Abstract
The interaction between cystatin C variants, in which the evolutionarily conserved Gly-11 residue was substituted by Ala, Glu or Trp, and the cysteine proteinases, papain, ficin, actinidin and cathepsin B, was characterized. The substitutions reduced the affinity of binding in a manner consistent with the Gly residue of the wild-type inhibitor, allowing the N-terminal region to adopt a conformation that was optimal for interaction with target proteinases. Replacement of Gly-11 by Ala resulted in only a 5- to 100-fold reduction in binding affinity. Comparison with the affinities of wild-type cystatin C lacking the N-terminal region indicated that even this small structural change affects the conformation of this region sufficiently to largely abolish its interaction with the weakly binding proteinases, actinidin and cathepsin B. However, the substitution allows interactions of appreciable strength between the N-terminal region and the tightly binding enzymes, papain or ficin. Replacement of Gly-11 with the larger Glu and Trp residues substantially decreased the affinity of binding to all enzymes, from 10(3)- to 10(5)-fold. These substitutions further affect the conformation of the N-terminal region, so that interactions of this region with papain and ficin are also essentially eliminated. The decreased affinities of the three cystatin C variants for papain, ficin and actinidin were due exclusively to increased dissociation rate constants. In contrast, the decreased affinity between cathepsin B and the Ala-11 variant, the only one for which rate constants could be determined with this enzyme, was due almost entirely to a decreased association rate constant. This behaviour is analogous to that observed for forms of cystatin C lacking the N-terminal region and supports the conclusion that the mode of interaction of this region with target proteinases varies with the enzyme as a result of structural differences in the active-site region of the latter.
Full text
PDF

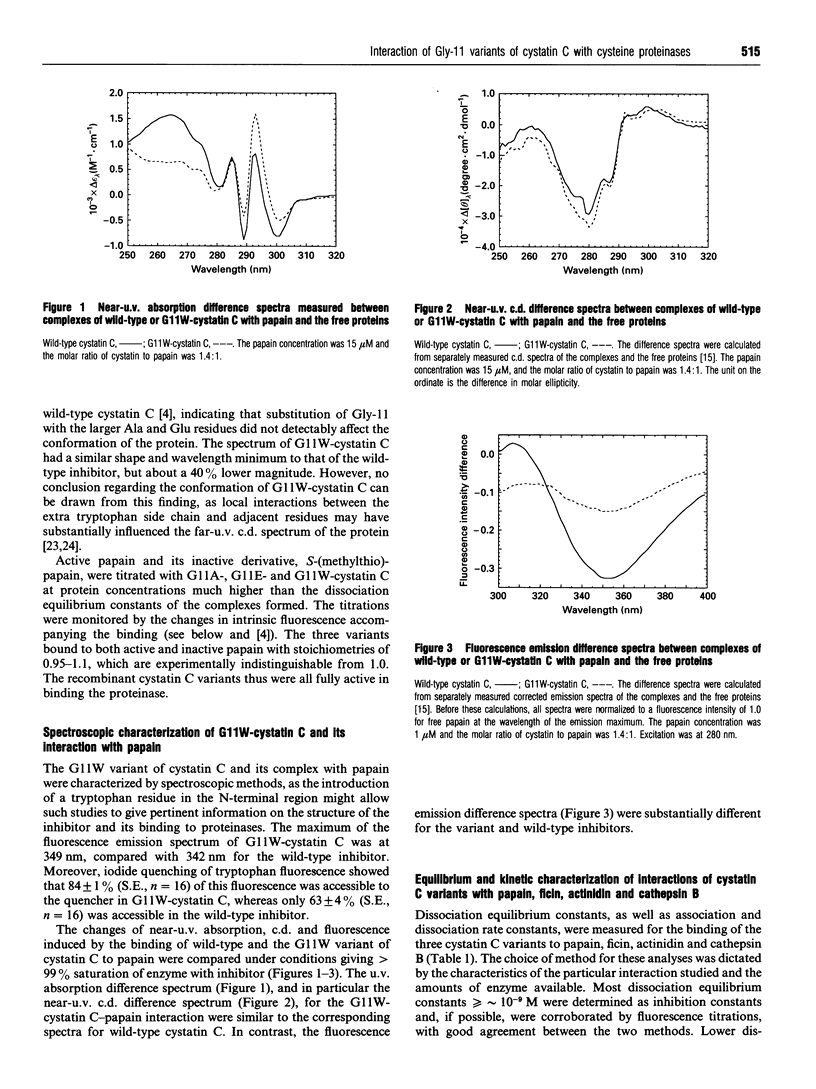
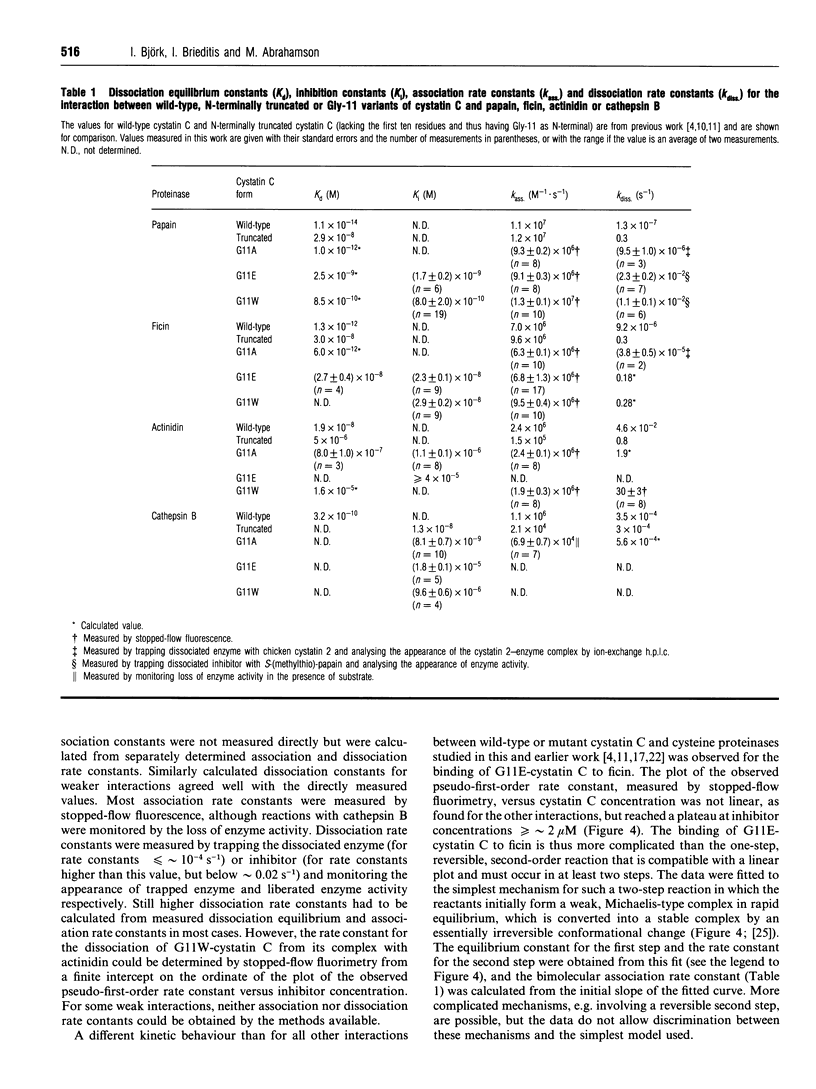
