Abstract
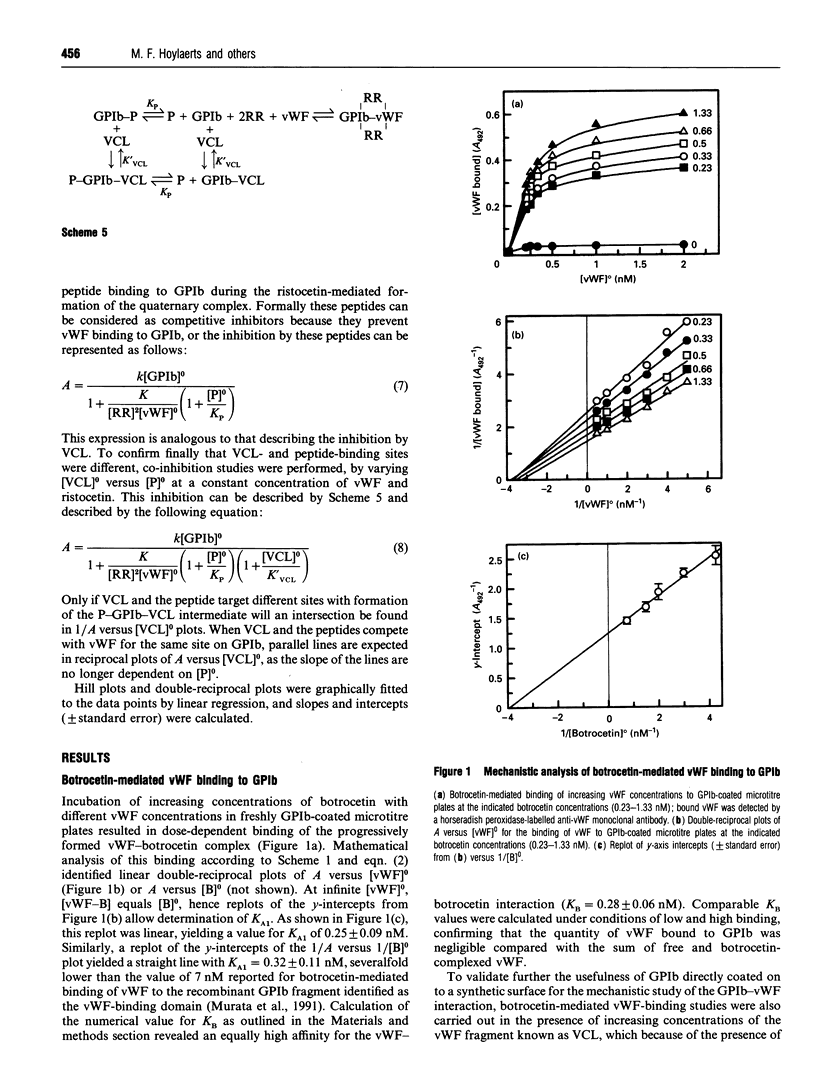
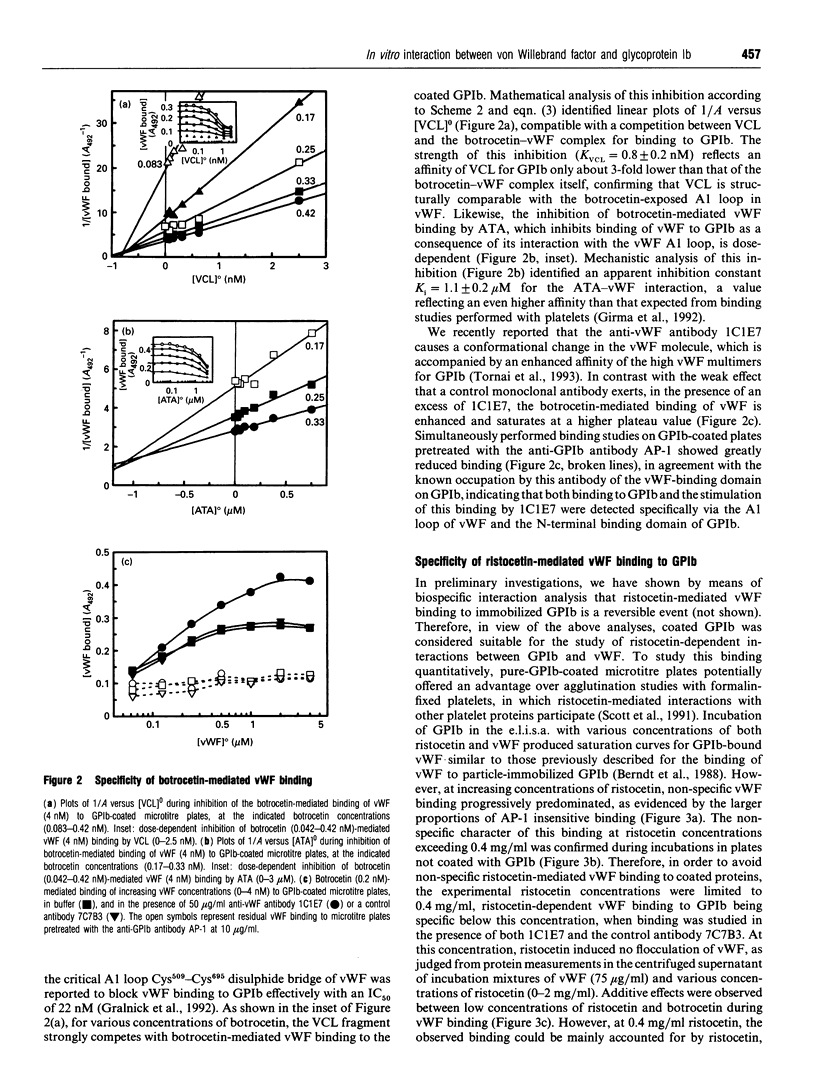
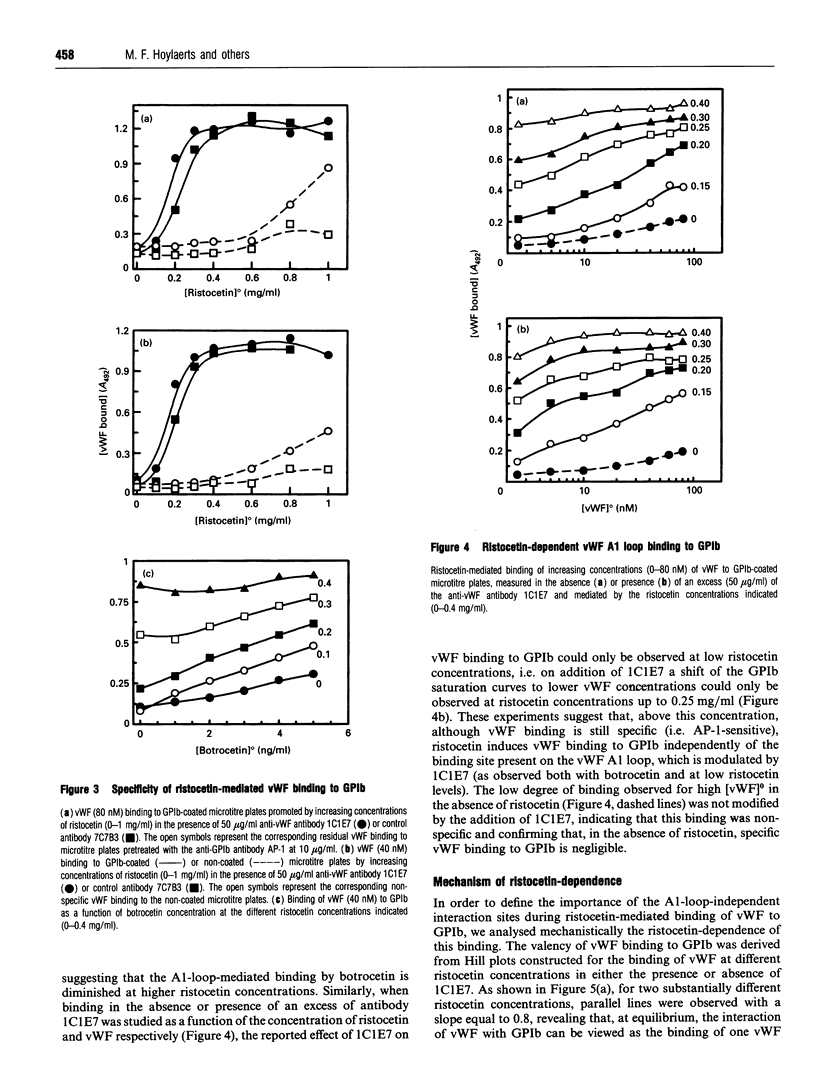
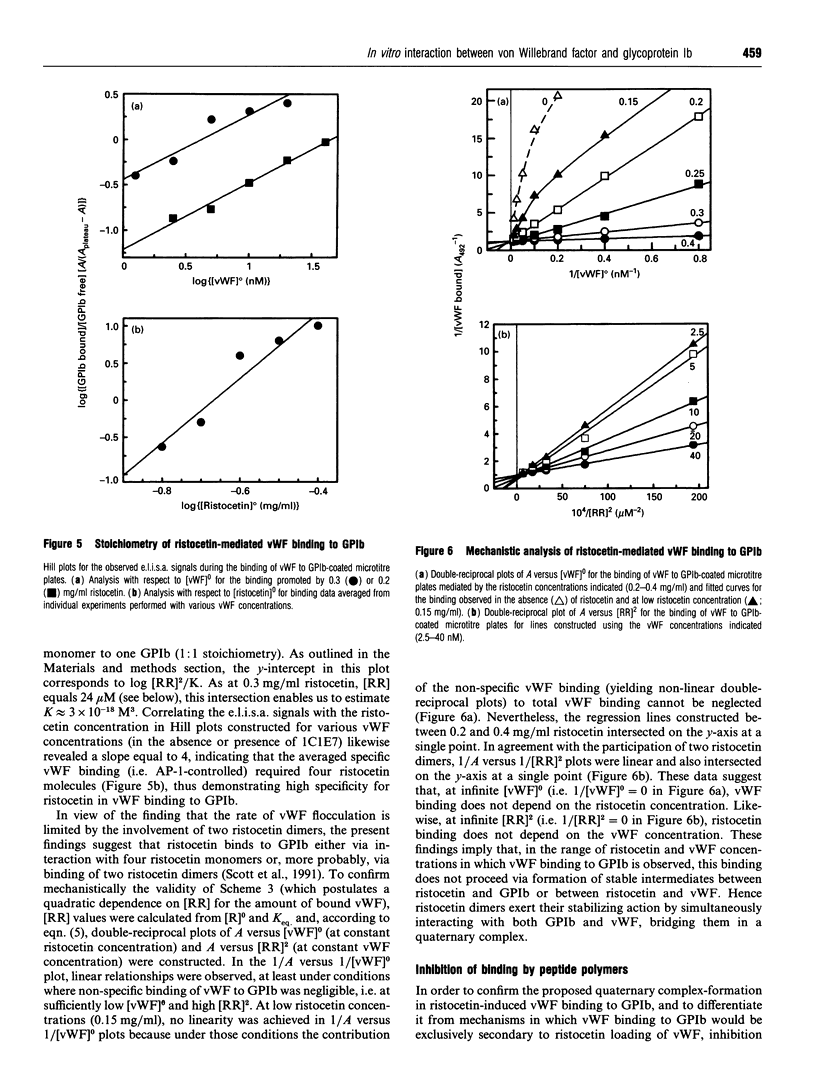
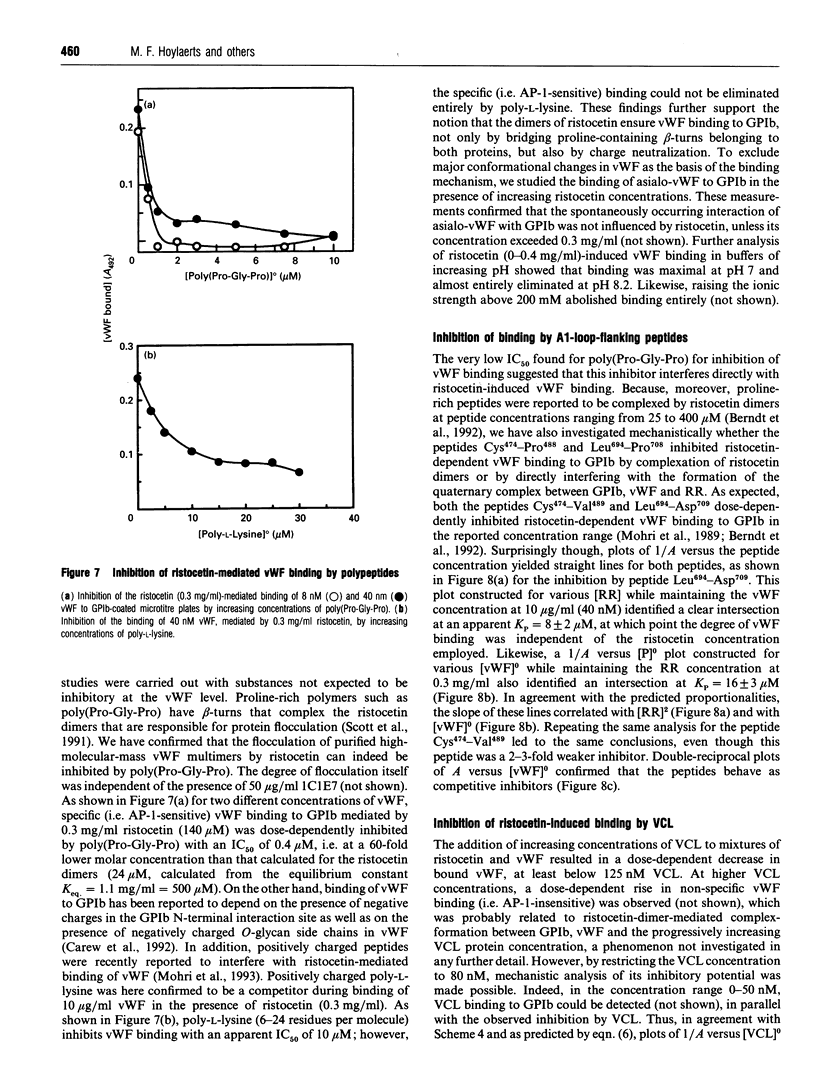
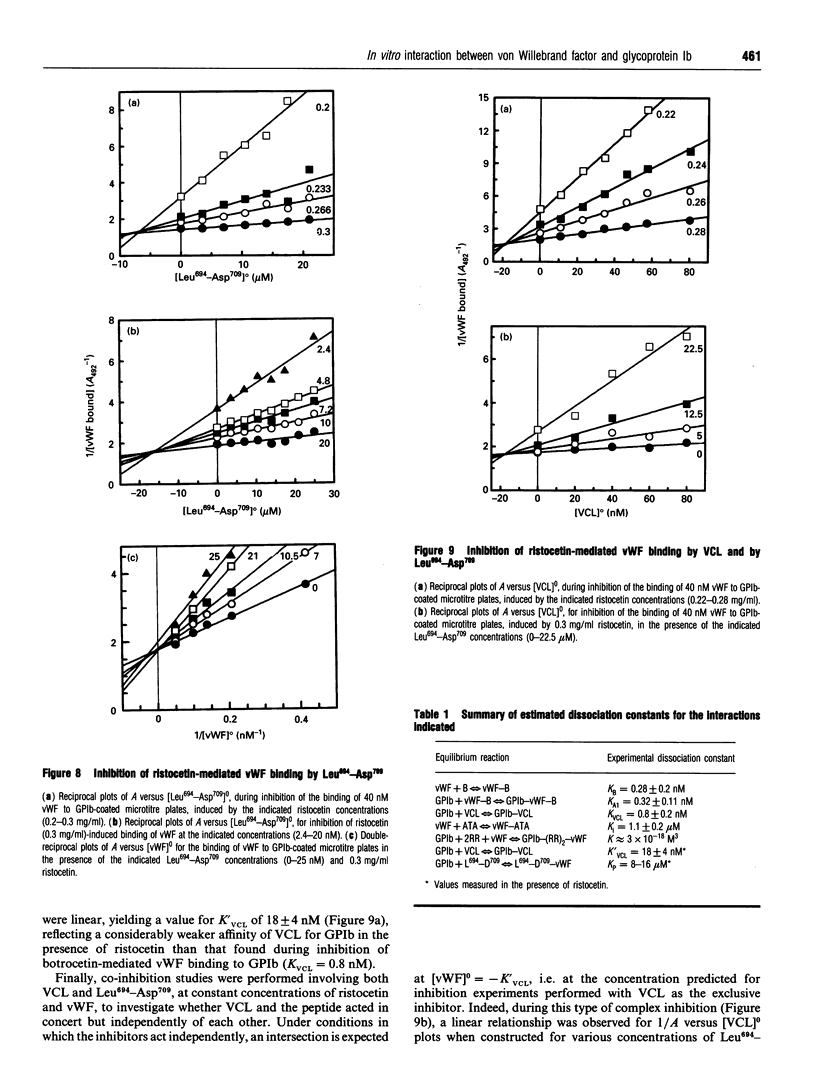
In the absence of high shear forces, the in vitro binding of human von Willebrand factor (vWF) to its platelet receptor glycoprotein Ib (GPIb) can be promoted by two well-characterized mediators, botrocetin and ristocetin. Using purified vWF and GPIb, we have investigated the mechanisms by which ristocetin mediates this binding. Specific binding of vWF monomers to GPIb occurred with a 1:1 stoichiometry, but high-affinity binding required the participation of two ristocetin dimers. Binding was strongly dependent on pH and inhibited by low poly-L-lysine concentrations, indicating ristocetin-dependent charge neutralization during the interaction. With increasing ristocetin concentrations, vWF binding depended progressively less on the involvement of its A1 loop, which is compatible with a model in which the two ristocetin dimers bridge the vWF-GPIb complex on secondary sites. In agreement with this model, the ristocetin-dimer-promoted stabilization of vWF on GPIb was abolished by low concentrations of poly(Pro-Gly-Pro), which is known to complex ristocetin dimers. Mechanistic analysis of the inhibition of vWF binding