Abstract
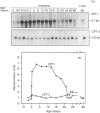
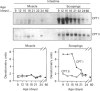
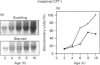
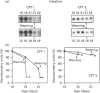
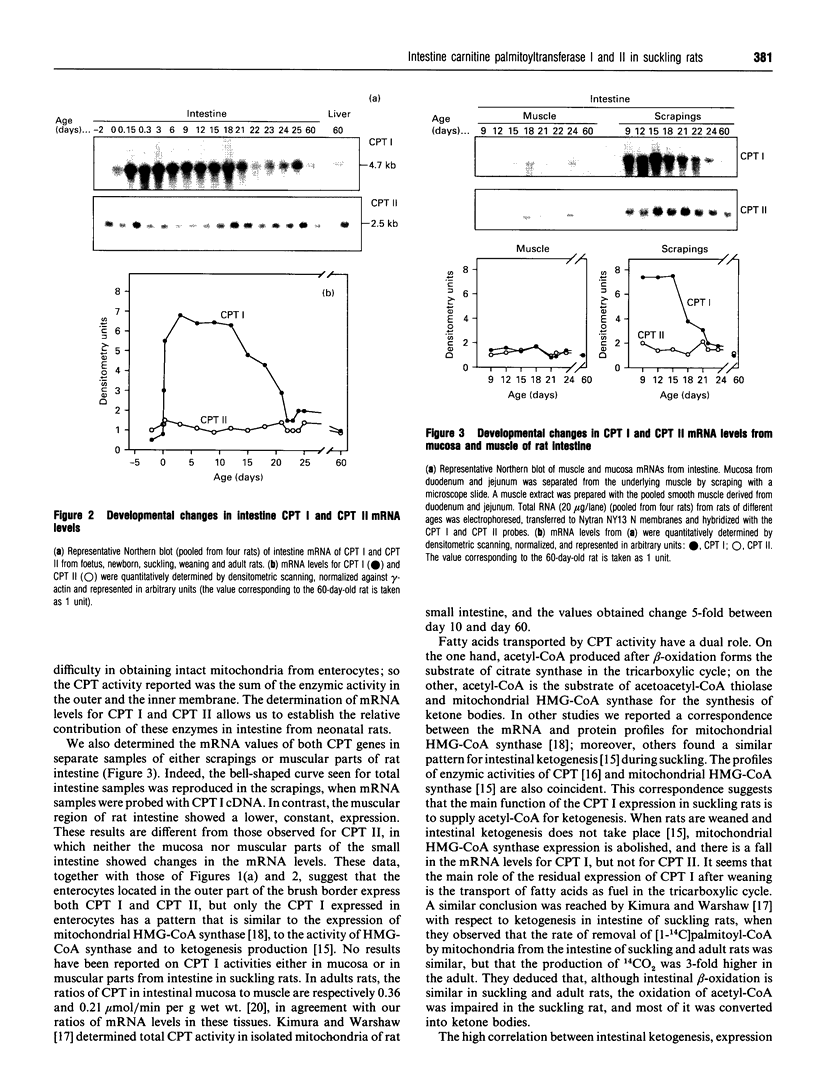
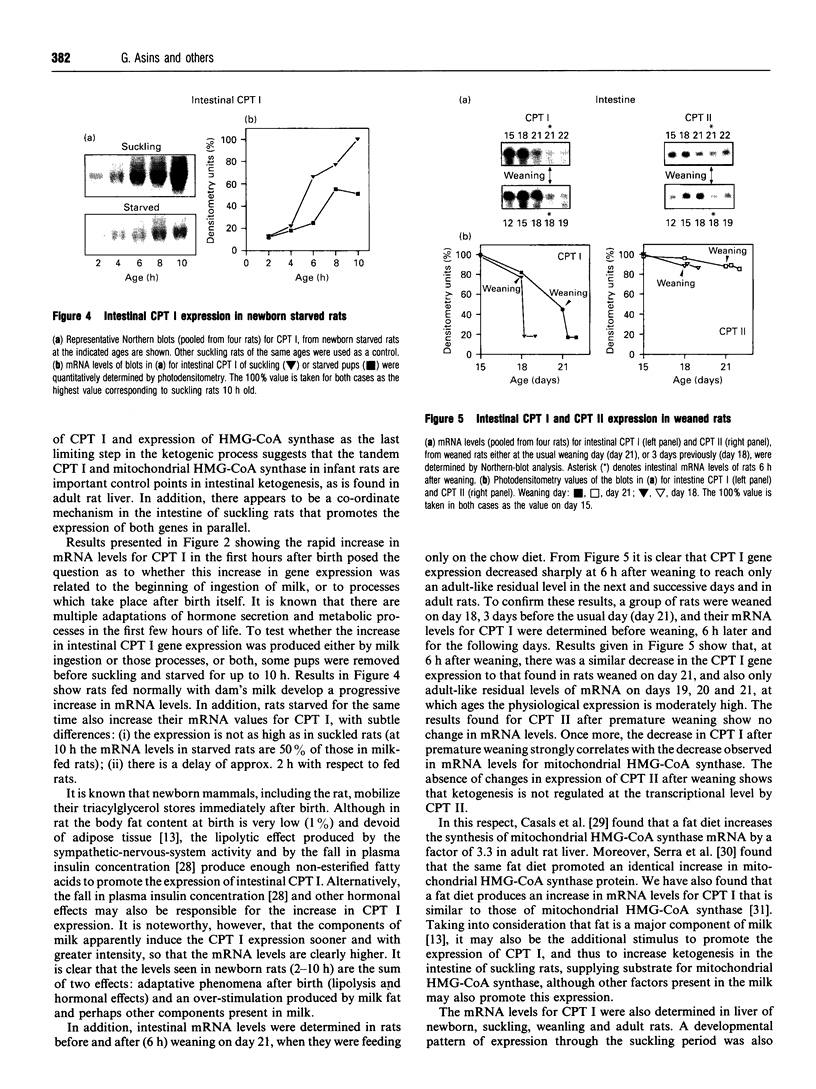
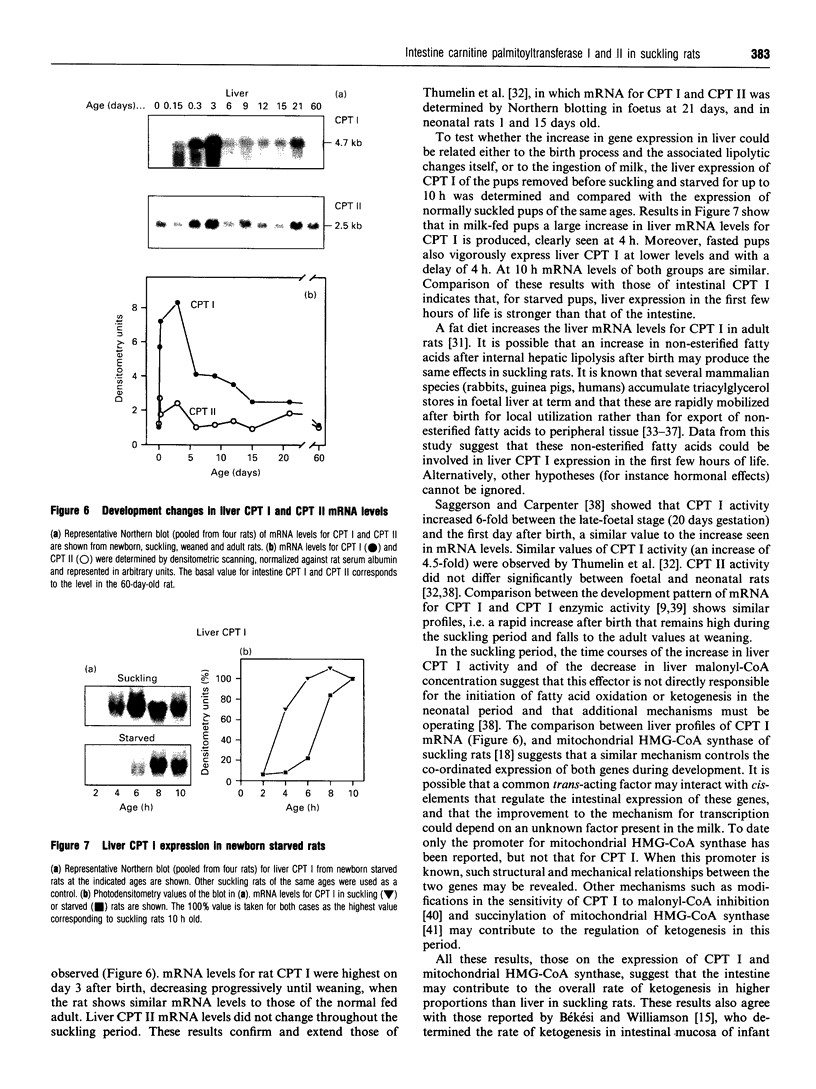
Carnitine palmitoyltransferase (CPT) I is expressed in the intestine of suckling rats; its mRNA increases very rapidly after birth, remains on a plateau until day 18 and decreases until weaning, when basal (adult) values are reached, which remain unchanged thereafter. CPT II mRNA values do not show any appreciable change in this period. CPT I and CPT II are expressed mainly in mucosa and, to a lesser extent, in the muscular part of the intestine. Intestinal expression of CPT I is maximal in duodenum and jejunum, whereas CPT II is expressed in a similar pattern throughout the whole intestine. Dam's milk may influence the intestinal expression of CPT I, since mRNA levels at birth are low but increase after the first lactation. Moreover, rats weaned at either day 18 or 21 decrease their mRNA levels. Apparently, CPT II gene expression is not influenced by the mother's milk. CPT I and CPT II are also expressed in the liver of suckling rats. Hepatic CPT I is maximal at day 3, and levels of CPT II mRNA do not change, in a similar fashion to that in intestine. The profile of expression of CPT I in liver and intestine strongly resembles that previously reported for mitochondrial 3-hydroxy-3-methyl-glutaryl-CoA synthase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asins G., Rosa J. L., Serra D., Gil-Gómez G., Ayté J., Bartrons R., Tauler A., Hegardt F. G. Gene expression of enzymes regulating ketogenesis and fatty acid metabolism in regenerating rat liver. Biochem J. 1994 Apr 1;299(Pt 1):65–69. doi: 10.1042/bj2990065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asins G., Serra D., Hegardt F. G. The effect of etomoxir on the mRNA levels of enzymes involved in ketogenesis and cholesterogenesis in rat liver. Biochem Pharmacol. 