Abstract
A comprehensive study of sorghum bran and flour was performed to explore the secondary metabolite profiles of differently coloured genotypes and to evaluate the variability in the antioxidant properties based on differences in polarity and solubility. This research included one red variety and one white variety. Among the samples, the red variety contained significantly greater amounts of secondary metabolites than did the white variety, with total polyphenol contents of 808.04 ± 63.89 mg.100 g-1 and 81.56 ± 3.87 mg.100 g-1, respectively. High-molecular-weight condensed tannin-type flavonoid extracts with high antioxidant activity were obtained by using relatively low-polarity acetone-water solvents, which was reflected by the measured antioxidant values. Among the methods used, the electron-donating Trolox equivalent antioxidant assay provided the highest antioxidant capacity, with values ranging from 118.5 to 182.6 μmol g−1 in the case of the red variety, in accordance with the electron donor properties of condensed tannins. Key secondary metabolites were identified using MS techniques and quantified using HPLC. Catechin and procyanidin B1 were found in the red variety at concentrations of 3.20 and 96.11 mg.100 g-1, respectively, while the concentrations in the white variety were under the limit of detection. All four tocopherols were found in sorghum, with the red variety containing a higher amount than the white variety, but the vitamin B complex concentrations were higher in the white variety. Overall, the red sorghum variety proved to be a better source of secondary metabolites with potential health benefits and could be used as a nutrient-rich food source.
Keywords: Sorghum, Secondary metabolites, Antioxidant, Vitamins, Polyphenols
1. Introduction
Currently, alternative grains and pseudocereals such as sorghum (Sorghum bicolor (L.) Moench.) are being cultivated in increasing amounts, as agricultural sustainability and adaptation to the ever-changing climate are becoming increasingly important [[1], [2], [3], [4]]. S. bicolor is an ancient grain originating from the tropical region of Northeast Africa and is now the fifth most widely grown cereal worldwide. In addition to its tolerance against drought, heat, and poor soil conditions, it is also a highly nutritious source of food for both humans and animals. In Asia and Africa, it is used mostly as a food source, while in Western countries, it is considered mainly as animal feed or industrial material [[5], [6], [7]].As the dietary habits of modern society have changed and the prevalence of food-related allergies and intolerances has increased, it has become necessary to provide a new, nutritious and safe ingredient for the food industry. Sorghum is one of the most important alternative cereal grains and is composed of highly beneficial starch, notably containing 15–30 % resistant starch, but it also contains as much as 65 % total starch [8,9]. This not only provides an easy food source for the human microbiota but also significantly decreases the glycaemic index of flour. Sorghum is also gluten free, so people with celiac disease can consume it. Furthermore, sorghum is highly rich in unique secondary metabolites, mainly polyphenolic components, with several verified positive physiological effects, such as anti-inflammatory and antioxidant effects [[10], [11], [12], [13], [14], [15], [16], [17]].
These metabolites can be grouped according to their solubility. Among water soluble compounds, the most important are small-molecular-weight phenolic acids (ferulic acid, caffeic acid, cinnamic acid), flavones (luteolin, apigenin), flavanones (naringenin), and B vitamins (B1, B2, B6). The less water soluble components include high-molecular-weight proanthocyanidin oligomers and polymers such as condensed tannins (CTs), while the apolar components include tocopherols (vitamin E) [[18], [19], [20], [21], [22]]. In terms of physiological effects, the most important group comprises flavonoids, which are responsible for most of the health benefits associated with sorghum [23,24].
Tannins are divided into hydrolysable tannins (HTs) and condensed tannins. In the case of sorghum, CTs are more relevant than HTs. During synthesis, naringenin and anthocyanidins are considered the main branching points for the synthesis of different flavonoids [25,26]. Sorghum contains a unique amount of CTs compared to other cereals, which makes it especially important in the feed and food industries. CTs are polyhydroxy-flavan-3-ol oligomers and polymers with C4–C6 or C4–C8 hydrogen bonds and a high molecular weight of 120–3000 Da. These compounds mostly accumulate in the pigmented testa layer, develop from the inner integument of the seed coat, and are absent in seeds without this layer. Procyanidin oligomers and polymers (B1, B2, etc.) are the main types of CTs in sorghum. The chemical structure of CTs influences the pharmacological characteristics of these compounds. Owing to their many free hydroxyl groups, they are important electron donors in redox processes, such as free radical scavenging; thus, they are important antioxidants and anti-inflammatory agents [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. Furthermore, the notable property of CTs is their ability to chelate metal ions and proteins by bonding to them via covalent bonds, thus inhibiting pathological bacterial activities and inhibiting enzymatic activities such as salivary and pancreatic amylases. This inhibition is often unwelcomed in animal husbandry since it not only prevents the growth of the animal but also, with excess gas production due to indigestible carbohydrate–tannin complexes in the gut, can also lead to the death of the animal. However, from the perspective of human dietary and therapeutic aspects, these properties, when combined with an appropriate CT concentration, can regulate energy intake during food consumption with slower, smoother glucose absorption in people with obesity and diabetes [[42], [43], [44], [45]]. Their diverse and complex chemical structure also influences their solubility in organic matter. Size and polymerization degree of CT compounds are key factors determining their activity, digestibility, and bioavailability in living systems. For this reason, choosing the correct type and ratio of solvents in chemical analysis is a necessary step to investigate their biological effect accurately [[46], [47], [48], [49]].
Cereals such as sorghum are usually B vitamin accumulators. These vitamins are stored in the bran of the seed; thus, refined flours tend to contain a low amount of B vitamins. The most important group of proteins are the enzyme cofactors. Thiamine (B1) is a cofactor of many enzymes (pyruvate dehydrogenase and α-ketoglutarate dehydrogenase) of the citric acid cycle and the pentose phosphate cycle of carbohydrate synthesis, as well as amino acid metabolism and gluconeogenesis [50,51]. Pyridoxine (B6) is a cofactor in the transsulfuration pathway and folate cycle and is a key cofactor of serine hydroxymethyltransferase, cystathionine-β-synthase and cystathionine-gamma-lyase enzymes in the methionine cycle. A lack of these cofactors can lead to neurological disorders, haematological problems, the development of mental illnesses, and a lack of endogenous synthesis of H2S, which can cause severe cardiovascular problems such as hypertension or atherosclerosis [[52], [53], [54]]. The recommended daily dose of these cofactors is 1–2 mg, depending on sex and age, and sorghum can provide 20, 7, and 30 % of the daily intake of B1, B2, and B6, respectively [22]. Another important secondary metabolite is tocopherols or vitamin E, which are fat-soluble, methylated phenol compounds with four known variants (α, β, γ, δ). α-tocopherol is considered to be a vitamin E with high antioxidant potential because only the carrier protein alpha-TTP can be carried throughout the human body; however, according to recent studies, other forms, such as β- and γ-tocopherols, are also potential antioxidants that are found mainly in the human gut [55,56].
The main aim of this study was to evaluate the secondary metabolite profile of sorghum, particularly that of condensed tannins, which are important compounds both for their anti-nutritive effects and for their great health benefits in terms of human and animal consumption. The main objectives of this research were to develop a selective preparation method for CTs and to investigate the bioactive profile and antioxidant properties of red and white sorghum varieties by spectrophotometric and HPLC methods.
2. Materials and methods
2.1. Experimental design
Red and white (Zádor, Albita) sorghum samples were obtained from small experimental field plot (47°17′27.2″N, 20°53′27.8″E) at the Research Institute of Karcag in 2019. All the samples had a moisture content typical of cereals (9.5–11 %) and did not receive any nutritional supplementation. The white variety “Albita” was domesticated mainly for animal feed and had an extremely low tannin content, while the red variety “Zádor” was intended for general use and had a low tannin content. The experimental area is located in Hortobágy and Nagykunság, Hungary, and has clay-loam meadow chernozem soil. According to our soil analysis, this soil is a good source of phosphorous and potassium and has a decent humus content. The temperature during the growing season was favourable, with an average temperature of 19 °C, while the rainfall levels were low during summer, which was complemented by relatively high precipitation in the spring pre-sowing season.
2.2. Sample preparation
The samples were peeled using a laboratory-scale Satake rice peeler (SATAKE, Hiroshima, Japan) at maximum output for 50 s. The bran was separated from the kernel, which was milled using a laboratory-scale roller mill. The flour and bran were stored at −20 °C in a plastic bag until further analysis. The moisture content of the flour was determined using a thermogravimetric method with a drying oven. A fixed amount of sample was dried in an oven for 3 h at 105 °C, after which the moisture content was calculated from the difference in weight.
2.3. Extraction of polyphenols using methanol as a solvent for preliminary measurement
Methanol extraction was used to determine the total amount of polyphenols in the sorghum samples. A 0.5 g sample was weighed, and 5 mL of a methanol-distilled water mixture (80:20 v/v%) was added. The mixture was vortexed for 30 s and put into an ultrasonic water bath (25 °C) for 20 min. After extraction, the samples were centrifuged using a Frontier 5000 centrifuge (Ohaus Europe, Nänikon, Switzerland) at a maximum of 4500 rpm for 10 min, after which the supernatants were saved for analysis. The results of this measurement together with other varieties are published in the following article referenced here as a part of another evaluation [57].
2.4. Extraction of condensed tannins using methanol for preliminary measurement
A preliminary measurement was performed to estimate the amount of condensed tannins in the studied samples. During extraction, 5 ml of methanol was added to 0.5 g of bran, which was subsequently vortexed for 30 s and put into an ultrasonic water bath for 20 min. The extracts were centrifuged at 4500 rpm for 10 min, and the supernatants were used for further analysis. The results of this measurement together with other varieties are published in the following article referenced here as a part of another evaluation [57].
2.5. Extraction of condensed tannins using an acetone-water solvent
Our main aim here was to ensure all CTs with different degree of polymerization, molecular weight, and solubility are extracted from the sample matrix to investigate the antioxidant activities. Acetone is an excellent solvent for the extraction of several polyphenolic compounds, especially larger tannin molecules, because of its lower polarity, thus it was chosen as a main solvent for extraction of sorghum tannins. Ratios of 50:50 to 90:10 acetone (AC)-distilled water (DW) were used to extract tannins from sorghum according to the methods of Hagerman (2002). Solvents with different acetone-water ratios were as follows (%): AC1, 50:50; AC2, 60:40; AC3, 70:30; AC4, 80:20; and AC5, 90:10 Since phenolic compounds accumulate mainly in the bran fraction and preliminary studies have shown that flour contains negligible amounts of condensed tannins, the bran fraction was used for preparation. Two-gram samples were weighed in triplicate into 50 ml centrifuge tubes, and 20 ml of solvent was added to each sample. The mixture was shaken using an ultrasonic water bath for 30 min, and the extracts were centrifuged at 2500 rpm for 10 min. The supernatant was saved, and extraction was carried out three additional times, for a total of 4 extraction steps. The extracted supernatants were pooled and evaporated to dryness using a rotary evaporator. Twenty millilitres of the extracts were separated for MS/MS analysis. The dried extracts were stored at −20 °C until analysis.
