Abstract
BACKGROUND:
Effectiveness and safety of advanced therapies for ulcerative colitis (UC) warrant assessment in the real world.
OBJECTIVE:
To perform a systematic review and summarize real-world evidence of advanced therapies approved for moderate-to-severe UC.
METHODS:
A systematic literature review was conducted using real-world studies of biologics or small molecules in UC using Embase, MEDLINE, and MEDLINE-In Process databases. Only products approved in any jurisdiction during the search were included. English-language full-papers (January 2005 to February 2022) and congress abstracts (January 2019 to February 2022) were included. Studies with less than 30 patients or only biologic-naive patients were excluded.
RESULTS:
A total of 139 studies were included out of 3,930 identified articles (75%, published between 2019 and 2022; 64%, retrospective observational; 53%, from 5 countries [Italy, United States, Spain, United Kingdom, and Belgium]). Most studies were single agent (highest: vedolizumab = 50, tofacitinib = 24, and adalimumab = 18), and rates of clinical remission (CR) and adverse events varied widely. From the published comparative effectiveness studies (16), the rates of CR were numerically higher with vedolizumab vs anti-tumor necrosis factor (TNF)-α agents. Compared with vedolizumab, the effectiveness of tofacitinib was numerically greater in CR (occasionally significant). Rates of steroid-free CR were comparable between ustekinumab and tofacitinib. Infliximab was the most effective anti-TNFα agent, as reported by 2 studies. Remarkably, adverse events were similar across therapies in comparative studies.
CONCLUSIONS:
Vedolizumab and tofacitinib were the most assessed therapies. In comparative studies, remission rates were numerically higher with tofacitinib vs vedolizumab and for vedolizumab vs anti-TNFα. Tofacitinib was comparable with ustekinumab for steroid-free CR. Safety was comparable across therapies. Future studies should explore the literature gaps identified, including limited comparative studies with small sample sizes, variations in study designs and patient characteristics, varied definitions of CR, and limited use of patient-reported outcome measures in real-world settings.
Plain language summary
We conducted a systematic literature review to understand the safety and effectiveness of available therapies in the real world for the treatment of moderate-to-severe ulcerative colitis. We found that vedolizumab and tofacitinib were the most assessed therapies. Remission rates were numerically higher with tofacitinib vs vedolizumab, and for vedolizumab vs anti-tumor necrosis factor-α (TNFα). Tofacitinib was comparable with ustekinumab. Studies have reported that infliximab was the most effective anti-TNFα agent. Safety was comparable across therapies.
Implications for managed care pharmacy
This systematic review explores the real-world effectiveness and safety of biologic therapies for moderate-to-severe ulcerative colitis. Vedolizumab and tofacitinib were the most assessed therapies, with higher remission rates observed for tofacitinib compared with vedolizumab and for vedolizumab compared with anti-TNFα. Tofacitinib and ustekinumab showed comparable outcomes for steroid-free clinical remission. The safety profiles of all the therapies were comparable in real-world scenarios. These findings offer valuable guidance for health care providers in optimizing treatment decisions for patients with ulcerative colitis.
Ulcerative colitis (UC) is a chronic inflammatory disease of unknown etiology that results in mucosal inflammation and ulceration of the colon. Many patients with UC experience frequent flares and hospitalizations, leading to a significant direct and indirect economic burden.1-4 The primary therapeutic goal in UC is to induce and maintain clinical, endoscopic, and steroid-free remission (SF-REM) in the long term.5,6
The disease is classified as mild, moderate, or severe based on its clinical presentation.2 The most commonly used criteria for defining moderate-to-severe UC include a total Mayo Clinic Score between 5 and 12, a rectal bleeding subscore (RBS) of at least 1, and a Mayo Clinic Endoscopic Score of at least 2.3,4 Several advanced therapies (ATs) are approved for patients with moderate-to-severe UC, including biologics such as tumor necrosis factor (TNF)-α antagonists (infliximab, adalimumab, and golimumab), interleukin-12/23 antagonist (ustekinumab) and a recent interleukin-23 antagonist (mirikizumab), anti-integrin agent (vedolizumab), and small molecules viz Janus-Kinase inhibitors (tofacitinib, filgotinib, and upadacitinib) and sphingosine 1-phosphate receptor modulator (ozanimod, and recently also etrasimod).7,8-11
Real-world evidence (RWE) is gaining importance in clinical practice, whereas randomized controlled trials (RCTs) are considered the gold standard for demonstrating the efficacy and safety of drugs. The generalizability of RCT’s findings remains an issue because of strict eligibility criteria, which render the patients unrepresentative of the heterogeneous population treated in routine clinical practice. RWE is derived from data sources, such as electronic health records, health surveys, and patient registries, and covers real-life information on treatment/patient pathways, health outcomes, and safety.12,13 However, the variability in the study designs and execution significantly limits conclusions drawn from such data.
Although an increasing number of ATs have become available for the treatment of moderate-to-severe UC, a comprehensive assessment of their effectiveness and safety in a real-world setting is limited. We sought to systematically review and summarize published RWE on the effectiveness and safety of ATs for moderate-to-severe UC.
Methods
DATA SOURCES AND SEARCHES
A systematic literature review (SLR) was conducted following the standards published by the Cochrane Collaboration14 and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.15 We performed a comprehensive literature search using Embase and MEDLINE databases through the Embase.com platform from January 1, 2005, to February 28, 2022. The MEDLINE Epub ahead of print, in-process, and nonindexed citations were searched on PubMed (February 28, 2022). The time frame was determined based on the first approval of infliximab in September 2005. Only products that were approved in any jurisdiction globally at the time of search were included. Supplementary Tables 1-3 (2.1MB, pdf) , available in online article, provide the details of the search strategy. Searches were limited to English-language articles published in 2005 and later for full-text publications, and from January 2019 to February 2022 for conference abstracts.