1994 Apr 20;47(8):1373–1379. doi: 10.1016/0006-2952(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Ayté J., Gil-Gómez G., Haro D., Marrero P. F., Hegardt F. G. Rat mitochondrial and cytosolic 3-hydroxy-3-methylglutaryl-CoA synthases are encoded by two different genes. Proc Natl Acad Sci U S A. 1990 May;87(10):3874–3878. doi: 10.1073/pnas.87.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito M., Whitelaw E., Williamson D. H. Regulation of ketogenesis during the suckling-weanling transition in the rat. Studies with isolated hepatocytes. Biochem J. 1979 Apr 15;180(1):137–144. doi: 10.1042/bj1800137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmer T., Havel R. J., Long J. A. Physiological fatty liver and hyperlipemia in the fetal guinea pig: chemical and ultrastructural characterization. J Lipid Res. 1972 May;13(3):371–382. [PubMed] [Google Scholar]
- Békési A., Williamson D. H. An explanation for ketogenesis by the intestine of the suckling rat: the presence of an active hydroxymethylglutaryl-coenzyme A pathway. Biol Neonate. 1990;58(3):160–165. doi: 10.1159/000243256. [DOI] [PubMed] [Google Scholar]
- Callikan S., Ferre P., Pegorier J. P., Girard J. R., Marliss E. B., Assan R. Fuel metabolism in fasted newborn rabbits. J Dev Physiol. 1979 Aug;1(4):267–281. [PubMed] [Google Scholar]
- Casals N., Roca N., Guerrero M., Gil-Gómez G., Ayté J., Ciudad C. J., Hegardt F. G. Regulation of the expression of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene. Its role in the control of ketogenesis. Biochem J. 1992 Apr 1;283(Pt 1):261–264. doi: 10.1042/bj2830261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Cook G. A., Gamble M. S. Regulation of carnitine palmitoyltransferase by insulin results in decreased activity and decreased apparent Ki values for malonyl-CoA. J Biol Chem. 1987 Feb 15;262(5):2050–2055. [PubMed] [Google Scholar]
- Decaux J. F., Ferré P., Robin D., Robin P., Girard J. Decreased hepatic fatty acid oxidation at weaning in the rat is not linked to a variation of malonyl-CoA concentration. J Biol Chem. 1988 Mar 5;263(7):3284–3289. [PubMed] [Google Scholar]