2.6. Extraction of flavonoid compounds for HPLC analysis
Acidified methanol extraction was used to extract flavonoids from sorghum samples because it is often used to ensure the stability of flavonoid compounds. Ten grams of bran was weighed and extracted with 100 ml of 5 % HCl-methanol for 2 h on a magnetic stirrer. The extracts were centrifuged at 4500 rpm for 10 min, after which the supernatants were removed and evaporated to dryness. The dried extracts were stored at −20 °C until analysis.
2.7. Extraction of tocopherols
The tocopherol distribution was analysed in the flour and bran fractions of the samples. Extraction was performed according to Bíró et al. (2015) with some modifications [58].Samples (20-20 g) were weighed and extracted with 150 ml of hexane for 25 min on a magnetic stirrer and 25 min in an ultrasonic bath (25 °C). The samples were centrifuged at 4500 rpm for 10 min, the supernatants were decanted, and the extraction was repeated with the same parameters. The supernatants were filtered through cellulose filtration paper (with 34–42 μm retention), evaporated to dryness and stored at −20 °C until further analysis.
2.8. Extraction of vitamin B
Four B vitamins, namely, thiamine (B1), niacin (B3), pyridoxine (B6), and riboflavin (B2), were evaluated in the sorghum samples. Extraction was performed according to the methods of Nemes (2015) with some modifications. Five grams of bran and flour were weighed, and 50 ml distilled water was added before extraction on a magnetic stirrer for 1 h. Extraction was performed in a dark bottle under a nitrogen atmosphere to protect the samples from light and oxidation. After extraction, the samples were centrifuged at 4500 rpm for 10 min, and the supernatants were filtered through cellulose filtration paper. The filtrates were evaporated to dryness and dissolved in 1 ml distilled water. The samples were centrifuged again at 10000 rpm for 5 min. The concentrated samples were purified on a Strata-X-C solid-phase extraction column (Phenomenex, Aschaffenburg, Germany). The columns were activated by 2 ml of methanol and 2 ml of 1 % formic acid before the samples were mixed with an equivalent amount of formic acid and eluted on the column using a vacuum pump. Two fractions were obtained using methanol and 2 % ammonium-hydroxid:methanol (1/1 v/v%) solutions. The fractions were evaporated to dryness and dissolved in 2 ml of distilled water. The samples were subsequently centrifuged again at 10000 rpm for 5 min. The samples were stored at −20 °C until analysis.
2.9. Measurement of the total phenol content of the methanol extracts
Total phenolic content (TPC) analysis was carried out by the Folin–Ciocalteu method according to Singleton and Rossi with modifications by Nemes et al. (2018) [59]. For measurement, a SpectroStar nanospectrophotometer (BMG Labtech, Ortenberg, Germany) was used with a microplate reader and a TPP-96 plate. An aliquot of 10 μl of methanol extract was added to the wells, together with the required reagents described previously. The absorbance values were measured at 765 nm after 10 min of incubation at 45 °C. Gallic acid was used as a standard (Merck, Darmstadt, Germany), and the results are expressed as milligrams per 100 g gallic acid equivalent (GAE). All reagents and chemicals were of analytical grade. The samples were stored at −20 °C until analysis. The results of this measurement together with other varieties are published in the following article referenced here as a part of another evaluation [57].
2.10. Measurement of extractable condensed tannins from methanol extracts
The vanillin-HCL method was used for tannin evaluation according to Price (1978), with some modifications [60]. An aliquot of 10 μl of methanol extract was put into a TPP-96 plate, together with the required reagents described previously. The mixture was incubated at room temperature for 15 min, after which the absorbance was measured at 500 nm. Catechin (Extrasynthese, Genay, France) was used as a standard. The results are expressed as milligrams per gram catechin equivalent. The samples were stored at −20 °C until analysis. The results of this measurement together with other varieties are published in the following article referenced here as a part of another evaluation [57].
2.11. Measurement of antioxidant capacity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
Acetone extracts were used for antioxidant capacity measurements via three commonly applied assays. DPPH antioxidant assays were performed according to Blois (1958) with modifications by Nemes et al. (2018) [59]. A 2,2 diphenyl-1-picrylhydrazyl (DPPH) radical solution was prepared immediately before the measurement and stored in an amber bottle. The dried extracts were dissolved in methanol and centrifuged again at 2500 rpm for 10 min. A 2 ml sample was transferred by an automatic pipette to an Eppendorf tube. Samples were analysed in triplicate at 517 nm after 30 min of incubation at 25 °C on a TPP-96 plate. Trolox, a vitamin E analogue, was used as a standard to prepare a calibration curve.
2.12. Measurement of antioxidant capacity using the TEAC assay
Similar to the DPPH assay, this method also uses an artificial free radical, 2-2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid), to measure antioxidant properties. This radical was prepared one day prior to mixing it with an equal volume of potassium persulfate solution. Before the measurement, the solution was diluted twentyfold with 80 % ethanol. The reaction mixture was put together again on a TPP-96 plate using re-dissolved acetone extracts, and absorbance values were taken at 734 nm after 30 min of incubation at 25 °C. A Trolox standard was used to prepare the calibration curve. The results are expressed as μmol Trolox equivalent (TE) g−1 values for both assays.
2.13. Measurement of antioxidant capacity using the FRAP assay
The third assay was the ferric reducing antioxidant power (FRAP) assay, an appropriate method for measuring water-soluble, iron-reducing antioxidant agents. Dried acetone extracts were dissolved in distilled water instead of methanol, and after centrifugation, the supernatant was used for analysis. The FRAP reagent was prepared immediately before measurement with acetate buffer, iron(III) chloride, and 2,3,5-triphenyltetrazolium chloride (TPTZ). TPP-96 plates were used for the reaction medium, and absorbance values were measured at 593 nm after 8 min of incubation at 37 °C. Ascorbic acid was used as a standard for the calibration curve. Values are expressed as μmol ascorbic acid equivalent (AAE) g−1.
2.14. Qualitative analysis of condensed tannins and flavonoids of sorghum using UHPLC-ESI-ORBITRAP-MS/MS
Qualitative analyses were performed using UHPLC‒ESI‒MS/MS with a Dionex Ultimate 3000RS UHPLC system (Thermo Fisher, USA) coupled to a Thermo Q Exactive Orbitrap hybrid mass spectrometer equipped with a Thermo Accucore C18 analytical column (2.1 mm × 100 mm, 2.6 μm particle size). The flow rate was maintained at 0.2 ml min−1. The column oven and postcolumn cooling temperatures were set to 25 °C ± 1 °C. The mobile phase consisted of methanol (A) and water (B) (both acidified with 0.1 % formic acid). The gradient program was as follows: 0–3 min, 95 % B; 3–43 min, 0 % B; 43–61 min, 0 % B; 61–62 min, 95 % B; and 62–70 min, 95 % B. The injection volume was 2 μl. The mass spectrometer was equipped with an ESI source; thus, the samples could be measured in both positive and negative ionization modes separately. The resolutions for both ionization modes were 35000 and 17500. The scanning mass interval and collision energy were set to 100–1500 m/z and 30 normalized collision energy (NCE) units, respectively. The data were acquired and processed using Thermo Trace Finder 2.1 software based on our own and internet databases (Metlin, Mass Bank of North America, m/z Cloud). After processing, the results were manually checked using Thermo Xcalibur 4.0 software (Thermo Fisher Scientific, USA).
2.15. Sorghum-specific condensed tannin and flavonoid measurements using an HPLC–PDA and UHPLC-PDA system
The previously acquired acetone-water extract (AC3) was used for quantification of specific flavonoids. The dried extract was resuspended and purified using a C18 silica solid-phase extraction column. Identification and measurement were performed via an RP-HPLC instrument equipped with a PDA detector on a Hypersil ODS 5 column. The mobile phases were methanol (Solvent A) and 3 % formic acid (Solvent B). Separation was performed using an eluent gradient with the following settings: 0–16 min, 15 % A; 16 min, 15–28 % A; 16–20 min, 28–30 % A; 20–25 min, 30–35 % A; 25–30 min, 35–40 % A; 30–33 min, 40–48 % A; 33–37 min, 48–50 % A; 37–40 min, 50 % A; 40–41 min, 50–15 % A; and 45 min, 15 %. A 10 μl sample was injected at a flow rate of 1 ml min−1. Detection was performed at wavelengths of 280, 340, and 350 nm. The components were identified and quantified by retention time, spectrum, and area under the curve using external standards such as procyanidin B1 and catechin (Extrasynthese, Genay, France).
Other flavonoid compounds, such as naringenin, apigenin, and luteolin, that are found in sorghum according to literature were also quantified. Samples prepared previously using acidified methanol were resuspended in methanol, and an RP-UHPLC-PDA system with a Hypersil ODS 5 column was used. All the parameters were the same as detailed above.
2.16. Measurement of tocopherols by a UHPLC-PDA system
The prepared dried extracts were resuspended in 2 ml of dichloromethane (DCM) and purified on an SPE silicate column. The purified samples were measured on an RP-UHPLC-PDA system with a Hypersil ODS 5 column. The mobile phase was an acetonitrile-methanol-dichloromethane (65:35:5) mixture. A 10 μl sample was injected with a 1 ml min−1 isocratic flow rate. Identification was performed by a PDA detector at 295 nm. The components were identified and quantified by retention time, spectra, and area under the curve using external standards of α-, β-, γ-, and δ-tocopherols (Merck, Darmstadt, Germany).
2.17. Measurement of vitamin B concentrations using an HPLC-UV/VIS system
Identification and quantification of vitamin B compounds were performed via an HPLC system (Waters 2695 Separation module) equipped with a two-channel UV/VIS detector (Waters, 2487). The mobile phase consisted of methanol (Solution A) and 50 mM sodium acetate buffer (Solution B) solution. A 10 μl sample was injected at a flow rate of 1 ml min−1 using the following eluent gradient: 0 min, 10 % A–90 % B; 4 min, 50 % A–50 % B; 15 min, 50 % A–50 % B; 16 min, 10 % A–90 % B; and 25 min, 10 % A–90 % B. The total length of the chromatogram was 40 min with 25 min of elution and 15 min of washing with ethanol. The following external standards were used for identification: thiamine-hydrochloride, pyridoxine, nicotinamide, and riboflavin (Merck, Darmstadt, Germany). The compounds were detected at 254 nm.