STUDY SELECTION CRITERIA
Supplementary Table 4 (2.1MB, pdf) provides the details of the inclusion and exclusion criteria (population, interventions/ATs, outcomes, study design, and time period). Studies were included if they had adult patients with moderate-to-severe UC, with prior exposure to biologics or mixed populations, and reported effectiveness and/or safety outcomes using a real-world observational study design (retrospective or prospective). Studies with less than 30 patients or only biologic-naive patients were excluded. Non-English articles or those published before 2005 were excluded. All the retrieved citations were screened by 2 reviewers as per predefined eligibility criteria and a third reviewer resolved the discrepancies by consensus after discussion. Data extraction was conducted from full-text publications. Multiple publications from the same study were linked and extracted as a single study. The PRISMA flow diagram is provided as Supplementary Figure 1 (2.1MB, pdf) .
DATA EXTRACTION AND QUALITY ASSESSMENT
Data extraction and quality checks were performed by individual reviewers; differences were reconciled by the third reviewer. Data on study characteristics and methods, patient and treatment characteristics, follow-up duration, time points of assessments, effectiveness outcomes, safety events, results, and conclusions were extracted into a Microsoft Excel spreadsheet. Included studies were critically appraised for methodological quality using the Newcastle–Ottawa Scale. The Newcastle–Ottawa Scale is a tool for assessing the quality of nonrandomized studies using a star system to evaluate selection, comparability, and outcome reporting.16 Data were analyzed qualitatively, and results are reported as numbers and/or percentages or as crude median, Q1, and Q3.
Results
A total of 3,930 records were identified. Of these, 3,066 were excluded during title and abstract screening, and 864 full-text publications were evaluated for inclusion. After full-text screening, 181 publications were included. A total of 139 distinct studies were included for data extraction and analysis after linking multiple publications from the same studies (Supplementary Figure 2A-B (2.1MB, pdf) ; Supplementary Table 5 (2.1MB, pdf) ).
QUALITY OF STUDIES
According to the Newcastle–Ottawa Scale,16 studies were scored from 0 to 9 stars. The quality scores for included studies ranged from 2 to 8 stars, with most studies (69%) assigned a rating of 6 or more, indicating good quality. Conference abstracts were generally rated lower. All comparative studies were assigned 5 or more stars, and 10 studies were given 7 or 8 stars (Supplementary Table 6 (2.1MB, pdf) ).
DESCRIPTION OF THE INCLUDED STUDIES
Among eligible included studies, 109 (78%) were full-text publications and 30 (22%) were conference abstracts; their information is provided in Supplementary Table 7 (2.1MB, pdf) . Most of the studies (75%) were published between 2019 and 2022, and 53% were from 5 countries (Italy, n = 23; United States, n = 19; Spain, n = 12; United Kingdom, n = 10; Belgium, n = 9). Sixty-four percent of studies were retrospective, and 33% were prospective observational in nature. Studies were predominantly (67%) multicenter. Data sources used included electronic medical/health/hospital records (75%), registries (12%), chart reviews (9%), claims databases, and global safety databases (2% each).
PATIENT CHARACTERISTICS
Across studies, the median age of patients ranged from 2617 to 5518 years (median [Q1, Q3]: 41 [39, 45.5] years). The proportion of male patients ranged from 25%19 to 73%,20 with 46% of studies having an equal or almost equal balance of sexes. Median body mass index ranged from 2121-23 to 2724 kg/m2 (median [Q1, Q3]: 24.5 [24, 25] kg/m2) in the 45 studies. Eighty-two percent of studies reported a duration of disease between 4 and 9 years (range: 225-26 -1227 years [median (Q1, Q3): 7 (5, 8.5) years]). The extent of disease (E1/E2/E3) was distributed as up to 20% of patients with E1 (proctitis [Proximal extent to the sigmoid colon]; median [Q1, Q3]: 6% [3%, 11%]), 21% to 50% with E2 (left-sided colitis [to the splenic flexure]; 37% [31%, 42%]), and more than 51% patients with E3 (extensive colitis [beyond the splenic flexure]; 56% [46%, 63%]) (Supplementary Table 7 (2.1MB, pdf) ). Patient’s characteristics are presented in Figure 1.
FIGURE 1.
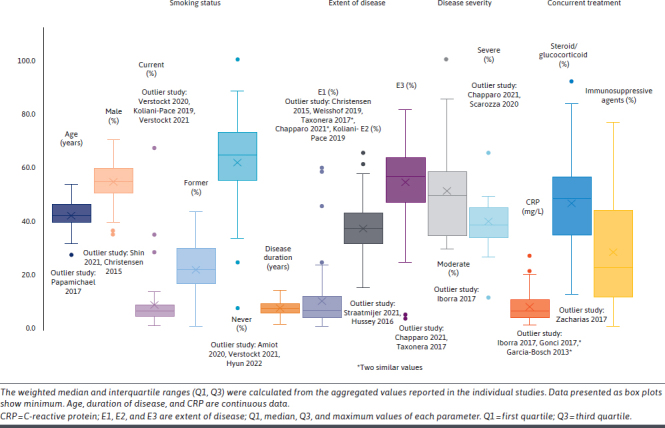
Patient Demographic and Clinical Characteristics at Baseline
Thirteen percent of studies had only biologic-exposed patients, 63% had more than 50% biologic-exposed patients, and 28% presented subgroup analyses for biologic-exposed patients. Of the biologic-exposed cohort, most patients had prior exposure to anti-TNFα agents in the range of 11%-100%, with a median (Q1, Q3) of 66% (44%, 94.2%). Other studies described patients previously treated with biologics such as vedolizumab, ustekinumab, and tofacitinib. Within these studies, the proportion of exposed patients varied (vedolizumab [median, 72% (57%, 81.5%); range, 2%-100%]; ustekinumab [median, 5% (3%, 8.5%); range, 1%-18%]; tofacitinib [median, 20% (8%, 30.7%); range, 10%-33%]).
INTERVENTIONS/ATs
Eighteen studies were comparative 18,19,23,24,27-40 (vedolizumab vs anti-TNFα agents18,28-36 [n = 10]; tofacitinib vs vedolizumab23,27,37,38 [n = 4]; different anti-TNFα agents19,24,39 [n =3]; tofacitinib vs ustekinumab40 [n = 1]), whereas 121 were single-arm studies (vedolizumab [n = 50], tofacitinib [n = 24], adalimumab [n = 18], golimumab [n = 14], infliximab [n = 10], ustekinumab [n = 5]). No real-world studies were yet found for filgotinib or ozanimod.