by the recombinant vWF fragment Leu504-Ser728 (VCL), which covers the entire A1 loop, revealed an affinity of VCL for GPIb comparable with that of the botrocetin-vWF complex for GPIb, and identified a specific but 20-fold lower affinity of VCL in the presence of ristocetin. The proline-rich peptides flanking the vWF A1 loop, Cys474-Val489 and Leu694-Asp709, inhibited vWF binding semispecifically by competitively interfering with the formation of the GPIb-vWF complex rather than by complexation of free ristocetin dimers. In conclusion, ristocetin-promoted binding of vWF to its GPIb receptor results from charge neutralization and interactions involving proline residues in the vicinity of the natural interaction sites present on both GPIb and the A1 domain of vWF.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma H., Sugimoto M., Ruggeri Z. M., Ware J. A role for von Willebrand factor proline residues 702-704 in ristocetin-mediated binding to platelet glycoprotein Ib. Thromb Haemost. 1993 Feb 1;69(2):192–196. [PubMed] [Google Scholar]
- Berndt M. C., Du X. P., Booth W. J. Ristocetin-dependent reconstitution of binding of von Willebrand factor to purified human platelet membrane glycoprotein Ib-IX complex. Biochemistry. 1988 Jan 26;27(2):633–640. doi: 10.1021/bi00402a021. [DOI] [PubMed] [Google Scholar]