2.18. Statistical analysis
The results were analysed using IBM SPSS Statistics version 24 software, using a completely randomized design, and GraphPad Prism 8 software. Two-way analysis of variance (ANOVA) with Tukey's test and unpaired t tests were applied for comparison of the data.
3. Results and discussion
3.1. Extraction efficiency and variety characterization
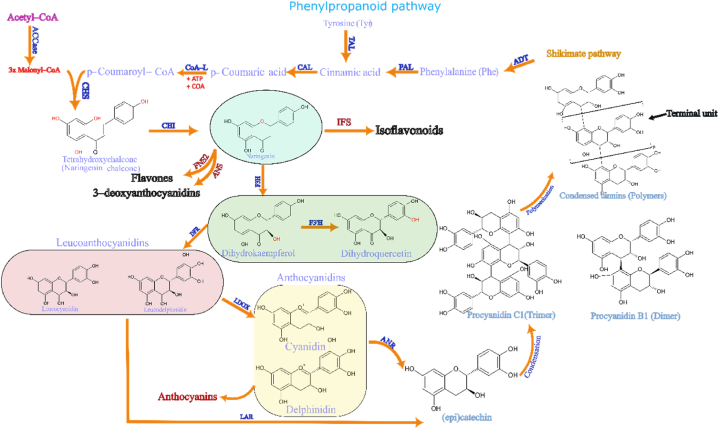
The polarity of the extractants strongly determines the amount and type of extractable compounds from any material. Due to their numerous polar hydroxyl groups, polyphenols and flavonoids are soluble mainly in polar solvents such as water, alcohol, or acetone. However, as the molecule size increases to that of oligomers and polymers, the solubility and thus extraction efficiency also change, which is increasingly important for condensed tannin (CT) - type molecules. These CTs are often oligomers and polymers made from several subunits and complex structures, which impedes their solubility in water compared to that of smaller polyphenols. Their synthesis pathway can be seen at Fig. 1.
Fig. 1.
Biosynthesis of proanthocyanidins through the shikimate-phenilpropanoid pathway. Note: The scheme was constructed based on the following methods [[25], [26], [61], [62], [63]]: ACCase: acetyl-CoA carboxylase; TAL: tyrosine ammonia lyase; PAL: phenylalanine ammonia lyase; CAL: cinnamate-4-hydroxylase; CoA-L: coenzyme-A ligase; CHS: chalcone synthase; CHI: chalcone isomerase; FNS: flavone synthase; IFS: isoflavonoid synthase; F3H: flavonoid-3-hydroxylase; F3′H: flavonol-3-hydroxylase; DFR: dihydroflavanol 4-reductase; LAR: leucocyanidin 4-reductase; LDOX: leucocyanidin deoxygenase; ANS: anthocyanidin synthase; ANR: anthocyanidin reductase; ADT: arogenate dehydratase.
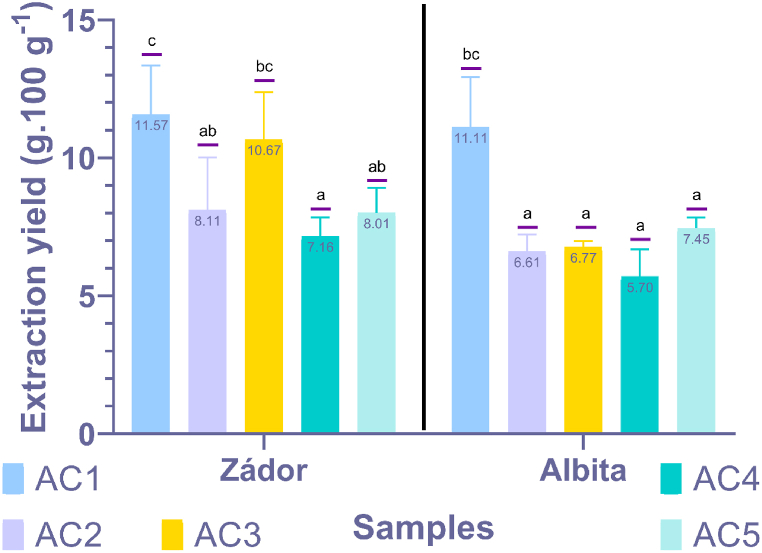
Several studies have suggested the use of acetone, a universal solvent with medium polarity, as an extractant for CTs and flavonoids. The amount of extracted material can be seen in Fig. 2. In terms of yield, there were several similarities between the varieties. In both cases, the AC1 extraction had the highest amount of extract, while the AC4 extraction had the lowest amount. In the case of AC1, due to its high polarity, in addition to flavonoids, several other water-soluble compounds were also extracted. Hence, a high amount of sample was extracted with the solvent AC1. By reducing the water ratio, yields were also reduced, but this also affected the composition of the extracts. Furthermore, the reduction in polarity allowed for more efficient extraction of high-molecular-weight tannin oligomers and polymers, as shown by the high extraction yield observed with AC3 extraction for the red sorghum variety, and these polymers were not present in the white sorghum variety. Therefore, we considered the AC3 extraction to be the most efficient among all the variations.
Fig. 2.
Extraction yield of different acetone-water ratios for red (Zádor) and white (Albita) sorghum. Note: Values with the same lowercase letters are not significantly different. Legend: AC1 = 50:50 acetone-water v/v%, AC5 = 90:10 acetone-water v/v%. Statistical analysis was conducted for each variety separately.
3.2. Measurement of antioxidant capacities
Sorghum is an excellent source of antioxidants, which makes it an important functional ingredient in the current food industry. Several analytical methods, including fluorescence-based methods and colorimetric methods, are available for measuring antioxidant potential. We used colorimetric methods for measurement. Among them, the TEAC and DPPH assays are considered mixed assays based on electron and hydrogen transportation, where TEAC is primarily an electron-donating compound and DPPH is somewhat similar to a hydrogen (proton)-donating molecule. These two methods are able to detect different types of antioxidants, and they are more suitable for measuring fat-soluble antioxidants. The FRAP assay is considered to be an electron transfer method, and it is an appropriate method for measuring water-soluble ferric ion-reducing antioxidants [64].
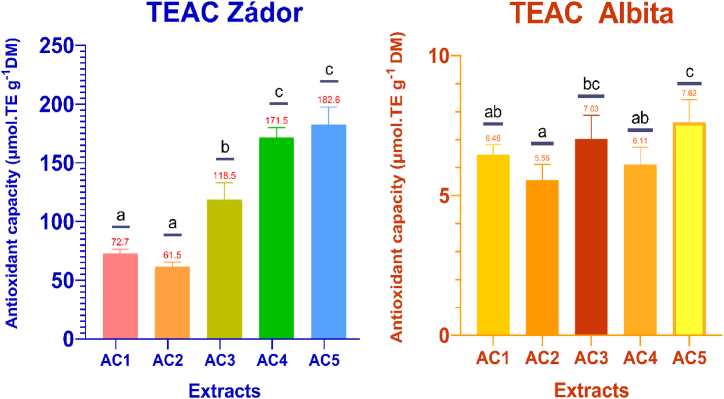
Flavonoids, especially CTs, are strong antioxidants that can quench several free radicals per molecule due to their several free hydroxyl groups. The measured antioxidant capacity values are shown in Fig. 3, Fig. 4, Fig. 5 for TEAC, DPPH, and FRAP, respectively. Due to differences between the two sorghum varieties, significant differences were observed between the antioxidant capacities of extracts from the Zádor and Albita varieties. Zádor is described as a “low-tannin genotype” by the breeder, while Albita is described as a “tannin-free variety” and contains extremely low amounts of tannins. Among the three applied methods, TEAC was the most efficient, with a maximum value of 182.64 ± 14.91 μmol TE g−1 DM for Zádor, and there were statistically significant differences in TEAC activity between the different extracts. The antioxidant activity of the AC3 extract of Zádor was significantly lower than that of the other extracts, with a value of 118.50 ± 14.49 μmol TE g−1 DM, despite AC3 being the solvent ratio recommended in the literature for the extraction of strong antioxidants such as tannins and flavonoids. The increase in antioxidant capacity can be explained by the increasing proportion of less polar oligomers and polymers with multiple radical binding ability. The results for the Albita variety showed similar antioxidant properties for every extraction, with slight differences attributed to the lack of tannins. The TEAC values were in accordance with the extraction solvent ratios: as the polarity of the solvents decreased, the ratio of larger, less water-soluble oligomers and polymers increased, represented by the increase in antioxidant power. Chiremba et al. (2009) published results with similar ABTS values from different sorghum varieties. TEAC values were reported between 13 and 370 μmol E g−1 [65]. Awika and his coworkers have compared several antioxidant methods for sorghum, and they published TEAC values between 230 and 768 μmol TE g−1 [66].
Fig. 3.
TEAC antioxidant properties of red (Zádor) and white (Albita) sorghum samples extracted by different solvents. Notes: Values with the same lowercase letters are not significantly different. Legend: AC1 = 50:50 acetone-water v/v%, AC5 = 90:10 acetone-water v/v%. Statistical analysis was conducted for each variety separately.
Fig. 4.
DPPH antioxidant properties of red (Zádor) and white (Albita) sorghum samples extracted by different solvents. Notes: Values with the same lowercase letters are not significantly different. Legend: AC1 = 50:50 acetone-water v/v%, AC5 = 90:10 acetone-water v/v%. Statistical analysis was conducted for each variety separately.
Fig. 5.
FRAP antioxidant properties of red (Zádor) and white (Albita) sorghum samples extracted with different solvents. Notes: Values with the same lowercase letters are not significantly different. Legend: AC1 = 50:50 acetone-water v/v%, AC5 = 90:10 acetone-water v/v%. Statistical analysis was conducted for each variety separately.