FOLLOW-UP AND OUTCOMES
In 76% of studies, the mean/median duration of follow-up was up to 54 weeks. Effectiveness outcomes were most frequently reported at 8-16 weeks, followed by 48-54 weeks; outcomes beyond 54 weeks were usually not reported. Clinical response (CRES), clinical remission (CR), SF-REM, and endoscopic remission were the most commonly reported outcomes, whereas histologic remission, patient-reported outcomes (PROs), and deep remission (combined clinical and endoscopic remission) were rarely reported. Primary nonresponse or loss of response was reported in 42 studies. Any adverse event (AE) (46%), UC-related colectomy/surgery (46%), discontinuation/withdrawal because of AE (37%), and infections (24%) were frequently reported safety outcomes. Major adverse cardiovascular events (MACEs), malignancies, and venous thromboembolism (VTE) were reported in 12% of studies.
Within studies reporting CR, the Partial Mayo Score (PMS) or total Mayo Clinic Score of less than or equal to 2 alone or combined with RBS and stool frequency subscore (SFS) of less than 1 were the most used definitions, followed by a PMS of less than or equal to 1, or Simple Colitis Clinical Activity Index (SCCAI) score of less than or equal to 2. SF-REM was reported as CR achieved without the use of steroids. Of the studies reporting endoscopic remission and mucosal healing (MH), a Mayo Endoscopic endoscopic subscore of 1 or 0 was the most commonly used definition; however, a few studies included histological remission in their definition of MH (Figure 2A). Few studies reported deep remission, usually defined as a combination of CR and endoscopic remission/MH (Figure 2B).
FIGURE 2.
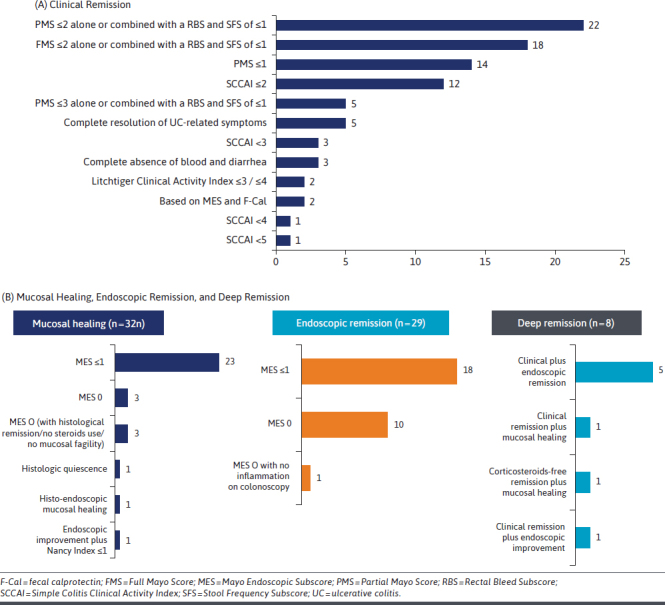
Number of Studies Following Definitions
RWE ON EFFECTIVENESS
Single-Treatment Studies
Vedolizumab. We identified 50 real-world studies of vedolizumab. The proportion of patients who achieved CR with vedolizumab after induction ranged from 18%22 to 87%41 at 8-12 weeks, and 32%42 to 56%43 at 14 weeks after initiation. CR was reported to be maintained in 36%44 to 71%45 of patients at 24 weeks and 28%46 to 77%45 at 52-54 weeks. Long-term CR was reported in 28%46,47 to 33%48 of patients at 104-108 weeks (Supplementary Figure 3 (2.1MB, pdf) ).
Tofacitinib. In the 24 studies describing the use of tofacitinib, rates of CR varied between 23%49 and 57%50 at 8 weeks and 32%51 and 65%52 at 16 weeks. CR was maintained in 23%17 to 62%53 of patients at 24-26 weeks and 27%54 to 64%55 of patients at 48-52 weeks or more. One study with long-term data reported 56%56 of patients achieving CR at 78 weeks and 54%56 to 70%50 at 104 weeks (Supplementary Figure 4 (2.1MB, pdf) ).
Ustekinumab. The CR varied from 35%57 to 43%55 in a few studies55,57,58 at 12-16 weeks. One study reported 39%57 of patients achieving CR at 24 weeks, whereas 3 studies reported 33%57 to 45%55 of patients achieving CR at 52 weeks. (Supplementary Figure 5 (2.1MB, pdf) ).
Anti-TNFα Agents. Forty-two studies were included for anti-TNFα agents (18 adalimumab, 14 golimumab, and 10 infliximab). In the single-arm studies of anti-TNFα agents, CR was ranging 16%59 to 65%60 at 8-14 weeks (induction), 27%61 to 86%62 at 24-30 weeks, and 20%59 to 90%62 at 52-54 weeks (maintenance). The long-term CR reported in studies varied greatly, with 25%63 to 68%64 of patients achieving this endpoint at 64-156 weeks (Supplementary Figure 6 (2.1MB, pdf) ).
Comparative Studies
Vedolizumab vs Anti-TNFα Agent. Ten studies compared vedolizumab with anti-TNFα agents18,28-36 and 3 of these were propensity-scored matched (PSM) comparisons.18,28,30 The PSM analysis from a study with 722 patients (454 vedolizumab, 268 anti-TNFs) showed that vedolizumab-treated patients were more likely to achieve CR (hazard ratio [HR] = 1.651; 95% CI = 1.229-2.217), SF-REM (HR = 1.828; 95% CI = 1.135-2.944), and steroid-free deep remission (HR = 2.819; 95% CI = 1.496-5.310) than those treated with anti-TNFα agents.28 Another study reported that rates of SF-REM in vedolizumab, adalimumab, and golimumab-treated patients were similar at 12 weeks but were significantly greater with vedolizumab at 52 weeks (52%, 31%, and 29%, respectively; P ≤ 0.002).18 In a multicenter chart review study from Germany (vedolizumab, n = 76; anti-TNFα, n = 57), the rates of CR at week 26 were numerically higher with vedolizumab vs anti-TNFα agents (54% vs 32%).31 Comparable rates of CRES, CR, and SF-REM between vedolizumab and anti-TNFα agents at 6, 24, and 52 weeks, respectively, were reported in a United Kingdom study (Table 1).34
TABLE 1.