- Berndt M. C., Ward C. M., Booth W. J., Castaldi P. A., Mazurov A. V., Andrews R. K. Identification of aspartic acid 514 through glutamic acid 542 as a glycoprotein Ib-IX complex receptor recognition sequence in von Willebrand factor. Mechanism of modulation of von Willebrand factor by ristocetin and botrocetin. Biochemistry. 1992 Nov 17;31(45):11144–11151. doi: 10.1021/bi00160a027. [DOI] [PubMed] [Google Scholar]
- Bolhuis P. A., Sakariassen K. S., Sander H. J., Bouma B. N., Sixma J. J. Binding of factor VIII-von Willebrand factor to human arterial subendothelium precedes increased platelet adhesion and enhances platelet spreading. J Lab Clin Med. 1981 Apr;97(4):568–576. [PubMed] [Google Scholar]
- Carew J. A., Quinn S. M., Stoddart J. H., Lynch D. C. O-linked carbohydrate of recombinant von Willebrand factor influences ristocetin-induced binding to platelet glycoprotein 1b. J Clin Invest. 1992 Dec;90(6):2258–2267. doi: 10.1172/JCI116112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y., Miyata S., Nishida S., Miura S., Kaneda M., Yoshioka A., Fukui H., Katayama M., Tuddenham E. G., Usami Y. The interaction of botrocetin with normal or variant von Willebrand factor (types IIA and IIB) and its inhibition by monoclonal antibodies that block receptor binding. Thromb Haemost. 1992 Oct 5;68(4):464–469. [PubMed] [Google Scholar]