- Duee P. H., Pegorier J. P., el Manoubi L., Herbin C., Kohl C., Girard J. Hepatic triglyceride hydrolysis and development of ketogenesis in rabbits. Am J Physiol. 1985 Nov;249(5 Pt 1):E478–E484. doi: 10.1152/ajpendo.1985.249.5.E478. [DOI] [PubMed] [Google Scholar]
- Duée P. H., Pégorier J. P., Bois-Joyeux B., Girard J. Fuel metabolism and energy stores in fasting or suckling newborn guinea pigs. J Dev Physiol. 1983 Dec;5(6):383–393. [PubMed] [Google Scholar]
- Esser V., Britton C. H., Weis B. C., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J Biol Chem. 1993 Mar 15;268(8):5817–5822. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferré P., Pégorier J. P., Williamson D. H., Girard J. R. The development of ketogenesis at birth in the rat. Biochem J. 1978 Dec 15;176(3):759–765. doi: 10.1042/bj1760759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P., Satabin P., Decaux J. F., Escriva F., Girard J. Development and regulation of ketogenesis in hepatocytes isolated from newborn rats. Biochem J. 1983 Sep 15;214(3):937–942. doi: 10.1042/bj2140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. C., Bailey E. Changes in the activities of the enzymes of hepatic fatty acid oxidation during development of the rat. Biochem J. 1976 Jan 15;154(1):49–56. doi: 10.1042/bj1540049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J., Ferré P., Pégorier J. P., Duée P. H. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol Rev. 1992 Apr;72(2):507–562. doi: 10.1152/physrev.1992.72.2.507. [DOI] [PubMed] [Google Scholar]
- Girard J. Metabolic adaptations to change of nutrition at birth. Biol Neonate. 1990;58 (Suppl 1):3–15. doi: 10.1159/000243294. [DOI] [PubMed] [Google Scholar]
- Hahn P., Chanez C., Hamilton J. Carnitine and carnitine transferases in the intestinal mucosa of suckling rats. Biol Neonate. 1985;48(2):77–84. doi: 10.1159/000242157. [DOI] [PubMed] [Google Scholar]
- Hahn P., Taller M. Ketone formation in the intestinal mucosa of infant rats. Life Sci. 1987 Sep 21;41(12):1525–1528. doi: 10.1016/0024-3205(87)90718-1. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Carrington J. M. Activity of 3-oxo acid CoA-transferase, D-3-hydroxybutyrate dehydrogenase, hexokinase and carnitine palmitoyltransferase in the stomach and small and large intestine of the rat. Biochem J. 1981 Nov 15;200(2):349–355. doi: 10.1042/bj2000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. T. Lipid metabolism and mobilization in the guinea pig during pregnancy. Biochem J. 1976 May 15;156(2):357–365. doi: 10.1042/bj1560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R. E., Warshaw J. B. Control of fatty acid oxidation by intramitochondrial [NADH]/[NAD+] in developing rat small intestine. Pediatr Res. 1988 Mar;23(3):262–265. doi: 10.1203/00006450-198803000-00006. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Woeltje K. F., Kuwajima M., Foster D. W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989 May;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- McMillin Wood J. Carnitine palymityltransferase in neonatal and adult heart and liver mitochondria. Effect of phospholipase C treatment. J Biol Chem. 1975 Apr 25;250(8):3062–3066. [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Rapid switch of hepatic fatty acid metabolism from oxidation to esterification during diurnal feeding of meal-fed rats correlates with changes in the properties of acetyl-CoA carboxylase, but not of carnitine palmitoyltransferase I. Biochem J. 1993 Apr 1;291(Pt 1):241–246. doi: 10.1042/bj2910241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M., Monkus E. Metabolism of subcutaneous adipose tissue in the immediate postnatal period of human newborns. 1. Developmental changes in lipolysis and glycogen content. Pediatr Res. 1972 Feb;6(2):73–80. doi: 10.1203/00006450-197202000-00001. [DOI] [PubMed] [Google Scholar]
- Pegorier J. P., Prip-Buus C., Duee P. H., Girard J. Hormonal control of fatty acid oxidation during the neonatal period. Diabete Metab. 1992;18(1 Pt 2):156–160. [PubMed] [Google Scholar]
- Quant P. A., Robin D., Robin P., Ferre P., Brand M. D., Girard J. Control of hepatic mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase during the foetal/neonatal transition, suckling and weaning in the rat. Eur J Biochem. 1991 Jan 30;195(2):449–454. doi: 10.1111/j.1432-1033.1991.tb15724.x. [DOI] [PubMed] [Google Scholar]