A Summary of Comparative Effectiveness Reported in Studies Comparing Vedolizumab vs Anti-TNFα Agents
| Outcome | Study name | Follow-up (weeks) | Vedolizumab | IFX | GOL | ADA | Anti-TNFα as class |
|---|---|---|---|---|---|---|---|
| Clinical remission | Lukin 202228 | 48 | 42% (187/453)a | 37% (61/163) | |||
| Helwig 202031 | 26 | 54%b (P = 0.0380) | 32%b | ||||
| Bertani 202032 | 54 | 75% | 44% | 61% | 65% | ||
| Davis 201934 | 12 | 48% (NS) | IFX/ADA/GOL: 40% | ||||
| 24 | 52% (NS) | IFX/ADA/GOL: 39% | |||||
| 52 | 51% | IFX/ADA/GOL: 28% | |||||
| Clinical response | Gagnon 202129 | 52 | 48% (19/39) | 63% (45/68) | 57% (33/58) | ||
| Macaluso 202018 | 12 | 71% | 68% | 69% | |||
| 52 | 72% (P < 0.001 vs GOL, ADA) | 40% (NS vs ADA) | 48% (P < 0.001) | ||||
| Davis 201934 | 12 | 57% (NS) | IFX/ADA/GOL: 55% | ||||
| 24 | 69% (NS) | IFX/ADA/GOL: 59% | |||||
| 52 | 51% (NS) | IFX/ADA/GOL: 42% | |||||
| Steroid-free clinical remission | Macaluso 202018 | 12 | 24% (NS vs GOL, ADA) | 31% (NS vs ADA) | 33% | ||
| 52 | 52% (P = 0.001 vs GOL; P = 0.002 vs ADA) | 29% (NS vs ADA) | 31% | ||||
| Davis 201934 | 12 | 33% (NS) | IFX/ADA/GOL: 31% | ||||
| 24 | 48% (NS) | IFX/ADA/GOL: 36% | |||||
| 52 | 14% (NS) | IFX/ADA/GOL: 25% |
Significant values are highlighted in bold.
aVedolizumab was shown to be associated with a higher probability of achieving clinical remission compared with infliximab (hazards ratio = 1.810; 95% CI = 1.225-2.675).
bCumulative rates estimated using nonparametric, stratified K-M approach to account for variability in patient follow-up and timing of outcome events.
ADA = adalimumab; GOL = golimumab; IFX = infliximab; NS = nonsignificant; S = significant; TNFα = tumor necrosis factor α.
Tofacitinib vs Vedolizumab. Four studies compared tofacitinib with vedolizumab,23,27,37,38 and among them including a prospective study from the Initiative on Crohn and Colitis registry with 65 patients on tofacitinib and 83 on vedolizumab, previously exposed to anti-TNFα. PSM results showed that tofacitinib-treated patients were more likely to achieve SF-REM (ie, SCCAI ≤2) compared with vedolizumab-treated patients at week 12 (odds ratio [OR] = 6.33, 95% CI = 3.81-10.50, P < 0.01), week 24 (OR = 3.02, 95% CI = 1.89-4.84, P < 0.01), and week 52 (OR = 1.86, 95% CI = 1.15-2.99, P = 0.01). Biochemical remission (C-reactive protein ≤5 mg/L or fecal calprotectin ≤250 µg/g) was also more frequently reported in tofacitinib-treated patients vs vedolizumab (P ≤ 0.03).27 A multicenter study from France with 87 patients receiving tofacitinib and 112 administered with vedolizumab, previously exposed to at least 1 anti-TNFα, described that 16-week SF-REM was numerically greater with tofacitinib than vedolizumab (54% vs 43%). The rates of 16-week SF-REM were numerically higher with tofacitinib after 1 (57% vs 51%, P = 0.77), 2 (55% vs 42%, P = 0.61), and at least 3 biologics (57% vs 6%, P = 0.007). Tofacitinib was more effective than vedolizumab in achieving SF-REM in patients with primary failure to at least 1 biologic (72% vs 31%, P = 0.049). Endoscopic improvement was more common in patients treated with tofacitinib (34% vs 7%, P = 0.048).38 In a single-center retrospective study from Japan in patients with UC who initiated tofacitinib (n = 38) and vedolizumab (n = 28), the rate of CR (ie, PMS ≤1 or decrease from baseline by ≥3 points) at week 6 was significantly higher with tofacitinib than vedolizumab (63% vs 36%, P = 0.027) (Figure 3).37
FIGURE 3.