- Fujimura Y., Titani K., Usami Y., Suzuki M., Oyama R., Matsui T., Fukui H., Sugimoto M., Ruggeri Z. M. Isolation and chemical characterization of two structurally and functionally distinct forms of botrocetin, the platelet coagglutinin isolated from the venom of Bothrops jararaca. Biochemistry. 1991 Feb 19;30(7):1957–1964. doi: 10.1021/bi00221a032. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Sadler J. E. von Willebrand disease: a database of point mutations, insertions, and deletions. For the Consortium on von Willebrand Factor Mutations and Polymorphisms, and the Subcommittee on von Willebrand Factor of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1993 Feb 1;69(2):177–184. [PubMed] [Google Scholar]
- Girma J. P., Fressinaud E., Christophe O., Rouault C., Obert B., Takahashi Y., Meyer D. Aurin tricarboxylic acid inhibits platelet adhesion to collagen by binding to the 509-695 disulphide loop of von Willebrand factor and competing with glycoprotein Ib. Thromb Haemost. 1992 Dec 7;68(6):707–713. [PubMed] [Google Scholar]
- Girma J. P., Takahashi Y., Yoshioka A., Diaz J., Meyer D. Ristocetin and botrocetin involve two distinct domains of von Willebrand factor for binding to platelet membrane glycoprotein Ib. Thromb Haemost. 1990 Oct 22;64(2):326–332. [PubMed] [Google Scholar]