- Quant P. A., Robin D., Robin P., Girard J., Brand M. D. A top-down control analysis in isolated rat liver mitochondria: can the 3-hydroxy-3-methylglutaryl-CoA pathway be rate-controlling for ketogenesis? Biochim Biophys Acta. 1993 Feb 13;1156(2):135–143. doi: 10.1016/0304-4165(93)90128-u. [DOI] [PubMed] [Google Scholar]
- Robles-Valdes C., McGarry J. D., Foster D. W. Maternal-fetal carnitine relationship and neonatal ketosis in the rat. J Biol Chem. 1976 Oct 10;251(19):6007–6012. [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Regulation of hepatic carnitine palmitoyltransferase activity during the foetal-neonatal transition. FEBS Lett. 1982 Dec 13;150(1):177–180. doi: 10.1016/0014-5793(82)81329-x. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra D., Asins G., Hegardt F. G. Ketogenic mitochondrial 3-hydroxy 3-methylglutaryl-CoA synthase gene expression in intestine and liver of suckling rats. Arch Biochem Biophys. 1993 Mar;301(2):445–448. doi: 10.1006/abbi.1993.1169. [DOI] [PubMed] [Google Scholar]
- Serra D., Casals N., Asins G., Royo T., Ciudad C. J., Hegardt F. G. Regulation of mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase protein by starvation, fat feeding, and diabetes. Arch Biochem Biophys. 1993 Nov 15;307(1):40–45. doi: 10.1006/abbi.1993.1557. [DOI] [PubMed] [Google Scholar]
- Thumelin S., Esser V., Charvy D., Kolodziej M., Zammit V. A., McGarry D., Girard J., Pegorier J. P. Expression of liver carnitine palmitoyltransferase I and II genes during development in the rat. Biochem J. 1994 Jun 1;300(Pt 2):583–587. doi: 10.1042/bj3000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumelin S., Forestier M., Girard J., Pegorier J. P. Developmental changes in mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene expression in rat liver, intestine and kidney. Biochem J. 1993 Jun 1;292(Pt 2):493–496. doi: 10.1042/bj2920493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Sen A., Cox W. F., McPhaul M. J., Slaughter C. A., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver mitochondrial carnitine palmitoyltransferase II. J Biol Chem. 1990 Jun 25;265(18):10720–10725. [PubMed] [Google Scholar]