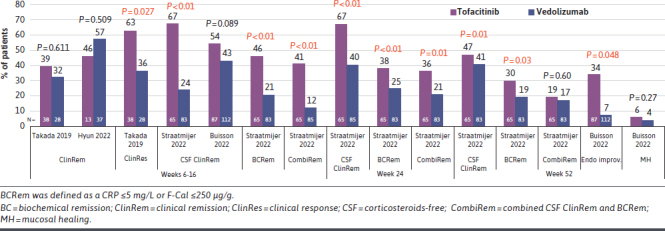
A Summary of Comparative Effectiveness Reported in Studies Comparing Tofacitinib vs Vedolizumab
Comparison Between Anti-TNFα Agents. Of the 3 studies comparing anti-TNFα agents,19,20,39 only 2 reported effectiveness.19,39 A single-center retrospective study from Italy comparing 3 anti-TNFα agents (infliximab, adalimumab, and golimumab) showed that overall CRES was 77% after induction, 81% at 30 weeks, and 77% at 52 weeks. The SF-REM was 40%, 46%, and 55% after induction, 30 and 52 weeks, respectively. The rates of CRES, CR, and SF-REM were greater with infliximab at all the time points compared with adalimumab and golimumab.20 The rate of treatment failure was higher (after induction), and rates of CRES and SF-CR (at the end of follow-up) were lower with golimumab at week 14 compared with infliximab and adalimumab.19 A multicenter study extracting web-based data from the Sicilian Network for inflammatory bowel disease compared adalimumab (n = 118) and golimumab (n = 79) in moderate-to-severe patients with UC, with 50%-63% of patients in each group previously exposed to biologics. PSM analysis showed that clinical benefit (ie, CRES plus SF-REM) was significantly higher with adalimumab than golimumab after 8 weeks (79% vs 63%, P = 0.026) and at the end of follow-up (median 34-40 weeks) (67% vs 47%, P = 0.008).39
Tofacitinib vs Ustekinumab. A single study from the United States was identified comparing tofacitinib with ustekinumab, which was conducted in biologic-experienced patients with UC (45 tofacitinib and 36 ustekinumab).40 The PSM results showed similar rates of SF-REM (ie, SCCAI ≤2 [44% tofacitinib vs 40% ustekinumab, P = 0.82]) and steroid-free CRES (46% tofacitinib vs 49% ustekinumab, P = 1.00) at 12-16 weeks, and SF-REM (60% tofacitinib vs 55% ustekinumab) at 52 weeks, after treatment initiation.40
RWE ON SAFETY
Single-Treatment Studies
Vedolizumab. A 4-year postmarketing safety data analysis on vedolizumab reported serious AEs (SAEs) in 10% of patients with UC. Most frequent AEs were gastrointestinal events (17%), infections (7%), and malignancies were reported in less than 1% of patients.65
Other real-world studies showed a wide proportion of patients reporting AEs (2%66 to 62%67) and SAEs (2%68 to 17%48). UC-related hospitalization ranged from 4%69 to 22%.25 The rate of colectomy varied between 1%69-71 and 26%,47 infections between 1%72 and 22%,67 whereas the serious infections were infrequent (3%-4%).44,48,73 Herpes-zoster virus (HZV) infection, VTE, and MACE were not reported in vedolizumab studies. Malignancy rates were low (Supplementary Table 8 (2.1MB, pdf) ).48,67 Discontinuation rate reported varied between 8%74 and 51%75 at at least 52 weeks.
Tofacitinib. A safety study of tofacitinib in patients with UC (27 months reporting period; 8,916 person-years [PYs] exposure) showed that reported AEs were consistent with those seen in RCTs and mostly were nonserious.76 Overall, 4,226 case reports were received and included 12,103 AEs, of which 1,839 were SAEs (27%; death: 0.4%). The reported incidence rate for gastrointestinal disorders, infections and infestations, and vascular disorders was 6.97, 3.28, and 1.26 per 100 PYs, respectively.76
AEs in single-arm studies ranged widely between 9%77 and 72%,78 and SAEs ranged between 5%56 and 16%.79 UC-related hospitalization was reported in 1%80 to 19%77 and UC-related colectomy ranged between 1%52 and 26%.79 Infections were reported in 3%51 to 24%,79 whereas information on serious infections was not reported commonly (HZV infection was reported in up to 8% of patients).17,50,51,56,79 The incidence rate of VTE was negligible less than or equal to 1%,17,50, 51,53,56,78,81,82 whereas MACEs and malignancies were rarely reported and had a very low incidence (Supplementary Table 9 (2.1MB, pdf) ).17,50,51,54,79,83 The discontinuation rate ranged between 31%56 and 61%83 at at least 52 weeks.
Ustekinumab. AEs were reported in 1%84 to 12%,55,57,58,84,85 whereas SAEs occurred in 4%85 to 6%.55 The rate of UC-related hospitalization was 3%85 to 6%,55 and colectomy rates varied between 2%85 and 9%.57 The infections were reported in 6%,55 although serious infections were infrequent. HZV infection, VTE, or malignancies were not reported. The rates of MACE were very infrequent (1% in one study85; Supplementary Table 10 (2.1MB, pdf) ), and the discontinuation rate was reported to be between 13%84 and 36%57 at at least 52 weeks.
Anti-TNFα Inhibitors. Reports of AEs varied between 4%61,86 and 38%87 (infliximab = 6%64 to 27%87; adalimumab = 4%61,86 to 38%87; golimumab = 8%88 to 22%88) and 0%88 and 11%89 for SAEs (infliximab = 4%64 to 11%90; adalimumab = 3%91 to 5%92; golimumab = 0%88 to 5%88). Colectomy rates were between 0%20 and 36%63 (infliximab = 1%26 to 36%63; adalimumab = 3%93 to 25%94; golimumab = 0%20 to 22%95), whereas UC-related hospitalizations were not commonly reported. Patients reporting infections in anti-TNFα agent studies ranged from 0%20 to 47%96 (adalimumab = 2%93 to 47%96; golimumab = 3%97 to 8%88), and serious infections varied between 0%61 and 6%26,91 (infliximab = 2%87 to 6%26; adalimumab = 0%61 to 6%91; with golimumab = 2%88). HZV infection (1%87 to 3%96) and malignancies (0%61 to 3%96) were very low. VTE and MACE were reported infrequently (Supplementary Table 11A-C (2.1MB, pdf) ). The discontinuation rate varied between 7%98 and 84%63 at at least 1 year.