- Grainick H. R., Williams S. B., Coller B. S. Asialo von Willebrand factor interactions with platelets. Interdependence of glycoproteins Ib and IIb/IIIa for binding and aggregation. J Clin Invest. 1985 Jan;75(1):19–25. doi: 10.1172/JCI111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S., McKeown L., Kramer W., Krutzsch H., Gorecki M., Pinet A., Garfinkel L. I. A monomeric von Willebrand factor fragment, Leu-504--Lys-728, inhibits von Willebrand factor interaction with glycoprotein Ib-IX [corrected]. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7880–7884. doi: 10.1073/pnas.89.17.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalafatis M., Takahashi Y., Girma J. P., Meyer D. Localization of a collagen-interactive domain of human von Willebrand factor between amino acid residues Gly 911 and Glu 1,365. Blood. 1987 Nov;70(5):1577–1583. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Light J., Williams C. E., Entwistle M. B. Ristocetin and von Willebrand factor analysis. Med Lab Sci. 1987 Jul;44(3):272–279. [PubMed] [Google Scholar]
- Meyer D., Girma J. P. von Willebrand factor: structure and function. Thromb Haemost. 1993 Jul 1;70(1):99–104. [PubMed] [Google Scholar]
- Mohri H., Fujimura Y., Shima M., Yoshioka A., Houghten R. A., Ruggeri Z. M., Zimmerman T. S. Structure of the von Willebrand factor domain interacting with glycoprotein Ib. J Biol Chem. 1988 Dec 5;263(34):17901–17904. [PubMed] [Google Scholar]