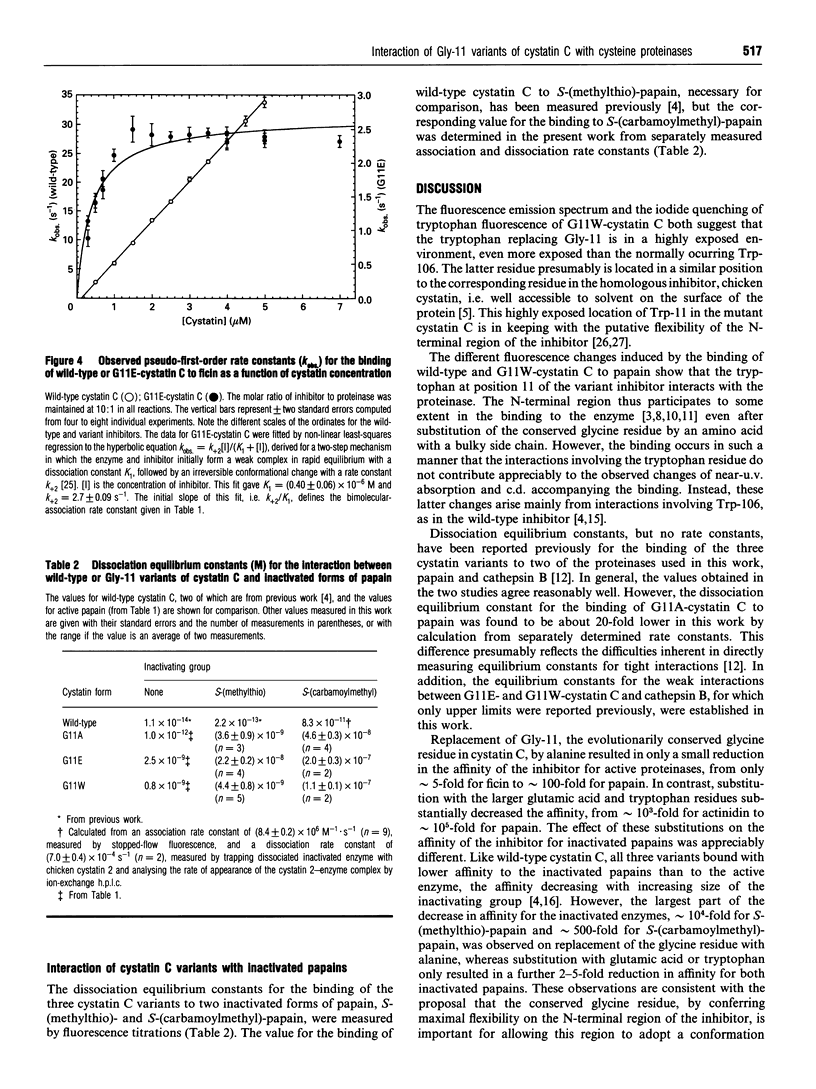

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson M., Barrett A. J., Salvesen G., Grubb A. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem. 1986 Aug 25;261(24):11282–11289. [PubMed] [Google Scholar]
- Abrahamson M., Dalbøge H., Olafsson I., Carlsen S., Grubb A. Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 1988 Aug 15;236(1):14–18. doi: 10.1016/0014-5793(88)80276-x. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Mason R. W., Hansson H., Buttle D. J., Grubb A., Ohlsson K. Human cystatin C. role of the N-terminal segment in the inhibition of human cysteine proteinases and in its inactivation by leucocyte elastase. Biochem J. 1991 Feb 1;273(Pt 3):621–626. doi: 10.1042/bj2730621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson M., Ritonja A., Brown M. A., Grubb A., Machleidt W., Barrett A. J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987 Jul 15;262(20):9688–9694. [PubMed] [Google Scholar]
- Anastasi A., Brown M. A., Kembhavi A. A., Nicklin M. J., Sayers C. A., Sunter D. C., Barrett A. J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983 Apr 1;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Alriksson E., Ylinenjärvi K. Kinetics of binding of chicken cystatin to papain. Biochemistry. 1989 Feb 21;28(4):1568–1573. doi: 10.1021/bi00430a022. [DOI] [PubMed] [Google Scholar]
- Björk I., Pol E., Raub-Segall E., Abrahamson M., Rowan A. D., Mort J. S. Differential changes in the association and dissociation rate constants for binding of cystatins to target proteinases occurring on N-terminal truncation of the inhibitors indicate that the interaction mechanism varies with different enzymes. Biochem J. 1994 Apr 1;299(Pt 1):219–225. doi: 10.1042/bj2990219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K. Interaction between chicken cystatin and the cysteine proteinases actinidin, chymopapain A, and ficin. Biochemistry. 