels through Tight Junctions. J. Mol. Biol. 2015;427:291–297. doi: 10.1016/j.jmb.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 167.Irudayanathan F.J., Trasatti J.P., et al. Nangia S. Molecular Architecture of the Blood Brain Barrier Tight Junction Proteins-A Synergistic Computational and In Vitro Approach. J. Phys. Chem. B. 2016;120:77–88. doi: 10.1021/acs.jpcb.5b09977. [DOI] [PubMed] [Google Scholar]
- 168.Fromm M., Piontek J., et al. Krug S.M. Tight junctions of the proximal tubule and their channel proteins. Pflügers Archiv. 2017;469:877–887. doi: 10.1007/s00424-017-2001-3. [DOI] [PubMed] [Google Scholar]
- 169.Milatz S., Piontek J., et al. Günzel D. Tight junction strand formation by claudin-10 isoforms and claudin-10a/-10b chimeras. Ann. N. Y. Acad. Sci. 2017;1405:102–115. doi: 10.1111/nyas.13393. [DOI] [PubMed] [Google Scholar]
- 170.Piontek A., Rossa J., et al. Piontek J. Polar and charged extracellular residues conserved among barrier-forming claudins contribute to tight junction strand formation. Ann. N. Y. Acad. Sci. 2017;1397:143–156. doi: 10.1111/nyas.13341. [DOI] [PubMed] [Google Scholar]
- 171.Rajagopal N., Nangia S. Obtaining Protein Association Energy Landscape for Integral Membrane Proteins. J. Chem. Theor. Comput. 2019;15:6444–6455. doi: 10.1021/acs.jctc.9b00626. [DOI] [PubMed] [Google Scholar]
- 172.Man V.H., Li M.S., et al. Nguyen P.H. Molecular mechanism of ultrasound interaction with a blood brain barrier model. J. Chem. Phys. 2020;153:045104. doi: 10.1063/5.0010667. [DOI] [PubMed] [Google Scholar]
- 173.Piontek J., Krug S.M., et al. Fromm M. Molecular architecture and assembly of the tight junction backbone. Biochim. Biophys. Acta Biomembr. 2020;1862:183279. doi: 10.1016/j.bbamem.2020.183279. [DOI] [PubMed] [Google Scholar]
- 174.Berselli A., Alberini G., et al. Maragliano L. Computational study of ion permeation through claudin-4 paracellular channels. Ann. N. Y. Acad. Sci. 2022;1516:162–174. doi: 10.1111/nyas.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hempel C., Rosenthal R., et al. Piontek J. Tight junction channels claudin-10b and claudin-15: Functional mapping of pore-lining residues. Ann. N. Y. Acad. Sci. 2022;1515:129–142. doi: 10.1111/nyas.14794. [DOI] [PubMed] [Google Scholar]
- 176.Yu A.S.L., Cheng M.H., et al. Coalson R.D. Molecular Basis for Cation Selectivity in Claudin-2-based Paracellular Pores: Identification of an Electrostatic Interaction Site. J. Gen. Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aung P.P., Mitani Y., et al. Yasui W. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Arch. 2006;448:428–434. doi: 10.1007/s00428-005-0120-2. [DOI] [PubMed] [Google Scholar]
- 178.Tamura A., Kitano Y., et al. Tsukita S. Megaintestine in claudin-15-deficient mice. Gastroenterology. 2008;134:523–534. doi: 10.1053/j.gastro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 179.Luettig J., Rosenthal R., et al. Schulzke J.D. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3 doi: 10.4161/21688370.2014.977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Radloff J., Falchuk E.L., et al. Amasheh S. Molecular Characterization of Barrier Properties in Follicle-Associated Epithelium of Porcine Peyer's Patches Reveals Major Sealing Function of Claudin-4. Front. Physiol. 2017;8:579. doi: 10.3389/fphys.2017.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tanaka H., Tamura A., et al. Tsukita S. Site-specific distribution of claudin-based paracellular channels with roles in biological fluid flow and metabolism. Ann. N. Y. Acad. Sci. 2017;1405:44–52. doi: 10.1111/nyas.13438. [DOI] [PubMed] [Google Scholar]
- 182.Ong M.L.D.M., Yeruva S., et al. Turner J.R. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab. Invest. 2020;100:483–490. doi: 10.1038/s41374-019-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Beggs M.R., Young K., et al. Alexander R.T. Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2111247118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hempstock W., Nagata N., et al. Hayashi H. The effect of claudin-15 deletion on cationic selectivity and transport in paracellular pathways of the cecum and large intestine. Sci. Rep. 2023;13:6799. doi: 10.1038/s41598-023-33431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Curry J.N., Tokuda S., et al. Yu A.S.L. Combinatorial expression of claudins in the proximal renal tubule and its functional consequences. Am. J. Physiol. Ren. Physiol. 2020;318:F1138–F1146. doi: 10.1152/ajprenal.00057.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Negri A.L., Del Valle E.E. Role of claudins in idiopathic hypercalciuria and renal lithiasis. Int. Urol. Nephrol. 2022;54:2197–2204. doi: 10.1007/s11255-022-03119-2. [DOI] [PubMed] [Google Scholar]
- 187.Dimke H., Griveau C., Prot-Bertoye C., et al. Claudin-19 localizes to the thick ascending limb where its expression is required for junctional claudin-16 localization. Ann. N. Y. Acad. Sci. 2023;1526:126–137. doi: 10.1111/nyas.15014. [DOI] [PubMed] [Google Scholar]
- 188.Furuse M., Nakatsu D., et al. Hayashi H. Reconstitution of functional tight junctions with individual claudin subtypes in epithelial cells. Cell Struct. Funct. 2023;48:1–17. doi: 10.1247/csf.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saito A.C., Higashi T., Chiba H. Tight-junction strand networks and tightness of the epithelial barrier. Microscopy. 2023;72:213–225. doi: 10.1093/jmicro/dfad008. [DOI] [PubMed] [Google Scholar]
- 190.McCrea H.J., Van Itallie C.M., Anderson J.M. Claudin-15 increases TER in MDCK cells. Mol. Biol. Cell. 2001;12:220A. [Google Scholar]
- 191.Naser A.N., Guiler W., et al. Chen Y.H. Nanoarchitecture and molecular interactions of epithelial cell junction proteins revealed by super-resolution microscopy. Ann. N. Y. Acad. Sci. 2022;1516:175–187. doi: 10.1111/nyas.14855. [DOI] [PMC free article] [PubMed] [Google Scholar]