Comparative Studies
Vedolizumab vs Anti-TNFα Agent. The safety events comparing vedolizumab and anti-TNFα agents are summarized in Supplementary Table 12 (2.1MB, pdf) . Most studies were descriptive, only reporting the proportion of patients with safety events. No statistically significant differences were reported for the risk of SAEs (HR = 0.899; 95% CI = 0.502-1.612) or serious infections (HR = 1.235; 95% CI = 0.608-2.511) between vedolizumab-treated (n = 454) and anti-TNFα–treated (n = 268) patients.28
Tofacitinib vs Vedolizumab. Two descriptive studies (one each from Korea23 and Japan37) reported that safety profiles of tofacitinib and vedolizumab were similar in terms of overall AEs, with no reported SAEs (Supplementary Table 13 (2.1MB, pdf) ). A study by Straatmijer et al using inverse probability of treatment weighing showed that vedolizumab-treated patients (n = 83) had an overall higher chance of experiencing AEs than tofacitinib-treated (n = 65) patients (OR = 1.83; 95% CI = 1.10-3.03; P = 0.02), although the number of severe AEs were similar between the 2 treatment groups (OR = 0.39; 95% CI = 0.03-4.33; P = 0.44).27
Comparison Between Anti-TNFα Agents. Safety events did not differ significantly across anti-TNFα agents. A study by Barberio et al reported a similar and generally good safety profile for adalimumab, golimumab, and infliximab (originator and biosimilar).19 Hoque et al described the 8% colectomy rate within 12 months following treatment with each of golimumab (87 patients) and adalimumab (96 patients).24 There were reports of 10 AEs in the adalimumab group with an incidence rate of 80.4/1,000 PYs and 4 AEs in the golimumab group with an incidence rate of 33.1/1,000 PYs. The difference between the 2 groups was not statistically significant (P = 0.247).39
Tofacitinib vs Ustekinumab. In a study comparing tofacitinib (45 patients) with ustekinumab (36 patients), AEs were comparable between tofacitinib (11%) and ustekinumab (11%; 6%, respectively). Infection, deep vein thrombosis, liver injury, refractory nausea/vomiting, and shingles were reported with tofacitinib, whereas rash and urinary tract infection were reported with ustekinumab. Drug discontinuation or total colectomy was reported in 51% and 36% for tofacitinib and ustekinumab, respectively.40
Discussion
Real-world data provide valuable evidence and insight to support the efficacy and tolerability of therapies observed in RCTs; trial patients represent only a proportion of the entire UC population in contrast to studies performed in real-world settings.99 Overviews of recently available ATs in patients with moderate-to-severe UC may be helpful to address this gap. Currently available SLR and meta-analyses for vedolizumab,100 tofacitinib,101,102 or ustekinumab103 primarily assessed evidence from single-arm studies. These studies are limited with smaller sample sizes and limited outcomes assessed. Our review provides a comprehensive qualitative overview of RWE on the effectiveness and safety of ATs, incorporating data from both peer-reviewed full-text manuscripts/articles and conference abstracts.
In this SLR, more than half of real-world studies originated from 5 countries, namely the United States, United Kingdom, Italy, Spain, and Belgium. The findings may be an overestimate because of the exclusion of non-English studies from the analysis. Vedolizumab and tofacitinib were the most frequently assessed/reported ATs in moderate-to-severe UC, whereas the RWE for ustekinumab in UC is limited. Ozanimod and filgotinib were recently approved; hence, RWE was not yet available during the time of literature search.10 Additionally, mirikizumab, upadacitinib, and etrasimod were not approved11 at the time of searches for this review.
There was a wide variation in the studies in terms of their design, sample size, follow-up duration, patient demographics and characteristics, disease duration, previous exposure to biologics, and outcomes assessed. Although CR was the most reported outcome,6,7 a marked variability was observed in the definition of CR across studies. A possible explanation may be the evolution of study endpoints in RCTs and subsequently in real-world analyses. Common definitions included a PMS of less than or equal to 2 either alone or combined with an RBS and SFS of less than or equal to 2, a PMS of less than or equal to 1, or a SCCAI less than or equal to 2. Considering these variations, we feel that a uniform criterion of CR would be helpful to enhance comparability across studies. We suggest using a PMS of less than or equal to 2 and no individual score greater than 1 and RBS of 0 or the PRO2 criterion in future studies, which is also aligned with the definition used in current RCTs.
A few comparative studies were included in this review, most of which had a short follow-up time. Overall, the rate of CR with tofacitinib was numerically higher than vedolizumab and comparable with ustekinumab. Clinical remission was numerically higher for vedolizumab vs anti-TNFα agents. These results should be interpreted cautiously because of the small sample sizes in these studies and marked variation in the baseline characteristics of the patients, although a few studies reported PSM comparisons.18,27,28,30,39,40 Comparative studies with PSM analysis are strongly suggested for future research.
European Medicines Agency guidance (2018) on the development of new treatments for UC stated that only patients with MH (ie, absence of macroscopic signs of active inflammation) and no, or very mild, symptoms and signs should be considered in remission.104 The guidelines released by the American College of Gastroenterology and the American Gastroenterological Association recommend the use of SF-REM as a marker of remission in patients with UC.6,105 However, a recent ECCO position paper and the STRIDE II guidelines have also placed emphasis on histological activity, which can persist despite clinical and endoscopic remission and is a known risk factor for disease flare. Histological remission (ie, the absence of inflammation and ulceration/erosion) is now under discussion as a target for UC therapy,106,107 however, it is rarely reported in the studies we reviewed for this SLR.39,55,83,108 Histological healing may be associated with improved clinical outcomes; therefore, it should be considered as an outcome in future real-world studies.
This SLR also highlighted that the use of PRO measures, apart from disease activity, was very limited in real-world studies. The literature indicates less use of PROs in phase 4 or observational studies compared with RCTs.109,110 PROs provide valuable insights on the experience of their therapies.111 The European Medicines Agency guidance and the STRIDE II guidelines also suggest that a symptomatic relief is best evaluated by PROs.104,107 Therefore, the use of PROs in RWE studies should be encouraged. This can be facilitated in several ways, including the development of standards for their use, collection, and analysis.112,113
There are considerable variations in AE and SAE reporting in single-arm studies. Comparative studies reported similar safety event rates across ATs, although most studies had small sample sizes and/or were not adjusted for covariates. There were zero or limited occurrences of MACE50,54,79 and malignancies.55,61,67,83,85
LIMITATIONS
There are limitations to this SLR conducted on real-world studies as the data collection is less stringent than RCT. The differences in study designs and patient characteristics across studies may result in considerable heterogeneity in reported outcomes. Thus, outcomes should be interpreted with caution if a quantitative assessment of heterogeneity was not performed. Some studies had small sample sizes, including those comparing tofacitinib with ustekinumab or vedolizumab. Only studies published in the English language were included; this may be considered a source of bias, although most articles are published in English. Despite these limitations, this SLR reports a large volume of RWE on the effectiveness and safety of ATs in moderate-to-severe UC, and the utility of this RWE in decision-making should not be underestimated.
Conclusions
This review comprising 139 real-world studies of ATs in moderate-to-severe UC highlights vedolizumab and tofacitinib as the most assessed ATs. Most studies (87%) assessed a single agent. The proportion of patients with CR and AEs varied widely., In comparative studies with largely bioexposed patients, CR rates were numerically higher with tofacitinib vs vedolizumab and comparable with ustekinumab. The CR was numerically higher for vedolizumab vs anti-TNFα agents. Generally, the safety events were similar across ATs. Several evidence gaps at the time of search were identified, such as the paucity of comparative studies, small sample sizes, variations in study design, patient characteristics, and definition of outcomes, limited use of PRO measures, and limited reporting of AEs such as MACE and malignancies. Further research should focus on addressing these issues.