An inverse relationship was observed for the DPPH assay, especially for the Zádor samples, which had a richer bioactive compound profile than the Albita samples. Here, extracts from AC1 to AC3, which have more polar compositions, had significantly greater antioxidant capacities than did AC4, and AC5 had a greater ratio of acetone. The DPPH radical scavenging capacity decreased in Zádor samples as the ratio of acetone increased. The highest DPPH values were experienced for AC4 and AC5 samples with values of 72.79 ± 1.59 and 67.84 ± 5.52 μmol TE g−1 DM respectively. For Albita, there were no significant differences between the extracts except for AC5, and the antioxidant potential of these extracts was markedly lower than that of Zádor extracts, with a maximum value of 5.20 ± 0.81 μmol TE g−1 DM in AC5. Furthermore, the DPPH radical scavenging capacity was considerably lower than the TEAC radical scavenging capacity for all samples. These differences are caused by the differences between the two assays. The DPPH assay is primarily based on proton (H+) donation, and because of the distinct shape of the DPPH radical used for measuring the size of the molecule, interactions with the radical are limited during the reaction. DPPH assays have provided lower values mostly because CT oligomers and polymers were too large for proper interaction during the incubation period. Thus, the TEAC assay is more appropriate than the DPPH assay for measuring sorghum antioxidants because it is based mainly on electron transfer. Extracts from AC1 and AC2 exhibited proton-donating tendencies, while those from AC4 and AC5 seemed to exhibit electron-donating characteristics. Other studies have reported significantly different DPPH radical scavenging capacity values for sorghum varieties than those reported in this study. Choi et al. (2019) reported DPPH radical scavenging values between 0.7 and 40 μmol TE g−1, while Awika et al. (2003) reported values between 6 and 716 μmol TE g−1 DM depending on the genotype and fraction examined [66,67]. Moreover, Dykes et al. (2005) published results similar to our values for red sorghum, where a 2-fold difference was observed between the TEAC and DPPH values [68].
There was no difference in the antioxidant power determined by the FRAP assay between the different extraction methods. The Zádor variety had higher antioxidant values than did the Albita variety, as was observed for the other assays. AC1 had the highest ferric reducing antioxidant power, at 38.97 ± 3.49 μmol.AAE g−1 DM.
Based on the results of these 3 antioxidant assays, sorghum was determined to have a significant amount of high-molecular-weight, non-water-soluble antioxidants, which are most accurately measured with the TEAC assay, as their ferric reducing ability is relatively low. The extract of AC3 produced high values for both the TEAC and DPPH assays, while the AC4 and AC5 extracts had high TEAC values. These findings indicate an increase in polymer size in components of the AC4 and AC5 extracts, while in the AC3 extract, there are likely CT oligomers of medium molecular size, and they are able to interact with both TEAC and DPPH radicals freely. These CTs contain several accessible hydroxyl groups, which makes them effective antioxidants with the ability to quench multiple free radicals at once. These characteristics can explain the especially high TEAC values measured for the AC3-AC5 extracts. Furthermore, we found high FRAP values for the Zádor variety, especially in of the AC1 and AC2 extracts, which implies that there are some water-soluble antioxidants that have the ability to reduce ferric ions. Several other articles have reported similar results. Sreeramulu et al. (2009) reported a FRAP value of 66.90 μmol g−1 in sorghum, while Huo et al. (2016) described varieties with a FRAP concentration of 77.01 μmol.FE g−1 [69,70].
All these results indicate that the red sorghum variety, Zádor, is rich in CT oligomers and polymers, as well as other valuable antioxidant compounds such as phenolic acids, flavonoids and vitamins. This property makes it an excellent candidate both for human physiology and animal health, and consuming these components can play a great role in the treatment of infection, inflammation, and related diseases. These compounds can also replace antibiotics additives in animal feeds because of their antibacterial and antiviral effects [71,72]. However, the anti-nutritive effects of CTs should be noted, as they can inhibit important enzyme activities and the digestion of macro- and micronutrients, thus decreasing digestion and growth efficiency. According to the results of this study and of other studies, we proceeded with the AC3 fraction for further analysis.
3.3. Measurement of the total phenol and condensed tannin contents of methanolic sorghum extracts
Colour is a key marker for evaluating the bioactivity of a seed or fruit. In addition to their important physiological properties, anthocyanins and proanthocyanidins also function as pigments, giving seeds a reddish hue. Polyphenols also dissolve well in alcohol; thus, they are easily extractable using an alcohol solution. The total phenol and condensed tannin contents of the two varieties are shown in Fig. 6. It is observed from the measured total phenol and CT contents that the two varieties are significantly different from each other. The total phenol content of the Zádor variety was 808.04 ± 63.89 mg.100 g-1 DM, while that of the Albita variety was only 81.56 ± 3.87 mg.100 g-1 DM. These compounds are stored mainly in the seed coat, with minimal amounts found in the endosperm. This was supported by our preliminary analysis, in which there was no traceable tannin content in the refined sorghum flour, and the total phenol content was low. The CT content of the samples varied between 8.81 ± 1.74 mg g−1 DM for the Zádor variety and 0.65 ± 0.05 mg g−1 DM for the Albita variety, as expected. The Albita variety was reported to be a tannin-free variety, and the low values can be explained by environmental effects or interference from other compounds in the applied measurement methods.
Fig. 6.
Total polyphenol and condensed tannin contents of red and white sorghum varieties. Note: *** = p < 0.001. The results of this measurement together with other varieties are published in the following article referenced here as a part of another evaluation [57].
Therefore, there are significant differences in the total phenol and tannin contents depending on the variety and seed colour, as supported by the literature. Our results were in line with those of previous research performed on sorghum. Dykes et al. (2005) evaluated several sorghum genotypes and reported that the highest tannin content occurred in red sorghum, in which the catechin equivalent (CE) concentration was 11–12 mg g−1. The plants of both varieties were shown to have a specific layer of tannin accumulation, named the pigmented testa under the seed coat [68]. In the case of white sorghum varieties, Afify et al. (2012) published research on exploring their bioactive profiles. The authors found CT contents as high as 0.013–0.21 mg g−1 CE [73]. Further research has also established that the change in tannin content occurs broadly depending on the genotype and environmental factors, exhibiting a range of 1.2–30 mg g−1 [74,75]. Shen et al. (2018) explored the total phenol and flavonoid contents of several sorghum varieties and genotypes and reported phenol contents between 174 and 1240 mg.GAE 100 g−1 DM [76]. Ghimire et al. (2021) also reported significant differences between sorghum varieties worldwide (USA, Korea, India), with values ranging from 10 to 300 mg.GAE g−1 [77]. These findings are of particular importance because they determine the utilization potential of a specific sorghum variety. Due to their complex structure, CTs can chelate most macro- and micronutrients as well as metal ions, thus producing hard-to-digest complexes; additionally, CTs have high physiological importance. The anti-nutritive effects of CTs are undesirable because they inhibit animal growth and decrease feed efficiency. For these reasons, white sorghum varieties are generally more commonly used than red sorghum varieties in animal husbandry as feed. Moreover, a low tannin content can be exploited to decrease energy intake by creating slowly digestible starch (SDS) and resistant starch (RS), slowing glucose absorption and thus lowering the glycaemic index of foods [[78], [79], [80]].
3.4. Determination of the bioactive profiles of red and white sorghum varieties using UHPLC-ESI-ORBITRAP-MS/MS
The MS/MS technique was used for qualitative analysis, and all identified compounds are shown in Table 1. The red variety contained multiple isomers of procyanidin dimers and trimers (procyanidin A, B, and C) with retention times of 12–19 min, as well as catechin monomers, which are the building blocks of these procyanidins. Sorghum-specific eriodictyol and its derivatives were also found in the Zádor variety. The Albita variety did not contain any procyanidin-type tannins, but both varieties had common flavones or flavanones (naringenin, luteolin, apigenin) and sorghum-specific 3-deoxyanthocyanidins (luteolinidin, apigeninidin, and their methylated forms). The extraction solvent did not influence the flavonoid profile of the different samples; thus, it was not selective for CTs.
Table 1.
Identified flavonoid compounds of the evaluated varieties.
| Flavonoid group | Name | Formula | Variety | Retention time | [M − H]- |
|---|---|---|---|---|---|
| Flavans, flavan-3-ols, flavan-4-ols |
Catechin | C15H14O6 | Zádor | 14.19 | 289.07121 |
| Luteoforol | C15H14O6 | Zádor, Albita | 19.55 | 289.07121 | |
| Luteoliflavan (Proluteolinidin) |
C15H14O5 |
Zádor, Albita |
21.50 |
273.07630 |
|
| Deoxyantocianidin |
Luteolinidin | C15H10O5 | Zádor, Albita | 21.53 | 269.04500 |
| Apigeninidin | C15H10O4 | Zádor, Albita | 22.59 | 253.05008 | |
| Methoxyapigeninidin | C16H12O4 | Zádor | 23.24 | 267.06573 | |
| Methoxyluteolinidin | C16H12O5 | Zádor, Albita | 22.34 | 283.06065 | |
| Gesnerin (Apigeninidin-5-O-glucoside) |
C21H20O9 |
Zádor, Albita |
26.98 |
415.10291 |
|
| Flavanone |
Eriodictyol-O-glucoside | C21H22O11 | Zádor | 19.35 | 449.10839 |
| 5-O-Glucosylluteoliflavan-(4->8)-eriodictyol isomer 1 | C42H44O21 | Zádor | 19.86 | 883.22968 | |
| 5-O-Glucosylluteoliflavan-(4->8)-eriodictyol isomer 2 | C42H44O21 | Zádor | 20.96 | 883.22968 | |
| Naringenin (4′,5,7-Trihydroxyflavanone) |
C15H12O5 |
Zádor, Albita |
27.79 |
271.06065 |
|
| Flavone |
Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | Zádor, Albita | 28.48 | 285.03991 |
| Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | Zádor, Albita | 30.30 | 269.04500 | |
| Tricin (3′,5′-Dimethoxy-4′,5,7-trihydroxyflavone) | C17H14O7 | Zádor, Albita | 30.49 | 329.06613 | |
| Chrysoeriol (3′-Methoxy-4′,5,7-trihydroxyflavone) |
C16H12O6 |
Zádor, Albita |
30.54 |
299.05556 |
|
| Proanthocyanidin | Procyanidin A isomer 1 | C30H24O12 | Zádor | 14.66 | 575.11896 |
| Procyanidin A isomer 2 | C30H24O12 | Zádor | 17.56 | 575.11896 | |
| Procyanidin A isomer 3 | C30H24O12 | Zádor | 18.01 | 575.11896 | |
| Procyanidin A isomer 4 | C30H24O12 | Zádor | 19.25 | 575.11896 | |
| Procyanidin A isomer 5 | C30H24O12 | Zádor | 23.24 | 575.11896 | |
| Procyanidin B isomer 1 | C30H26O12 | Zádor | 12.31 | 577.13460 | |
| Procyanidin B isomer 2 | C30H26O12 | Zádor | 13.08 | 577.13460 | |
| Procyanidin B isomer 3 | C30H26O12 | Zádor | 17.91 | 577.13460 | |
| Procyanidin C isomer 1 | C45H38O18 | Zádor | 13.39 | 865.19799 | |
| Procyanidin C isomer 2 | C45H38O18 | Zádor | 13.75 | 865.19799 | |
| Procyanidin C isomer 3 | C45H38O18 | Zádor | 14.96 | 865.19799 |
Xiong and his co-researchers (2020) studied sorghum varieties from Australia via an HPLC-DAD-ESI-QTOF-MS/MS system for their phenolic acid and flavonoid profiles. These authors found flavone, flavanone, 3-deoxyanthocyanidins and their derivatives, such as apigenin, luteolin, naringenin, and eriodictyol, in brown and red sorghum varieties. Kang et al. (2016) reported similar results. Both studies revealed similar compounds in different varieties, as we observed in our research, and we also identified some procyanidin isomers only in the red genotype [81,82].