1990 Feb 20;29(7):1770–1776. doi: 10.1021/bi00459a016. [DOI] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K. Interaction of chicken cystatin with inactivated papains. Biochem J. 1989 May 15;260(1):61–68. doi: 10.1042/bj2600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Laber B., Stubbs M., Huber R., Turk V. Mechanism of interaction of cysteine proteinases and their protein inhibitors as compared to the serine proteinase-inhibitor interaction. Biol Chem Hoppe Seyler. 1990 May;371 (Suppl):111–118. [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Thiele U., Huber R., Karshikov A., Brzin J., Kos J., Turk V. The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988 Aug;7(8):2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann T., Mitschang L., Hofmann M., Kos J., Turk V., Auerswald E. A., Jaenicke R., Oschkinat H. The structures of native phosphorylated chicken cystatin and of a recombinant unphosphorylated variant in solution. J Mol Biol. 1993 Dec 20;234(4):1048–1059. doi: 10.1006/jmbi.1993.1658. [DOI] [PubMed] [Google Scholar]
- Hall A., Abrahamson M., Grubb A., Trojnar J., Kania P., Kasprzykowska R., Kasprzykowski F. Cystatin C based peptidyl diazomethanes as cysteine proteinase inhibitors: influence of the peptidyl chain length. J Enzyme Inhib. 1992;6(2):113–123. doi: 10.3109/14756369209040742. [DOI] [PubMed] [Google Scholar]
- Hall A., Dalbøge H., Grubb A., Abrahamson M. Importance of the evolutionarily conserved glycine residue in the N-terminal region of human cystatin C (Gly-11) for cysteine endopeptidase inhibition. Biochem J. 1993 Apr 1;291(Pt 1):123–129. doi: 10.1042/bj2910123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay L. A., Puett D. Circular dichroism of corticotropin, fragment 1-24, and model compounds. An assessment of the contributions of the peptide chromophore and armoatic residues. Biopolymers. 1976 Jan;15(1):43–59. doi: 10.1002/bip.1976.360150106. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Rivier J., Puett D. Conformational studies on somatostatin and analogues. Biochemistry. 1977 Nov 1;16(22):4895–4900. doi: 10.1021/bi00641a024. [DOI] [PubMed] [Google Scholar]