- Mohri H., Zimmerman T. S., Ruggeri Z. M. Synthetic peptides inhibit the interaction of von Willebrand factor-platelet membrane glycoproteins. Peptides. 1993 Mar-Apr;14(2):125–129. doi: 10.1016/0196-9781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- Montgomery R. R., Kunicki T. J., Taves C., Pidard D., Corcoran M. Diagnosis of Bernard-Soulier syndrome and Glanzmann's thrombasthenia with a monoclonal assay on whole blood. J Clin Invest. 1983 Feb;71(2):385–389. doi: 10.1172/JCI110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M., Ware J., Ruggeri Z. M. Site-directed mutagenesis of a soluble recombinant fragment of platelet glycoprotein Ib alpha demonstrating negatively charged residues involved in von Willebrand factor binding. J Biol Chem. 1991 Aug 15;266(23):15474–15480. [PubMed] [Google Scholar]
- Pareti F. I., Niiya K., McPherson J. M., Ruggeri Z. M. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987 Oct 5;262(28):13835–13841. [PubMed] [Google Scholar]
- Rabinowitz I., Randi A. M., Shindler K. S., Tuley E. A., Rustagi P. K., Sadler J. E. Type IIB mutation His-505-->Asp implicates a new segment in the control of von Willebrand factor binding to platelet glycoprotein Ib. J Biol Chem. 1993 Sep 25;268(27):20497–20501. [PubMed] [Google Scholar]
- Ruggeri Z. M., Ware J. The structure and function of von Willebrand factor. Thromb Haemost. 1992 Jun 1;67(6):594–599. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S., Russell S., Bader R., De Marco L. von Willebrand factor binding to platelet glycoprotein Ib complex. Methods Enzymol. 1992;215:263–275. doi: 10.1016/0076-6879(92)15069-o. [DOI] [PubMed] [Google Scholar]