During digestion, the polymerization degree and size of a molecule are among the factors that determine whether a nutrient can be absorbed in the gut. CTs and other flavonoid oligomers can be absorbed, generally, as dimers or trimers due to partial or complete hydrolysis steps. Tannins above this size are strong chelators that inhibit enzymes and can form complexes. These products are digested only in the colon by fermentation to produce beneficial short-chain fatty acids (SCFAs) [83].
3.5. Quantitative measurement of sorghum flavonoids using the HPLC-PDA and UHPLC-PDA systems
Sorghum-specific flavonoids, namely, procyanidin B1, catechin, naringin, luteolin, and apigenin, were quantified using HPLC, the results of which can be found in Table 2. There were significant differences between the varieties. The red variety, Zádor, contained a high amount of procyanidin B1, with a concentration of 81.03 mg.100 g-1, and catechin, with 11.84 mg. 100 g−1 DM. This variety also contained a higher concentration of naringenin than did the Albita variety. Despite the presence of apigenin, which was above the limit of detection in this system for both varieties, the concentration was below the MS/MS detection limit.
Table 2.
Red (Zádor) and white (Albita) sorghum flavonoid content measured by HPLC-PDA.
| Variety | Component | Concentration mg.100 g-1 DW |
|---|---|---|
| Zádor | Procyanidin B1 | 81.03 |
| Catechin | 11.84 | |
| Naringenin | 19.88 | |
| Albita | Naringenin | 2.05 |
Taleon et al. (2014) published similar results for seven sorghum varieties (red, yellow) for the flavonoid profile [41]. Our findings were consistent with those of other studies on naringenin and procyanidin B1 concentrations. The highest value of naringenin measured was 640 μg g−1 DM, while the total procyanidin B1 content was as high as 10 mg g−1 depending on the variety [75,84,85].
These results further support that white sorghum varieties are more suitable than coloured varieties for animal feed use due to the low CT content, but these varieties have poorer antioxidant properties. Red varieties can be used for food fortification with antioxidants, but there should be awareness about the CT content of these seeds. Additionally, a sufficient naringenin content in seeds is a key factor for 3-deoxyanthocyanidin synthesis.
3.6. Evaluation of tocopherol distribution in red and white sorghum seeds
Whole-grain flours are important sources of fibre and vitamin because they contain whole seed components rather than only selected components. Sorghum flour is usually available as a whole-grain flour, is an important source of vitamin B and vitamin E and is also rich in vitamin A and other carotenoids. Vitamin E comprises 4 distinct forms of tocopherol (α, β, γ, δ) commonly found in plants. These tocopherols are soluble in fat; thus, they accumulate in oily seeds or in the seed germ [[86], [87], [88], [89]]. Among tocopherols, α-, and γ-tocopherols are considered as primary vitamin E source with high antioxidant power, as they have the highest vitamin activity in blood plasma. Vitamin E is also important for maintaining redox homeostasis and preventing lipid peroxidation [[90], [91], [92], [93]]. However, recent studies have shown that these tocopherols can also exhibit beneficial effects mainly in the colon; regulating gut microbiota and preventing colon cancer [[94], [95], [96], [97], [98], [99]].The tocopherol distribution was determined from both the bran and flour fractions, and the results are shown in Fig. 7. All four tocopherols were detectable in both varieties using HPLC. Due to structural similarities, β - and γ-tocopherols were difficult to separate, and they constituted the vast majority of the tocopherol content for both varieties, with values of 6.68–7.75 μg g−1 DM for Zádor bran and flour and 2.95–5.78 μg g−1 DM for Albita bran and flour. Additionally, there were significant differences between varieties (p < 0.001). Delta-tocopherol was found only in the flour fraction, with concentrations of 0.42 and 0.53 μg g−1 in Albita and Zádor flours, respectively. There was no statistical significance between the varieties. The alpha-tocopherol content ranged between 2.75 and 1.37 μg g−1 in the bran samples, with significant differences (p < 0.001), and between 1.7 and 1.44 μg g−1 in the flours, with no significant differences. These results suggest that germ fragmentation occurs during the peeling process, which results in the loss of tocopherols in refined flour.
Fig. 7.
Distribution of tocopherols in red (Zádor) and white (Albita) sorghum bran and flour. Note: ****p < 0.0001, ***p < 0.001. ns = not significant.
Pinherio et al. (2021) reported similar alpha-tocopherol contents in sorghum at a concentration of 2.8 μg g−1, while other tocopherol forms were present at concentrations below 1 μg g−1. In contrast, Cardoso et al. (2015) studied more than 100 sorghum genotypes with beta-, gamma-, and delta-tocopherol contents ranging from 0 to 7.80, 1.74–21.09, and 0–3.79 μg g−1, respectively [55,100,101]. According to our results and the literature, S. bicolor belongs to the gamma- and beta-tocopherol accumulator group, and sorghum consumption can be beneficial for colon health.
3.7. Determination of vitamin B complex concentrations in sorghum seeds
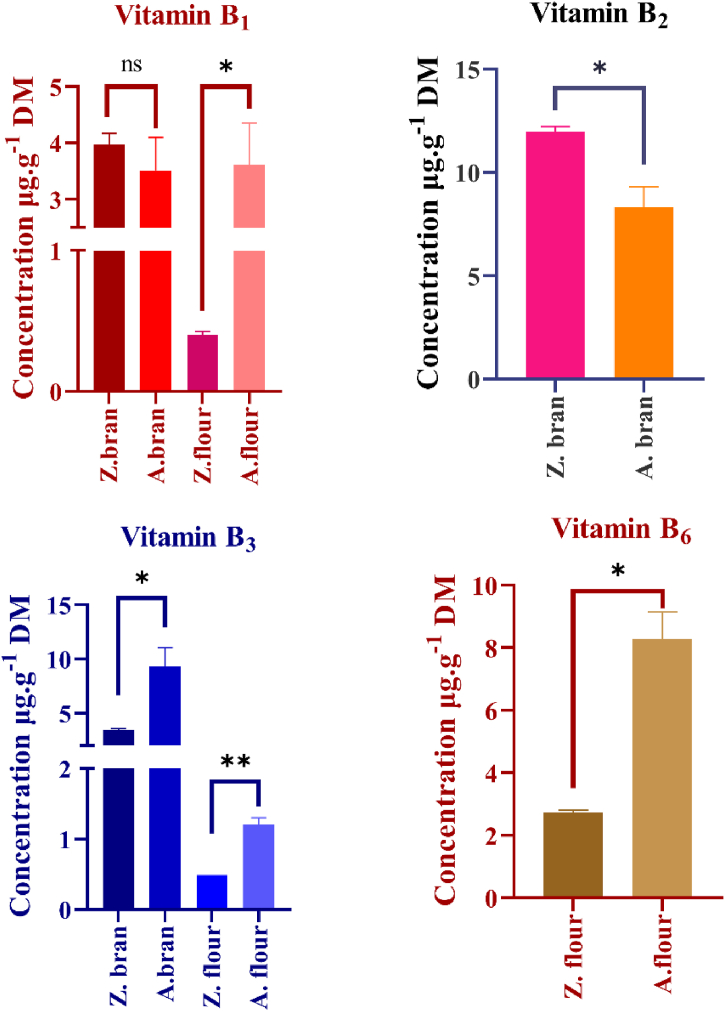
Another important vitamin group in cereals is vitamin B complexes. B vitamins commonly found in cereals have been identified using an HPLC-DAD system, and the results are shown in Fig. 8. Thiamine (B1), riboflavin (B2), niacin (B3), and pyridoxine (B6) are commonly found in cereal grains. Among these compounds, pyridoxine is particularly important because it is a relatively heat stable vitamin and does not degrade during the thermal processing of flours. Niacin was also abundant in the bran fraction of the two varieties, with concentrations of 3.45 ± 0.11 μg g−1 and 9.32 ± 1.71 μg g−1 for Zádor and Albita varieties, respectively. The thiamine content was similar in both bran, with no significant differences, while significant difference (p < 0.05) was observed in flours. Riboflavin was found in higher amount with 12 and 8.35 μg g−1 concentrations (p < 0.05) in Zádor and Albita bran. Pyridoxine was found in both flours, and the Albita variety flour contained more pyridoxine than did the Zádor flour. The vitamin content of the flours was lower than that of the bran samples, as vitamin B complexes have been determined to be synthesized and stored mainly in the seed coat. The lower values observed in the case of the Zádor variety can be attributed to the presence of CTs, which can also form complexes with micronutrients.
Fig. 8.
Vitamin B profiles of red (Zádor) and white (Albita) sorghum varieties. Notes: Z. = Zádor, A. = Albita, *: p < 0.05, **: p < 0.01, ***: p < 0.001.
During sample preparation, fractionation was also performed with methanol and ammonium hydroxide. B vitamins were detected in both fractions at different concentrations. The results can be seen in Table 3. Most B vitamins could be found in both fractions, while riboflavin was detected only in bran fractions. Overall, the Albita variety contained more B vitamins than did the Zádor variety. This can be attributed to the presence of CTs in the red variety. There was no clear difference between the methanol and ammonium fractions.
Table 3.