- Larsson L. J., Lindahl P., Hallén-Sandgren C., Björk I. The conformational changes of alpha 2-macroglobulin induced by methylamine or trypsin. Characterization by extrinsic and intrinsic spectroscopic probes. Biochem J. 1987 Apr 1;243(1):47–54. doi: 10.1042/bj2430047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Abrahamson M., Björk I. Interaction of recombinant human cystatin C with the cysteine proteinases papain and actinidin. Biochem J. 1992 Jan 1;281(Pt 1):49–55. doi: 10.1042/bj2810049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Alriksson E., Jörnvall H., Björk I. Interaction of the cysteine proteinase inhibitor chicken cystatin with papain. Biochemistry. 1988 Jul 12;27(14):5074–5082. doi: 10.1021/bi00414a019. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Nycander M., Ylinenjärvi K., Pol E., Björk I. Characterization by rapid-kinetic and equilibrium methods of the interaction between N-terminally truncated forms of chicken cystatin and the cysteine proteinases papain and actinidin. Biochem J. 1992 Aug 15;286(Pt 1):165–171. doi: 10.1042/bj2860165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machleidt W., Thiele U., Laber B., Assfalg-Machleidt I., Esterl A., Wiegand G., Kos J., Turk V., Bode W. Mechanism of inhibition of papain by chicken egg white cystatin. Inhibition constants of N-terminally truncated forms and cyanogen bromide fragments of the inhibitor. FEBS Lett. 1989 Jan 30;243(2):234–238. doi: 10.1016/0014-5793(89)80135-8. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Björk I. Binding of low-affinity and high-affinity heparin to antithrombin. Ultraviolet difference spectroscopy and circular dichroism studies. Biochemistry. 1978 Aug 8;17(16):3339–3344. doi: 10.1021/bi00609a026. [DOI] [PubMed] [Google Scholar]
- Popović T., Brzin J., Ritonja A., Turk V. Different forms of human cystatin C. Biol Chem Hoppe Seyler. 1990 Jul;371(7):575–580. doi: 10.1515/bchm3.1990.371.2.575. [DOI] [PubMed] [Google Scholar]
- Stubbs M. T., Laber B., Bode W., Huber R., Jerala R., Lenarcic B., Turk V. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 1990 Jun;9(6):1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


