Abstract
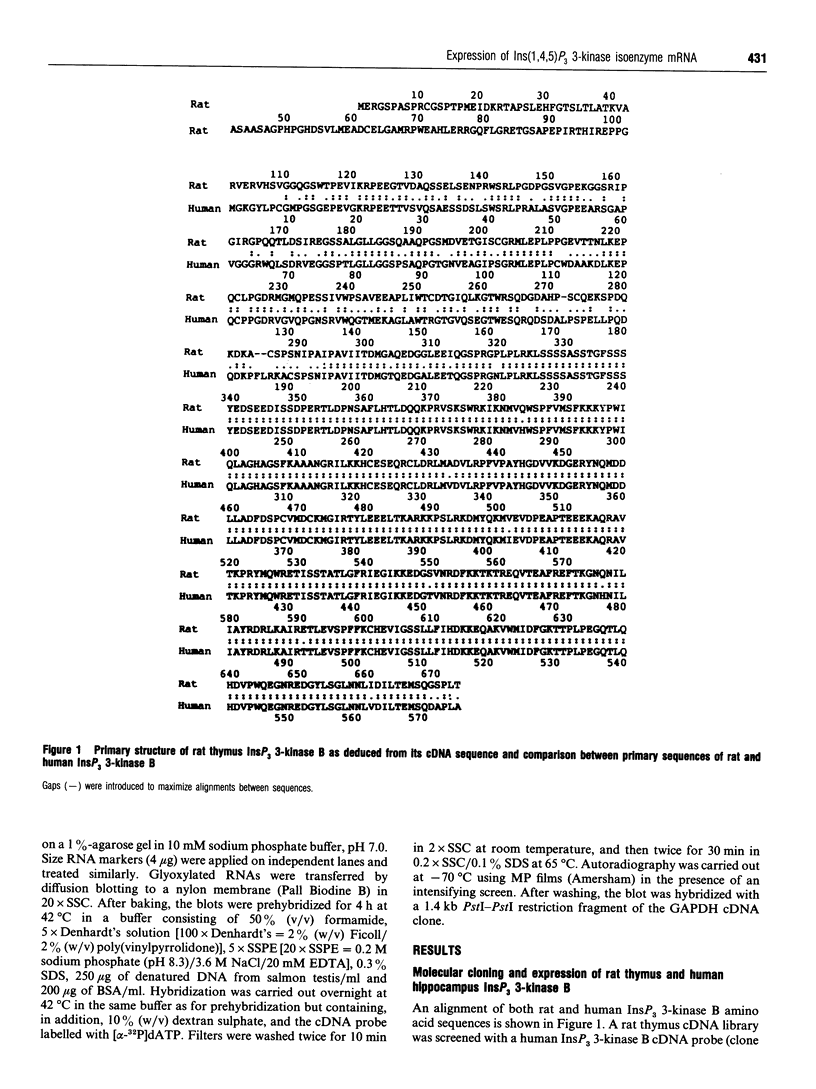
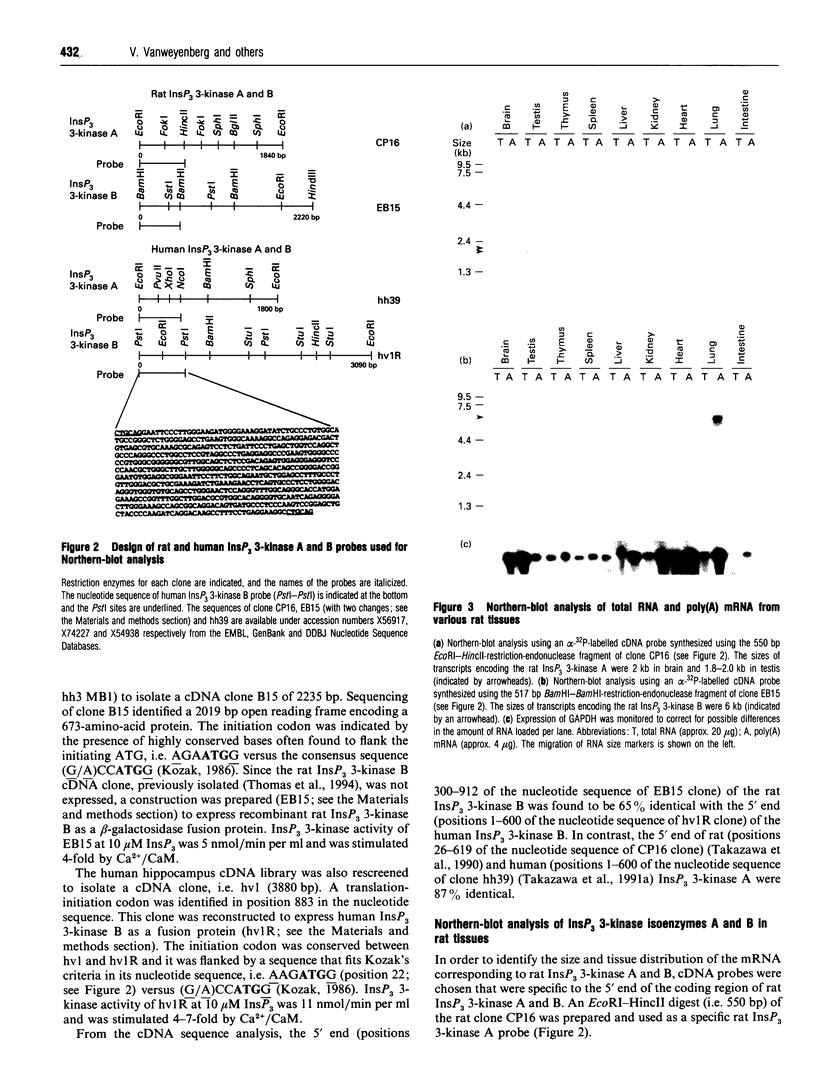
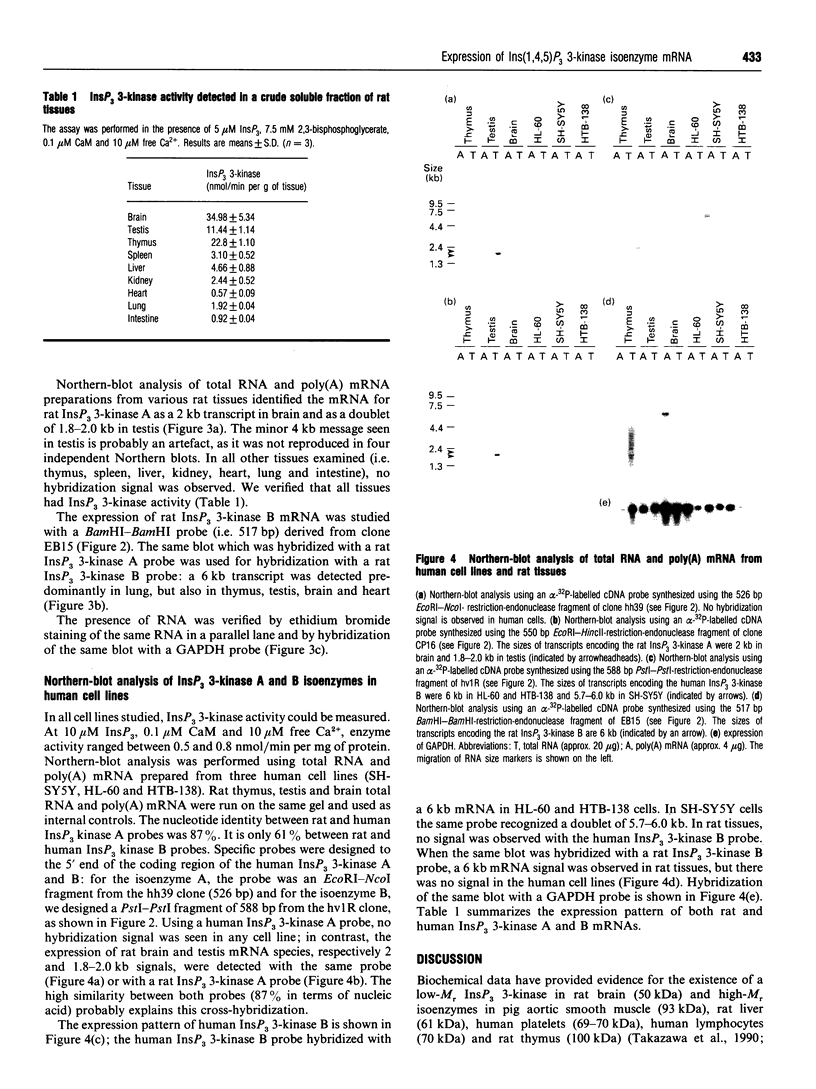
The phosphorylation of Ins(1,4,5)P3 (InsP3) to Ins(1,3,4,5)P4 (InsP4) is catalysed by InsP3 3-kinase. Molecular-biological data have shown the presence of two human isoenzymes of InsP3 3-kinase, namely InsP3 3-kinases A and B. We have isolated from a rat thymus cDNA library a 2235 bp cDNA (clone B15) encoding rat InsP3 3-kinase B. Northern-blot analysis of mRNA isolated from rat tissues (thymus, testis, brain, spleen, liver, kidney, heart, lung and intestine) revealed that a rat InsP3 3-kinase B probe hybridized to a 6 kb mRNA in lung, thymus, testis, brain and heart. In contrast, Northern-blot analysis of the same tissues probed under stringent conditions with a rat InsP3 3-kinase A probe hybridized to a 2 kb mRNA only in brain and a 1.8-2.0 kb mRNA species in testis. Northern-blot analysis of three human cell lines (HL-60, SH-SY5Y and HTB-138) probed with a human InsP3 3-kinase B probe showed the presence of a 6 kb mRNA in all cell lines, except in the human neuroblastoma cell line (SH-SY5Y), where two mRNA species of 5.7 and 6 kb were detected. Using the same blot, no hybridization signal could be seen with a human InsP3 3-kinase A probe. Altogether, our data are consistent with the notion that the two InsP3 3-kinase isoenzymes, A and B, are specifically expressed in different tissues and cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Wang X., Jin Y., Hui P. Transcriptional regulation and increased functional expression of the inositol trisphosphate receptor in retinoic acid-treated HL-60 cells. J Biol Chem. 1992 Oct 15;267(29):20959–20964. [PubMed] [Google Scholar]
- Chadwick C. C., Timerman A. P., Saito A., Mayrleitner M., Schindler H., Fleischer S. Structural and functional characterization of an inositol polyphosphate receptor from cerebellum. J Biol Chem. 1992 Feb 15;267(5):3473–3481. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Communi D., Takazawa K., Erneux C. Lys-197 and Asp-414 are critical residues for binding of ATP/Mg2+ by rat brain inositol 1,4,5-trisphosphate 3-kinase. Biochem J. 1993 May 1;291(Pt 3):811–816. doi: 10.1042/bj2910811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D., Vanweyenberg V., Erneux C. Purification and biochemical properties of a high-molecular-mass inositol 1,4,5-trisphosphate 3-kinase isoenzyme in human platelets. Biochem J. 1994 Mar 15;298(Pt 3):669–673. doi: 10.1042/bj2980669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave A., Patwardhan A., Broomhead L., Roufogalis B. A purification strategy for inositol 1,4,5-trisphosphate 3-kinase from rat liver based upon heparin interaction chromatography. Cell Signal. 1992 May;4(3):303–312. doi: 10.1016/0898-6568(92)90070-o. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate binding sites in neuronal and non-neuronal tissues. Properties, comparisons and potential physiological significance. Biochem J. 1992 Nov 15;288(Pt 1):149–154. doi: 10.1042/bj2880149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Patel Y., Kakkar V. V., Irvine R. F., Authi K. S. Specific binding sites for inositol 1,3,4,5-tetrakisphosphate are located predominantly in the plasma membranes of human platelets. Biochem J. 1994 Mar 15;298(Pt 3):739–742. doi: 10.1042/bj2980739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Santos C. S., Communi D., Ludgate M., Vanweyenberg V., Takazawa K., Erneux C. Identification of high molecular weight forms of inositol 1,4,5-trisphosphate 3-kinase in rat thymus and human lymphocytes. Cell Signal. 1994 Mar;6(3):335–344. doi: 10.1016/0898-6568(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Danoff S. K., Ferris C. D., Donath C., Fischer G. A., Munemitsu S., Ullrich A., Snyder S. H., Ross C. A. Inositol 1,4,5-trisphosphate receptors: distinct neuronal and nonneuronal forms derived by alternative splicing differ in phosphorylation. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Takazawa K. Intracellular control of inositol phosphates by their metabolizing enzymes. Trends Pharmacol Sci. 1991 May;12(5):174–176. doi: 10.1016/0165-6147(91)90539-5. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Hill T. D., Dean N. M., Boynton A. L. Inositol 1,3,4,5-tetrakisphosphate induces Ca2+ sequestration in rat liver cells. Science. 1988 Nov 25;242(4882):1176–1178. doi: 10.1126/science.2847317. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lambert D. G., Challiss R. A., Nahorski S. R. Accumulation and metabolism of Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in muscarinic-receptor-stimulated SH-SY5Y neuroblastoma cells. Biochem J. 1991 Feb 1;273(Pt 3):791–794. doi: 10.1042/bj2730791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Sim S. S., Kim J. W., Moon K. H., Kim J. H., Rhee S. G. Purification and properties of D-myo-inositol 1,4,5-trisphosphate 3-kinase from rat brain. Susceptibility to calpain. J Biol Chem. 1990 Jun 5;265(16):9434–9440. [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Jul 25;265(21):12679–12685. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nghiem P., Saati S. M., Martens C. L., Gardner P., Schulman H. Cloning and analysis of two new isoforms of multifunctional Ca2+/calmodulin-dependent protein kinase. Expression in multiple human tissues. J Biol Chem. 1993 Mar 15;268(8):5471–5479. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reiser G., Schäfer R., Donié F., Hülser E., Nehls-Sahabandu M., Mayr G. W. A high-affinity inositol 1,3,4,5-tetrakisphosphate receptor protein from brain is specifically labelled by a newly synthesized photoaffinity analogue, N-(4-azidosalicyl)aminoethanol(1)-1-phospho-D-myo-inositol 3,4,5-trisphosphate. Biochem J. 1991 Dec 1;280(Pt 2):533–539. doi: 10.1042/bj2800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., Danoff S. K., Schell M. J., Snyder S. H., Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S. S., Kim J. W., Rhee S. G. Regulation of D-myo-inositol 1,4,5-trisphosphate 3-kinase by cAMP-dependent protein kinase and protein kinase C. J Biol Chem. 1990 Jun 25;265(18):10367–10372. [PubMed] [Google Scholar]
- Sonnenburg W. K., Seger D., Beavo J. A. Molecular cloning of a cDNA encoding the "61-kDa" calmodulin-stimulated cyclic nucleotide phosphodiesterase. Tissue-specific expression of structurally related isoforms. J Biol Chem. 1993 Jan 5;268(1):645–652. [PubMed] [Google Scholar]
- Südhof T. C., Newton C. L., Archer B. T., 3rd, Ushkaryov Y. A., Mignery G. A. Structure of a novel InsP3 receptor. EMBO J. 1991 Nov;10(11):3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa K., Erneux C. Identification of residues essential for catalysis and binding of calmodulin in rat brain inositol 1,4,5-trisphosphate 3-kinase. Biochem J. 1991 Nov 15;280(Pt 1):125–129. doi: 10.1042/bj2800125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa K., Passareiro H., Dumont J. E., Erneux C. Ca2+/calmodulin-sensitive inositol 1,4,5-trisphosphate 3-kinase in rat and bovine brain tissues. Biochem Biophys Res Commun. 1988 Jun 16;153(2):632–641. doi: 10.1016/s0006-291x(88)81142-2. [DOI] [PubMed] [Google Scholar]