Distribution of vitamin B complexes in red and white sorghum varieties. Notes: Values are shown in μg.g−1dry matter.
| Variety | Sample type | Fraction | Niacin | Pyridoxine | Thiamine | Riboflavin |
|---|---|---|---|---|---|---|
| Albita | Bran | MeOH | 9.32 ± 1.71 | nd. | nd. | 8.31 ± 1.00 |
| NH4 | nd. | nd. | 3.51 ± 0.58 | nd. | ||
| Flour | MeOH | 0.71 ± 0.12 | 2.89 ± 0.51 | 2.41 ± 1.16 | nd. | |
| NH4 | 0.50 ± 0.02 | 5.39 ± 1.37 | 1.21 ± 0.40 | nd. | ||
| Zádor | Bran | MeOH | nd. | nd. | 3.77 ± 0.18 | 1.89 ± 0.08 |
| NH4 | 3.47 ± 0.10 | nd. | 0.31 ± 0.02 | 10.10 ± 0.33 | ||
| Flour | MeOH | 0.22 ± 0.02 | 0.53 ± 0.0.2 | 0.40 ± 0.03 | nd. | |
| NH4 | 0.34 ± 0.07 | 2.02 ± 0.21 | nd. | nd. |
The literature data suggest that the B vitamin composition of sorghum is quite diverse. According to the literature, the thiamine concentration ranged from 2.32 to 23 μg g−1, the pyridoxine concentration ranged from 1 to 8.1 μg g−1, and the riboflavin concentration ranged from 0.5 to 1.42 μg g−1. Further studies revealed high niacin concentrations, with values between 17 and 30 μg g−1 [100,[102], [103], [104]]. Compared to these results, we found a slight increase in the presence of riboflavin in the bran and an increase in the thiamine content. Overall, these results suggest that sorghum flour is more advantageous for consumption as a whole grain, as it can be a decent B vitamin source for human consumption.
4. Conclusion
In this study, the bioactive composition of locally produced red (Zádor) and white (Albita) sorghum varieties were analysed with respect to their potential use in human food products and animal feed. We have found that Zádor is a rich source of compounds with strong antioxidant properties, most of them belonging to flavonoids and tocopherols. Measured antioxidant capacity was 182.64 ± 14.91 μmol TE g−1 DM, and 72.79 ± 1.59 μmol TE g−1 DM for TEAC, and DPPH respectively. Albita had significantly lower antioxidant capacity with 7.62 ± 0.80 μmol TE g−1 DM, and 5.20 ± 0.81 μmol TE g−1 DM values for TEAC and DPPH. Among flavonoids procyanidin type condensed tannin dimers, and trimers were most abundantly present in case of Zádor, while Albita proved to be tannin free. These condensed tannins are responsible for the high antioxidant values of Zádor, due to their multiple bonding sites. The investigated varieties were also rich in vitamins such as vitamin E, and several type of vitamin B. Beta -, and gamma tocopherols were the most abundant tocopherols with 6.68–7.75 μg g−1 DM in Zádor, and 2.95–5.78 μg g−1 DM in Albita bran and flour fraction respectively. Both flour and bran samples contained significant amount of tocopherols. During milling, the germ can be fragmented and sieved from the flour during refining, thus causing significant losses in the tocopherol content of sorghum flours. Among B vitamins riboflavin and niacin were the most abundant B vitamins with 12 and 9.32 μg g−1 DM concentrations in Zádor and Albita brans respectively. Contrary to tocopherol composition, Albita showed higher vitamin B content compared to Zádor.
Based on these results, we concluded although both varieties are fit for human consumption. Zádor has a higher bioactive value due to its high antioxidant and tocopherol content, and it can be recommended as a food ingredient for gluten free product development. This opportunity is further strengthen by the fact, that it is more and more important to consume food products with high additional values such as food rich in minerals or antioxidants. However, the anti-nutritional effects of CTs such as slower glucose metabolism and absorption should be taken into account during consumption or utilization. Meanwhile the other variety, Albita is considered as a tannin free variety, and it is a suitable candidate for animal feeding purposes. Furthermore, its rich vitamin B content also makes Albita a good fit for human consumption as well, despite its lower antioxidant value. It also should be mentioned, that sorghum has excellent tolerance against droughts and other severe environmental conditions, which in turn makes its production more cost effective and cheaper compared to other grains, and in many areas it is considered as an alternative to maize by farmers and agricultural companies as well.
Funding
Financial support was received from TKP2021-NKTA funding scheme provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund.
Data availability
Data used for this research hasn't been deposited into a public repository. However, data used in the study is available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Róbert Nagy: Writing – review & editing, Writing – original draft, Visualization, Resources, Investigation, Formal analysis. Andrea Kun-Nemes: Validation, Methodology, Investigation. Erzsébet Szőllősi: Validation, Methodology, Investigation. Piroska Bíróné Molnár: Methodology. Zoltán Cziáky: Validation, Methodology, Investigation. Eszter Murányi: Resources. Péter Sipos: Supervision. Judit Remenyik: Writing – review & editing, Writing – original draft, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank our colleagues from the Department of Applied Plant Biology (Faculty of Agricultural and Food Sciences and Environmental Management, University of Debrecen) and our colleagues from the Research Institute of Karcag (Hungarian University of Agriculture and Life Sciences) for providing the samples and the weather data for the research. We would also like to thank our coauthor Dr. Zoltán Cziáky and the Agricultural and Molecular Research and Service Group (Institute of Technology and Agricultural Sciences, University of Nyíregyháza) for all their support in the investigation.
References
- 1.Kakabouki I., Tataridas A., Mavroeidis A., Kousta A., Roussis I., Katsenios N., Efthimiadou A., Papastylianou P. Introduction of alternative crops in the Mediterranean to satisfy EU Green Deal goals. A review. Agron. Sustain. Dev. 2021;41:71. doi: 10.1007/s13593-021-00725-9. [DOI] [Google Scholar]
- 2.Mavroeidis A., Roussis I., Kakabouki I. The role of alternative crops in an upcoming global food crisis: a concise review. Foods. 2022;11:3584. doi: 10.3390/foods11223584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermuth J., Janovská D., Čepková P.H., Usťak S., Strašil Z., Dvořáková Z., Hermuth J., Janovská D., Čepková P.H., Usťak S., Strašil Z., Dvořáková Z. Alternative Crops and Cropping Systems. IntechOpen; 2016. Sorghum and foxtail millet—promising crops for the changing climate in central Europe. [DOI] [Google Scholar]
- 4.Trnka M., Feng S., Semenov M.A., Olesen J.E., Kersebaum K.C., Rötter R.P., Semerádová D., Klem K., Huang W., Ruiz-Ramos M., Hlavinka P., Meitner J., Balek J., Havlík P., Büntgen U. Mitigation efforts will not fully alleviate the increase in water scarcity occurrence probability in wheat-producing areas. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aau2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bean S.R., Wilson J.D., Moreau R.A., Galant A., Awika J.M., Kaufman R.C., Adrianos S.L., Ioerger B.P. Sorghum. John Wiley & Sons, Ltd; 2019. Structure and composition of the sorghum grain; pp. 173–214. [DOI] [Google Scholar]
- 6.FAOSTAT, (n.d.). https://www.fao.org/faostat/en/#data/QCL/visualize (accessed October 6, 2022).
- 7.Hossain MdS., Islam MdN., Rahman MdM., Mostofa M.G., Khan MdA.R. Sorghum: a prospective crop for climatic vulnerability, food and nutritional security. J. Agri. Food Res. 2022;8 doi: 10.1016/j.jafr.2022.100300. [DOI] [Google Scholar]
- 8.Taylor J.R.N., Duodu K.G. Resistant-type starch in sorghum foods—factors involved and health implications. Starch - Stärke n/a. 2022 doi: 10.1002/star.202100296. [DOI] [Google Scholar]
- 9.Teixeira N. de C., Queiroz V.A.V., Rocha M.C., Amorim A.C.P., Soares T.O., Monteiro M.A.M., de Menezes C.B., Schaffert R.E., Garcia M.A.V.T., Junqueira R.G. Resistant starch content among several sorghum (Sorghum bicolor) genotypes and the effect of heat treatment on resistant starch retention in two genotypes. Food Chem. 2016;197:291–296. doi: 10.1016/j.foodchem.2015.10.099. [DOI] [PubMed] [Google Scholar]
- 10.Dalton T.J., Hodjo M. In: Sorghum in the 21st Century: Food – Fodder – Feed – Fuel for a Rapidly Changing World. Tonapi V.A., Talwar H.S., Are A.K., Bhat B.V., Reddy ChR., Dalton T.J., editors. Springer; Singapore: 2020. Trends in global production, consumption, and utilization of sorghum; pp. 3–15. [DOI] [Google Scholar]
- 11.Loh W., Tang M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Publ. Health. 2018;15:2043. doi: 10.3390/ijerph15092043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gargano D., Appanna R., Santonicola A., De Bartolomeis F., Stellato C., Cianferoni A., Casolaro V., Iovino P. Food allergy and intolerance: a narrative review on nutritional concerns. Nutrients. 2021;13:1638. doi: 10.3390/nu13051638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adebo O.A. African sorghum-based fermented foods: past, current and future prospects. Nutrients. 2020;12:1111. doi: 10.3390/nu12041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aruna C., Visarada K.B.R.S. In: Breeding Sorghum for Diverse End Uses. Aruna C., Visarada K.B.R.S., Bhat B.V., Tonapi V.A., editors. Woodhead Publishing; 2019. Chapter 13 - sorghum grain in food and brewing industry; pp. 209–228. [DOI] [Google Scholar]
- 15.Dayakar Rao B. In: Breeding Sorghum for Diverse End Uses. Aruna C., Visarada K.B.R.S., Bhat B.V., Tonapi V.A., editors. Woodhead Publishing; 2019. Chapter 23 - sorghum value chain for food and fodder security; pp. 409–419. [DOI] [Google Scholar]
- 16.Przybylska-Balcerek Anna, Frankowski Jakub, Stuper-Szablewska Kinga. Bioactive compounds in sorghum. Eur. Food Res. Technol. 2019;245:1075–1080. doi: 10.1007/s00217-018-3207-0. [DOI] [Google Scholar]
- 17.Girard A.L., Awika J.M. Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J. Cereal. Sci. 2018;84:112–124. doi: 10.1016/j.jcs.2018.10.009. [DOI] [Google Scholar]
