Abstract
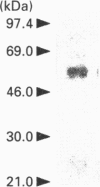
A phosphatidic-acid-hydrolysing phospholipase A2 was purified from rat brain and characterized. This phospholipase A2 was purified by sequential cation, hydrophobic, heparin and gel-filtration chromatography. The purified protein had a mass of approximately 58 kDa as assayed by SDS/PAGE, had a pH optimum of 6.0, and was Ca(2+)-independent. This enzyme was apparently phosphatidic-acid-selective and had little measurable catalytic activity when phosphatidylcholine, phosphatidylethanolamine or diacylglycerol was used as substrate. On the basis of its physical and catalytic properties, we conclude that this phospholipase A2 is unique from those previously purified, and we speculate that it may be important for the production of the bioactive lipid lysophosphatidic acid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Bomalaski J. S., Clark M. A. Phospholipase A2 and arthritis. Arthritis Rheum. 1993 Feb;36(2):190–198. doi: 10.1002/art.1780360208. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Durieux M. E., Lynch K. R. Signalling properties of lysophosphatidic acid. Trends Pharmacol Sci. 1993 Jun;14(6):249–254. doi: 10.1016/0165-6147(93)90021-b. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Kindom S. E., Peterson D. A., Peller J., Krantz K. E., White J. G. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am J Pathol. 1979 Aug;96(2):423–438. [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta. 1989 Feb 20;1001(3):282–285. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- Jalink K., Moolenaar W. H., Van Duijn B. Lysophosphatidic acid is a chemoattractant for Dictyostelium discoideum amoebae. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1857–1861. doi: 10.1073/pnas.90.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J Biol Chem. 1990 Jul 25;265(21):12232–12239. [PubMed] [Google Scholar]
- Kume N., Gimbrone M. A., Jr Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest. 1994 Feb;93(2):907–911. doi: 10.1172/JCI117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The phosphatidylinositol cycle and the regulation of arachidonic acid production. Nature. 1981 Jul 23;292(5821):367–369. doi: 10.1038/292367a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis C. R., Walsh J. P., Bell R. M. sn-1,2-Diacylglycerol kinase of Escherichia coli. Purification, reconstitution, and partial amino- and carboxyl-terminal analysis. J Biol Chem. 1985 Apr 10;260(7):4091–4097. [PubMed] [Google Scholar]
- Mauco G., Chap H., Simon M. F., Douste-Blazy L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie. 1978 Sep 29;60(6-7):653–661. doi: 10.1016/s0300-9084(78)80784-6. [DOI] [PubMed] [Google Scholar]
- Murakami M., Kudo I., Fujimori Y., Suga H., Inoue K. Group II phospholipase A2 inhibitors suppressed lysophosphatidylserine-dependent degranulation of rat peritoneal mast cells. Biochem Biophys Res Commun. 1991 Dec 16;181(2):714–721. doi: 10.1016/0006-291x(91)91249-c. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Phosphatidic acid may stimulate membrane receptors mediating adenylate cyclase inhibition and phospholipid breakdown in 3T3 fibroblasts. J Biol Chem. 1987 Apr 25;262(12):5522–5529. [PubMed] [Google Scholar]
- Nakano T., Raines E. W., Abraham J. A., Klagsbrun M., Ross R. Lysophosphatidylcholine upregulates the level of heparin-binding epidermal growth factor-like growth factor mRNA in human monocytes. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1069–1073. doi: 10.1073/pnas.91.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. M., Ramirez F. E., Kumar C. C., Thomson F. J., Clark M. A. Activation of serum response element-regulated genes by lysophosphatidic acid. Nucleic Acids Res. 1994 Feb 11;22(3):450–452. doi: 10.1093/nar/22.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevin R., MacNulty E. E., Palmer S., Wakelam M. J. Differences in the regulation of endothelin-1- and lysophosphatidic-acid-stimulated Ins(1,4,5)P3 formation in rat-1 fibroblasts. Biochem J. 1991 Dec 15;280(Pt 3):609–615. doi: 10.1042/bj2800609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Schimmel R. J., Honeyman T. W., McMahon K. K., Serio R., Clark R. B. Inhibition of cyclic AMP accumulation in hamster adipocytes with phosphatidic acid: differences and similarities with alpha adrenergic effects. J Cyclic Nucleotide Res. 1980;6(6):437–449. [PubMed] [Google Scholar]
- Thompson F. J., Clark M. A. Purification of a lysophosphatidic acid-hydrolysing lysophospholipase from rat brain. Biochem J. 1994 Jun 1;300(Pt 2):457–461. doi: 10.1042/bj3000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigyi G., Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992 Oct 25;267(30):21360–21367. [PubMed] [Google Scholar]
- Tokumura A., Fukuzawa K., Isobe J., Tsukatani H. Lysophosphatidic acid-induced aggregation of human and feline platelets: structure-activity relationship. Biochem Biophys Res Commun. 1981 Mar 31;99(2):391–398. doi: 10.1016/0006-291x(81)91758-7. [DOI] [PubMed] [Google Scholar]
- Tokumura A., Fukuzawa K., Tsukatani H. Contractile actions of lysophosphatidic acids with a chemically-defined fatty acyl group on longitudinal muscle from guinea-pig ileum. J Pharm Pharmacol. 1982 Aug;34(8):514–516. doi: 10.1111/j.2042-7158.1982.tb04776.x. [DOI] [PubMed] [Google Scholar]
- VOGT W. Pharamacologically active acidic phospholipids and glycolipids. Biochem Pharmacol. 1963 Apr;12:415–420. doi: 10.1016/0006-2952(63)90074-1. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Hordijk P. L., Medema R. H., Bos J. L., Moolenaar W. H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]