ACKNOWLEDGMENTS
Raju Gautam was an employee of EVERSANA Pvt. Ltd, Mumbai, India, which was a paid consultant to Pfizer in connection with the development of this manuscript. Editorial support was provided by Drs Deshpande and Varshney from EVERSANA.
Funding Statement
This systematic literature review was sponsored and funded by Pfizer.
DATA AVAILABILITY
All relevant data underlying this article are available in the article and in its online Supplementary Materials.
REFERENCES
- 1.Crohn’s & Colitis Foundation of America. The facts about inflammatory bowel diseases. 2014. Accessed December 19, 2022. crohnscolitisfoundation.org [Google Scholar]
- 2.Magro F, Gionchetti P, Eliakim R, et al. ; European Crohn’s and Colitis Organisation [ECCO] . Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649-70. doi:10.1093/ecco-jcc/jjx008 [DOI] [PubMed] [Google Scholar]
- 3.D’Haens GR, Sandborn WJ, Zou G, et al. Randomised non-inferiority trial: 1600 mg versus 400 mg tablets of mesalazine for the treatment of mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther. 2017;46(3):292-302. doi:10.1111/apt.14164 [DOI] [PubMed] [Google Scholar]
- 4.Sands BE, Schreiber S, Lirio RA. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. Reply. N Engl J Med. 2020;382(1):93-4. doi:10.1056/NEJMc1915739 [DOI] [PubMed] [Google Scholar]
- 5.Feuerstein JD, Isaacs KL, Schneider Y, et al. ; AGA Institute Clinical Guidelines Committee . AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450-61. doi:10.1053/j.gastro.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burri E, Maillard MH, Schoepfer AM, et al. ; Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology . Treatment algorithm for mild and moderate-to-severe ulcerative colitis: An update. Digestion. 2020;101(suppl 1):2-15. doi:10.1159/000504092 [DOI] [PubMed] [Google Scholar]
- 7.Kayal M, Shah S. Ulcerative colitis: Current and emerging treatment strategies. J Clin Med. 2019;9(1):94. doi:10.3390/jcm9010094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferretti F, Cannatelli R, Monico MC, Maconi G, Ardizzone S. An update on current pharmacotherapeutic options for the treatment of ulcerative colitis. J Clin Med. 2022;11(9):2302. doi:10.3390/jcm11092302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeposia® (ozanimod). Highlights of prescribing information. US FDA; 2021. Accessed January 12, 2022. fda.gov [Google Scholar]
- 10.Jyseleca® (filgotinib). Prescribing information. EMA; 2021. Accessed January 12, 2022. europa.eu [Google Scholar]
- 11.Rinvoq® (upadacitinib). Highlights of prescribing information. US FDA; 2022. Accessed July 25, 2022. fda.gov [Google Scholar]
- 12.Chodankar D. Introduction to real-world evidence studies. Perspect Clin Res. 2021;12(3):171-4. doi:10.4103/picr.picr_62_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naidoo P, Bouharati C, Rambiritch V, et al. Real-world evidence and product development: Opportunities, challenges and risk mitigation. Wien Klin Wochenschr. 2021;133(15-16):840-6. doi:10.1007/s00508-021-01851-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane, 2021. Accessed January 12, 2022. www.training.cochrane.org/handbook
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. doi:10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Ottawa Hospital. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Accessed January 12, 2022. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 17.Avni-Biron I, Bar-Gil Shitrit A, Koslowsky B, et al. Short-term effectiveness and safety of tofacitinib in ulcerative colitis - real world data from tertiary medical centers in Israel. Dig Liver Dis. 2022;54(2):192-7. doi:10.1016/j.dld.2021.11.009 [DOI] [PubMed] [Google Scholar]
- 18.Macaluso FS, Ventimiglia M, Fries W, et al. ; Sicilian Network for Inflammatory Bowel Disease (SN-IBD) . A propensity score weighted comparison of vedolizumab, adalimumab, and golimumab in patients with ulcerative colitis. Dig Liver Dis. 2020;52(12):1461-6. doi:10.1016/j.dld.2020.06.014 [DOI] [PubMed] [Google Scholar]
- 19.Taxonera C, Iborra M, Bosca-Watts MM, et al. Early dose optimization of golimumab induces late response and long-term clinical benefit in moderately to severely active ulcerative colitis. Curr Med Res Opin. 2019;35(7):1297-304. doi:10.1080/03007995.2019.1579557 [DOI] [PubMed] [Google Scholar]
- 20.Barberio B, Zingone F, Frazzoni L, et al. Real-life comparison of different anti-TNF biologic therapies for ulcerative colitis treatment: A retrospective cohort study. Dig Dis. 2021;39(1):16-24. doi:10.1159/000508865 [DOI] [PubMed] [Google Scholar]
- 21.Saito D, Matsuura M, Ozaki R, et al. Clinical response of vedolizumab at week 6 predicted endoscopic remission at week 24 in ulcerative colitis. JGH Open. 2021;5(9):1056-62. doi:10.1002/jgh3.12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye BD, Cheon JH, Song KH, et al. The real-world outcomes of vedolizumab in patients with ulcerative colitis in Korea: A multicenter retrospective study. Therap Adv Gastroenterol. 2021;14:17562848211024769. doi:10.1177/17562848211024769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyun HK, Zhang HS, Yu J, et al. Comparative effectiveness of second-line biological therapies for ulcerative colitis and Crohn’s disease in patients with prior failure of anti-tumour necrosis factor treatment. BMC Gastroenterol. 2022;22(1):143. doi:10.1186/s12876-022-02225-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoque S, Puenpatom A, Boccaletti S, et al. Treatment persistence and colectomy-free outcomes in patients with ulcerative colitis receiving golimumab or adalimumab: A UK experience. BMJ Open Gastroenterol. 2020;7(1):e000476. doi:10.1136/bmjgast-2020-000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koliani-Pace JL, Singh S, Luo M, et al. Changes in vedolizumab utilization across US academic centers and community practice are associated with improved effectiveness and disease outcomes. Inflamm Bowel Dis. 2019;25(11):1854-61. doi:10.1093/ibd/izz071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer A, Rudant J, Drouin J, Coste J, Carbonnel F, Weill A. The effectiveness and safety of infliximab compared with biosimilar CT-P13, in 3112 patients with ulcerative colitis. Aliment Pharmacol Ther. 2019;50(3):269-77. doi:10.1111/apt.15323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straatmijer T, Biemans VB, Visschedijk M, et al. ; Initiative on Crohn and Colitis. Superior effectiveness of tofacitinib compared to vedolizumab in anti-TNF experienced ulcerative colitis patients: A nationwide Dutch Registry study. Clin Gastroenterol Hepatol. 2023;21(1):182-91.e2. doi:10.1016/j.cgh.2022.04.038 [DOI] [PubMed] [Google Scholar]