- 18.Wu G., Bennett S.J., Bornman J.F., Clarke M.W., Fang Z., Johnson S.K. Phenolic profile and content of sorghum grains under different irrigation managements. Food Res. Int. 2017;97:347–355. doi: 10.1016/j.foodres.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Kang J., Price W.E., Ashton J., Tapsell L.C., Johnson S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016;211:215–226. doi: 10.1016/j.foodchem.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Khoddami A., Truong H.H., Liu S.Y., Roberts T.H., Selle P.H. Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broiler chickens. Anim. Feed Sci. Technol. 2015;210:190–199. doi: 10.1016/j.anifeedsci.2015.09.029. [DOI] [Google Scholar]
- 21.Cardoso L. de M., Pinheiro S.S., de Carvalho C.W.P., Queiroz V.A.V., de Menezes C.B., Moreira A.V.B., de Barros F.A.R., Awika J.M., Martino H.S.D., Pinheiro-Sant’Ana H.M. Phenolic compounds profile in sorghum processed by extrusion cooking and dry heat in a conventional oven. J. Cereal. Sci. 2015;65:220–226. doi: 10.1016/j.jcs.2015.06.015. [DOI] [Google Scholar]
- 22.Pinheiro S.S., Anunciação P.C., Cardoso L. de M., Della Lucia C.M., de Carvalho C.W.P., Queiroz V.A.V., Pinheiro Sant'Ana H.M. Stability of B vitamins, vitamin E, xanthophylls and flavonoids during germination and maceration of sorghum (Sorghum bicolor L.) Food Chem. 2021;345 doi: 10.1016/j.foodchem.2020.128775. [DOI] [PubMed] [Google Scholar]
- 23.Ofosu F.K., Elahi F., Daliri E.B.-M., Yeon S.-J., Ham H.J., Kim J.-H., Han S.-I., Oh D.-H. Flavonoids in decorticated sorghum grains exert antioxidant, antidiabetic and antiobesity activities. Molecules. 2020;25:2854. doi: 10.3390/molecules25122854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awika J. ACS Symposium Series. 2011. Sorghum flavonoids: unusual compounds with promising implications for health; pp. 171–200. [DOI] [Google Scholar]
- 25.Tohge T., Watanabe M., Hoefgen R., Fernie A. Shikimate and phenylalanine Biosynthesis in the green lineage. Front. Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00062. https://www.frontiersin.org/articles/10.3389/fpls.2013.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackerman A., Wenndt A., Boyles R. The sorghum grain mold disease complex: pathogens, host responses, and the bioactive metabolites at play. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.660171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macáková K., Kolečkář V., Cahlíková L., Chlebek J., Hošťálková A., Kuča K., Jun D., Opletal L. Recent Advances in Medicinal Chemistry. Bentham Science Publishers, Emirate of Sharjah, United Arab Emirates; 2014. Chapter 6. Tannins and their influence on health; p. 469. [Google Scholar]
- 28.Das A.K., Islam MdN., Faruk MdO., Ashaduzzaman Md, Dungani R. Review on tannins: extraction processes, applications and possibilities. South Afr. J. Bot. 2020;135:58–70. doi: 10.1016/j.sajb.2020.08.008. [DOI] [Google Scholar]
- 29.Sharma K.P. Tannin degradation by phytopathogen's tannase: a Plant's defense perspective. Biocatal. Agric. Biotechnol. 2019;21 doi: 10.1016/j.bcab.2019.101342. [DOI] [Google Scholar]
- 30.Panzella L., Napolitano A. Condensed tannins, a viable solution to meet the need for sustainable and effective multifunctionality in food packaging: structure, sources, and properties. J. Agric. Food Chem. 2022;70:751–758. doi: 10.1021/acs.jafc.1c07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dykes L. Tannin analysis in sorghum grains. Methods Mol. Biol. 2019;1931:109–120. doi: 10.1007/978-1-4939-9039-9_8. [DOI] [PubMed] [Google Scholar]
- 32.Yan Z., Zhong Y., Duan Y., Chen Q., Li F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Animal Nutrition. 2020;6:115–123. doi: 10.1016/j.aninu.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truong V.-L., Jeong W.-S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Wellness. 2022;11:502–511. doi: 10.1016/j.fshw.2021.12.008. [DOI] [Google Scholar]
- 34.Oyenihi A.B., Smith C. Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? J. Ethnopharmacol. 2019;229:54–72. doi: 10.1016/j.jep.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Montenegro-Landívar M.F., Tapia-Quirós P., Vecino X., Reig M., Valderrama C., Granados M., Cortina J.L., Saurina J. Polyphenols and their potential role to fight viral diseases: an overview. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patra S., Pradhan B., Nayak R., Behera C., Das S., Patra S.K., Efferth T., Jena M., Bhutia S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: clinical evidences and molecular mechanisms of action. Phytomedicine. 2021;90 doi: 10.1016/j.phymed.2021.153554. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 38.Mithul Aravind S., Wichienchot S., Tsao R., Ramakrishnan S., Chakkaravarthi S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021;142 doi: 10.1016/j.foodres.2021.110189. [DOI] [PubMed] [Google Scholar]
- 39.Grosso G. Effects of polyphenol-rich foods on human health. Nutrients. 2018;10:1089. doi: 10.3390/nu10081089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang B., Zhang Y., Xing X., Wang S. Health benefits of dietary polyphenols: insight into interindividual variability in absorption and metabolism. Curr. Opin. Food Sci. 2022 doi: 10.1016/j.cofs.2022.100941. [DOI] [Google Scholar]
- 41.Taleon V., Dykes L., Rooney W.L., Rooney L.W. Environmental effect on flavonoid concentrations and profiles of red and lemon-yellow sorghum grains. J. Food Compos. Anal. 2014;34:178–185. doi: 10.1016/j.jfca.2014.03.003. [DOI] [Google Scholar]
- 42.Etuk E., Okeudo N., Esonu B.O., Udedibie A. Antinutritional factors in sorghum: chemistry, mode of action and effects on livestock and poultry. Online J. Anim. Feed Res. 2012;2 [Google Scholar]
- 43.Zarei M., Amirkolaei A.K., Trushenski J.T., Sealey W.M., Schwarz M.H., Ovissipour R. Sorghum as a potential valuable aquafeed ingredient: nutritional quality and digestibility. Agriculture. 2022;12:669. doi: 10.3390/agriculture12050669. [DOI] [Google Scholar]
- 44.Manzoor F., Nisa M.U., Hussain H.A., Khan M.K., Ahmad R.S., Ahmad N., Imran M., Umbreen H. Effect of hydrolysable tannin on nutrient intake obesity and other associated metabolic risk factors in polycystic rats. Translational Medicine Communications. 2021;6:10. doi: 10.1186/s41231-021-00089-y. [DOI] [Google Scholar]
- 45.Li Y., Zhu L., Guo C., Xue M., Xia F., Wang Y., Jia D., Li L., Gao Y., Shi Y., He Y., Yuan C. Dietary intake of hydrolyzable tannins and condensed tannins to regulate lipid metabolism. Mini Rev. Med. Chem. 2022;22:1789–1802. doi: 10.2174/1389557522666211229112223. [DOI] [PubMed] [Google Scholar]
- 46.Fraga-Corral M., Otero P., Cassani L., Echave J., Garcia-Oliveira P., Carpena M., Chamorro F., Lourenço-Lopes C., Prieto M.A., Simal-Gandara J. Traditional applications of tannin rich extracts supported by scientific data: chemical composition, bioavailability and bioaccessibility. Foods. 2021;10:251. doi: 10.3390/foods10020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molino S., Pilar Francino M., Ángel Rufián Henares J. Why is it important to understand the nature and chemistry of tannins to exploit their potential as nutraceuticals? Food Res. Int. 2023;173 doi: 10.1016/j.foodres.2023.113329. [DOI] [PubMed] [Google Scholar]
- 49.Saminathan M., Tan H.Y., Sieo C.C., Abdullah N., Wong C.M.V.L., Abdulmalek E., Ho Y.W. Polymerization degrees, molecular weights and protein-binding affinities of condensed tannin fractions from a leucaena leucocephala hybrid. Molecules. 2014;19:7990–8010. doi: 10.3390/molecules19067990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerns J.C., Gutierrez J.L. Thiamin. Adv. Nutr. 2017;8:395–397. doi: 10.3945/an.116.013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martel J.L., Kerndt C.C., Doshi H., Franklin D.S. StatPearls. StatPearls Publishing; Treasure Island (FL): 2023. Vitamin B1 (thiamine)http://www.ncbi.nlm.nih.gov/books/NBK482360/ [PubMed] [Google Scholar]
- 52.Dalto D.B., Matte J.-J. Pyridoxine (vitamin B6) and the glutathione peroxidase system; a link between one-carbon metabolism and antioxidation. Nutrients. 2017;9:189. doi: 10.3390/nu9030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parra M., Stahl S., Hellmann H. Vitamin B6 and its role in cell metabolism and physiology. Cells. 2018;7:84. doi: 10.3390/cells7070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mani S., Li H., Untereiner A., Wu L., Yang G., Austin R.C., Dickhout J.G., Lhoták Š., Meng Q.H., Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 55.Cardoso L. de M., Pinheiro S.S., da Silva L.L., de Menezes C.B., de Carvalho C.W.P., Tardin F.D., Queiroz V.A.V., Martino H.S.D., Pinheiro-Sant’Ana H.M. Tocochromanols and carotenoids in sorghum (Sorghum bicolor L.): diversity and stability to the heat treatment. Food Chem. 2015;172:900–908. doi: 10.1016/j.foodchem.2014.09.117. [DOI] [PubMed] [Google Scholar]
- 56.Rizvi S., Raza S.T., Ahmed F., Ahmad A., Abbas S., Mahdi F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ Med J. 2014;14:e157–e165. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997530/ [PMC free article] [PubMed] [Google Scholar]
- 57.Nagy R., Murányi E., Bíróné Molnár P., Szepesi J., Győri Z., Veres S., Remenyik J., Sipos P. Assessment of bioactive profile of sorghum brans under the effect of growing conditions and nitrogen fertilization. Agriculture. 2023;13:760. doi: 10.3390/agriculture13040760. [DOI] [Google Scholar]
- 58.Bíró A., Nemes A., Remenyik J. Sour cherry seed as an industrial gamma tocopherol source. Acta Agraria Debreceniensis. 2015:27–33. doi: 10.34101/actaagrar/63/1831. [DOI] [Google Scholar]
- 59.Nemes A., Szőllősi E., Stündl L., Biró A., Homoki J.R., Szarvas M.M., Balogh P., Cziáky Z., Remenyik J. Determination of flavonoid and proanthocyanidin profile of Hungarian sour cherry. Molecules. 2018;23:3278. doi: 10.3390/molecules23123278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price M.L., Van Scoyoc S., Butler L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978;26:1214–1218. doi: 10.1021/jf60219a031. [DOI] [Google Scholar]
- 61.Dias M.C., Pinto D.C.G.A., Silva A.M.S. Plant flavonoids: chemical characteristics and biological activity. Molecules. 2021;26:5377. doi: 10.3390/molecules26175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biochemistry and Molecular Biology of Plants, (n.d.). https://www.nhbs.com/biochemistry-and-molecular-biology-of-plants-book (accessed December 6, 2022).