- Scott J. P., Montgomery R. R., Retzinger G. S. Dimeric ristocetin flocculates proteins, binds to platelets, and mediates von Willebrand factor-dependent agglutination of platelets. J Biol Chem. 1991 May 5;266(13):8149–8155. [PubMed] [Google Scholar]
- Tornai I., Arnout J., Deckmyn H., Peerlinck K., Vermylen J. A monoclonal antibody recognizes a von Willebrand factor domain within the amino-terminal portion of the subunit that modulates the function of the glycoprotein IB- and IIB/IIIA-binding domains. J Clin Invest. 1993 Jan;91(1):273–282. doi: 10.1172/JCI116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornai I., Declerck P. J., Smets L., Arnout J., Deckmyn H., Caekebeke-Peerlinck K. M., Vermylen J. Measurement of von Willebrand factor antigen in plasma and platelets with an enzyme-linked immunosorbent assay based on two murine monoclonal antibodies. Haemostasis. 1991;21(3):125–134. doi: 10.1159/000216216. [DOI] [PubMed] [Google Scholar]
- Vermylen J., Donati M. B., De Gaetano G., Verstraete M. Aggregation of human platelets by bovine or human factor VIII: role of carbohydrate side chains. Nature. 1973 Jul 20;244(5412):167–168. doi: 10.1038/244167a0. [DOI] [PubMed] [Google Scholar]