- Takazawa K., Perret J., Dumont J. E., Erneux C. Molecular cloning and expression of a human brain inositol 1,4,5-trisphosphate 3-kinase. Biochem Biophys Res Commun. 1991 Jan 31;174(2):529–535. doi: 10.1016/0006-291x(91)91449-m. [DOI] [PubMed] [Google Scholar]
- Takazawa K., Perret J., Dumont J. E., Erneux C. Molecular cloning and expression of a new putative inositol 1,4,5-trisphosphate 3-kinase isoenzyme. Biochem J. 1991 Sep 15;278(Pt 3):883–886. doi: 10.1042/bj2780883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa K., Vandekerckhove J., Dumont J. E., Erneux C. Cloning and expression in Escherichia coli of a rat brain cDNA encoding a Ca2+/calmodulin-sensitive inositol 1,4,5-trisphosphate 3-kinase. Biochem J. 1990 Nov 15;272(1):107–112. doi: 10.1042/bj2720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theibert A. B., Estevez V. A., Ferris C. D., Danoff S. K., Barrow R. K., Prestwich G. D., Snyder S. H. Inositol 1,3,4,5-tetrakisphosphate and inositol hexakisphosphate receptor proteins: isolation and characterization from rat brain. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3165–3169. doi: 10.1073/pnas.88.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S., Brake B., Luzio J. P., Stanley K., Banting G. Isolation and sequence of a full length cDNA encoding a novel rat inositol 1,4,5-trisphosphate 3-kinase. Biochim Biophys Acta. 1994 Jan 13;1220(2):219–222. doi: 10.1016/0167-4889(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Hirata M., Kuriyama H. Purification and characterization of inositol 1,4,5-trisphosphate 3-kinase from pig aortic smooth muscle. Biochem J. 1988 Apr 1;251(1):129–134. doi: 10.1042/bj2510129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Sugiyama T., Hikichi K., Mattei M. G., Hasegawa K., Sekine S., Sakurada K., Miyawaki A., Furuichi T., Hasegawa M. Cloning and characterization of human type 2 and type 3 inositol 1,4,5-trisphosphate receptors. Receptors Channels. 1994;2(1):9–22. [PubMed] [Google Scholar]