- 63.Rippin, Sharma A.K., Beniwal V. Biosynthesis and medicinal applications of proanthocyanidins: a recent update. Biocatal. Agric. Biotechnol. 2022;45 doi: 10.1016/j.bcab.2022.102500. [DOI] [Google Scholar]
- 64.Munteanu I.G., Apetrei C. Analytical methods used in determining antioxidant activity: a review. Int. J. Mol. Sci. 2021;22:3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiremba Taylor, Duodu G. Phenolic content, antioxidant activity, and consumer acceptability of sorghum cookies. Cereal Chem. 2009;86:590–594. doi: 10.1094/CCHEM-86-5-0590. [DOI] [Google Scholar]
- 66.Awika J.M., Rooney L.W., Wu X., Prior R.L., Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003;51:6657–6662. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- 67.Choi S.C., Kim J.M., Lee Y.G., Kim C. Antioxidant activity and contents of total phenolic compounds and anthocyanins according to grain colour in several varieties of sorghum bicolor (L.) Moench. Cereal Res. Commun. 2019;47:228–238. doi: 10.1556/0806.47.2019.14. [DOI] [Google Scholar]
- 68.Dykes L., Rooney L.W., Waniska R.D., Rooney W.L. Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. J. Agric. Food Chem. 2005;53:6813–6818. doi: 10.1021/jf050419e. [DOI] [PubMed] [Google Scholar]
- 69.Sreeramulu D., Reddy C.V.K., Raghunath M. Antioxidant activity of commonly consumed cereals, millets, pulses and legumes in India. Indian J. Biochem. Biophys. 2009;46:112–115. [PubMed] [Google Scholar]
- 70.Hou F., Su D., Xu J., Gong Y., Zhang R., Wei Z., Chi J., Zhang M. Enhanced extraction of phenolics and antioxidant capacity from sorghum (sorghum bicolor L. Moench) shell using ultrasonic-assisted ethanol–water binary solvent. J. Food Process. Preserv. 2016;40:1171–1179. doi: 10.1111/jfpp.12699. [DOI] [Google Scholar]
- 71.Ma M., Chambers J.K., Uchida K., Ikeda M., Watanabe M., Goda Y., Yamanaka D., Takahashi S.-I., Kuwahara M., Li J. Effects of supplementation with a quebracho tannin product as an alternative to antibiotics on growth performance, diarrhea, and overall health in early-weaned piglets. Animals. 2021;11:3316. doi: 10.3390/ani11113316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Q., Liu X., Zhao G., Hu T., Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. 2018;4:137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Afify A.E.-M.M., El-Beltagi H.S., El-Salam S.M.A., Omran A.A. Biochemical changes in phenols, flavonoids, tannins, vitamin E, β-carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pac. J. Trop. Biomed. 2012;2:203–209. doi: 10.1016/S2221-1691(12)60042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palacios C.E., Nagai A., Torres P., Rodrigues J.A., Salatino A. Contents of tannins of cultivars of sorghum cultivated in Brazil, as determined by four quantification methods. Food Chem. 2021;337 doi: 10.1016/j.foodchem.2020.127970. [DOI] [PubMed] [Google Scholar]
- 75.Xiong Y., Zhang P., Warner R.D., Fang Z. Sorghum grain: from genotype, nutrition, and phenolic profile to its health benefits and food applications. Compr. Rev. Food Sci. Food Saf. 2019;18:2025–2046. doi: 10.1111/1541-4337.12506. [DOI] [PubMed] [Google Scholar]
- 76.Shen, H. R S., C L., W W., H C., J S., S C., X Y. Phenolic compositions and antioxidant activities differ significantly among sorghum grains with different applications. Molecules. 2018;23 doi: 10.3390/molecules23051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghimire B.-K., Seo J.-W., Yu C.-Y., Kim S.-H., Chung I.-M. Comparative study on seed characteristics, antioxidant activity, and total phenolic and flavonoid contents in accessions of sorghum bicolor (L.) Moench. Molecules. 2021;26:3964. doi: 10.3390/molecules26133964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amoako D.B., Awika J.M. Polymeric tannins significantly alter properties and in vitro digestibility of partially gelatinized intact starch granule. Food Chem. 2016;208:10–17. doi: 10.1016/j.foodchem.2016.03.096. [DOI] [PubMed] [Google Scholar]
- 79.Amoako D.B., Awika J.M. Resistant starch formation through intrahelical V-complexes between polymeric proanthocyanidins and amylose. Food Chem. 2019;285:326–333. doi: 10.1016/j.foodchem.2019.01.173. [DOI] [PubMed] [Google Scholar]
- 80.Omar N., Ismail C.A.N., Long I. Tannins in the treatment of diabetic neuropathic pain: research progress and future challenges. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.805854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong Y., Zhang P., Warner R.D., Shen S., Johnson S., Fang Z. HPLC-DAD-ESI-QTOF-MS/MS qualitative analysis data and HPLC-DAD quantification data of phenolic compounds of grains from five Australian sorghum genotypes. Data Brief. 2020;33 doi: 10.1016/j.dib.2020.106584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang J., Price W.E., Ashton J., Tapsell L.C., Johnson S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MS(n) Food Chem. 2016;211:215–226. doi: 10.1016/j.foodchem.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 83.Serrano J., Puupponen-Pimiä R., Dauer A., Aura A.-M., Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009;53:S310–S329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- 84.Awika J., Rooney L., Waniska R. Anthocyanins from black sorghum and their antioxidant properties. Food Chem. 2005;90:293–301. doi: 10.1016/j.foodchem.2004.03.058. [DOI] [Google Scholar]
- 85.Dykes L., Peterson G.C., Rooney W.L., Rooney L.W. Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem. 2011;128:173–179. doi: 10.1016/j.foodchem.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 86.Abbasi A.-R., Hajirezaei M., Hofius D., Sonnewald U., Voll L.M. Specific roles of α- and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007;143:1720. doi: 10.1104/pp.106.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mène-Saffrané L. Vitamin E Biosynthesis and its regulation in plants. Antioxidants. 2017;7:2. doi: 10.3390/antiox7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niu Y., Zhang Q., Wang J., Li Y., Wang X., Bao Y. Vitamin E synthesis and response in plants. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.994058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fritsche S., Wang X., Jung C. Recent advances in our understanding of tocopherol Biosynthesis in plants: an overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants. 2017;6 doi: 10.3390/antiox6040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reboul E. Vitamin E bioavailability: mechanisms of intestinal absorption in the spotlight. Antioxidants. 2017;6 doi: 10.3390/antiox6040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reboul E. Vitamin E intestinal absorption: regulation of membrane transport across the enterocyte. IUBMB Life. 2019;71:416–423. doi: 10.1002/iub.1955. [DOI] [PubMed] [Google Scholar]
- 92.Galli F., Bonomini M., Bartolini D., Zatini L., Reboldi G., Marcantonini G., Gentile G., Sirolli V., Di Pietro N. Vitamin E (Alpha-Tocopherol) metabolism and nutrition in chronic kidney disease. Antioxidants. 2022;11:989. doi: 10.3390/antiox11050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tucker J.M., Townsend D.M. Alpha-tocopherol: roles in prevention and therapy of human disease. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2005;59:380. doi: 10.1016/j.biopha.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu K.Y., Nakatsu C.H., Jones-Hall Y., Kozik A., Jiang Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2021;163:180–189. doi: 10.1016/j.freeradbiomed.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 95.Ciarcià G., Bianchi S., Tomasello B., Acquaviva R., Malfa G.A., Naletova I., Mantia A.L., Giacomo C.D. Vitamin E and non-communicable diseases: a review. Biomedicines. 2022;10 doi: 10.3390/biomedicines10102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gothandapani D., Makpol S. Effects of vitamin E on the gut microbiome in ageing and its relationship with age-related diseases: a review of the current literature. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241914667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang Q., Im S., Wagner J.G., Hernandz M.L., Peden D.B. Gamma-tocopherol, a major form of vitamin E in diets: insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radical Biol. Med. 2022;178:347. doi: 10.1016/j.freeradbiomed.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y., Sen A., Ren J., Askew L.M., Sidahmed E., Brenner D.E., Ruffin Mack T., Turgeon D.K., Djuric Z. Effects of vitamin E supplements and diet on colonic α- and γ-tocopherol concentrations in persons at increased colon cancer risk. Nutr. Cancer. 2015;67:73. doi: 10.1080/01635581.2015.965333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dolfi S.C., Yang Z., Lee M.-J., Guan F., Hong J., Yang C.S. Inhibitory effects of different forms of tocopherols, tocopherol phosphates, and tocopherol quinones on growth of colon cancer cells. J. Agric. Food Chem. 2013;61:8533–8540. doi: 10.1021/jf401076g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pinheiro S.S., Anunciação P.C., Cardoso L. de M., Della Lucia C.M., de Carvalho C.W.P., Queiroz V.A.V., Pinheiro Sant'Ana H.M. Stability of B vitamins, vitamin E, xanthophylls and flavonoids during germination and maceration of sorghum (Sorghum bicolor L.) Food Chem. 2021;345 doi: 10.1016/j.foodchem.2020.128775. [DOI] [PubMed] [Google Scholar]
- 101.Li Z., Zhao X., Zhang X., Liu H. Bioactive compounds and biological activities of sorghum grains. Foods. 2021;10:2868. doi: 10.3390/foods10112868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohammed Z.S., Mabudi A.H., Murtala Y., Jibrin S., Sulaiman S., Salihu J. Nutritional analysis of three commonly consumed varieties of sorghum (sorghum bicolor L.) in bauchi state, Nigeria. J. Appl. Sci. Environ. Manag. 2019;23:1329–1334. doi: 10.4314/jasem.v23i7. [DOI] [Google Scholar]
- 103.Espitia-Hernández P., Chávez González M.L., Ascacio-Valdés J.A., Dávila-Medina D., Flores-Naveda A., Silva T., Ruelas Chacón X., Sepúlveda L. Sorghum (Sorghum bicolor L.) as a potential source of bioactive substances and their biological properties. Crit. Rev. Food Sci. Nutr. 2022;62:2269–2280. doi: 10.1080/10408398.2020.1852389. [DOI] [PubMed] [Google Scholar]
- 104.Hassan S. Nutritional, functional and bioactive properties of sorghum (sorghum bicolor I. Moench) with its future outlooks. Rev. Artic. 2023;5:1030. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used for this research hasn't been deposited into a public repository. However, data used in the study is available from the corresponding author on reasonable request.