- 28.Lukin D, Faleck D, Xu R, et al. Comparative safety and effectiveness of vedolizumab to tumor necrosis factor antagonist therapy for ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20(1):126-35. doi:10.1016/j.cgh.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gagnon AL, Beauchesne W, Tessier L, et al. Adalimumab, infliximab, and vedolizumab in treatment of ulcerative colitis: A long-term retrospective study in a tertiary referral center. Crohns Colitis 360. 2021;3(4):otab049. doi:10.1093/crocol/otab049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rundquist S, Sachs MC, Eriksson C, Olén O, Montgomery S, Halfvarson J; SWIBREG Study Group. Drug survival of anti-TNF agents compared with vedolizumab as a second-line biological treatment in inflammatory bowel disease: Results from nationwide Swedish registers. Aliment Pharmacol Ther. 2021;53(4):471-83. doi:10.1111/apt.16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helwig U, Mross M, Schubert S, et al. Real-world clinical effectiveness and safety of vedolizumab and anti-tumor necrosis factor alpha treatment in ulcerative colitis and Crohn’s disease patients: A German retrospective chart review. BMC Gastroenterol. 2020;20(1):211. doi:10.1186/s12876-020-01332-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertani L, Blandizzi C, Mumolo MG, et al. Fecal calprotectin predicts mucosal healing in patients with ulcerative colitis treated with biological therapies: A prospective study. Clin Transl Gastroenterol. 2020;11(5):e00174. doi:10.14309/ctg.0000000000000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Favale A, Onali S, Caprioli F, et al. ; Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) . Comparative efficacy of vedolizumab and adalimumab in ulcerative colitis patients previously treated with infliximab. Inflamm Bowel Dis. 2019;25(11):1805-12. doi:10.1093/ibd/izz057 [DOI] [PubMed] [Google Scholar]
- 34.Davis R, McParland P, Dodd S, et al. Comparative effectiveness of antitumour necrosis factor agents and vedolizumab in ulcerative colitis. Eur J Gastroenterol Hepatol. 2019;31(6):661-7. doi:10.1097/MEG.0000000000001395 [DOI] [PubMed] [Google Scholar]
- 35.Di Ruscio M, Variola A, Geccherle A, et al. Histological remission in patients with moderate-to-severe ulcerative colitis undergoing biological therapy: A single-centre experience. J Crohns Colitis. 2019;13(suppl 1):S441-2. doi:10.1093/ecco-jcc/jjy222.770 [Google Scholar]
- 36.Subramanian S, Davis R, MacParland P, et al. Comparative efficacy of anti-tumour necrosis factor agents and vedolizumab in ulcerative colitis. J Crohns Colitis. 2019;13(suppl 1):S321. doi:10.1093/ecco-jcc/jjy222.547 [Google Scholar]
- 37.Takada Y, Naganuma M, Mutaguchi M, et al. The comparison of short-term efficacy of treatments between tofacitinib and vedolizumab in patients with ulcerative colitis. Am J Gastroenterol. 2019;114(suppl 1):S24-5. doi:10.14309/01.ajg.0000613340.02177.3d [Google Scholar]
- 38.Buisson A, Nachury M, Fumery M, et al. Real-world multicenter comparison of effectiveness between tofacitinib and vedolizumab in patients with ulcerative colitis after failure to at least one anti-TNF agent. J Crohns Colitis. 2022;16(suppl 1):I120. doi:10.1093/ecco-jcc/jjab232.115 [Google Scholar]
- 39.Renna S, Mocciaro F, Ventimiglia M, et al. A real life comparison of the effectiveness of adalimumab and golimumab in moderate-to-severe ulcerative colitis, supported by propensity score analysis. Dig Liver Dis. 2018;50(12):1292-8. doi:10.1016/j.dld.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 40.Dalal RS, Mitri J, Goodrick H, Allegretti JR. Real-world comparison of tofacitinib vs ustekinumab among bio-exposed patients with ulcerative colitis: A propensity score analysis. Inflamm Bowel Dis. 2021;27(10):1694-7. doi:10.1093/ibd/izab097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plevris N, Jenkinson PW, Chuah CS, et al. Association of trough vedolizumab levels with clinical, biological and endoscopic outcomes during maintenance therapy in inflammatory bowel disease. Frontline Gastroenterol. 2019;11(2):117-23. doi:10.1136/flgastro-2019-101197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scarozza P, Marafini I, Laudisi F, et al. Extent of mucosal inflammation in ulcerative colitis influences the clinical remission induced by vedolizumab. J Clin Med. 2020;9(2):385. doi:10.3390/jcm9020385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bamias G, Kokkotis G, Gizis M, et al. Predictors of response to vedolizumab in patients with ulcerative colitis: Results from the Greek VEDO-IBD cohort. Dig Dis Sci. 2022;67(3):1007-17. doi:10.1007/s10620-021-06907-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narula N, Peerani F, Meserve J, et al. Vedolizumab for ulcerative colitis: Treatment outcomes from the VICTORY consortium. Am J Gastroenterol. 2018;113(9):1345-54. doi:10.1038/s41395-018-0162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinglas J, Gonczi L, Verdon C, et al. Low rate of drug discontinuation, frequent need for dose adjustment, and no association with development of new arthralgia in patients treated with vedolizumab: Results from a tertiary referral IBD center. Dig Dis Sci. 2020;65(7):2046-53. doi:10.1007/s10620-019-05982-z [DOI] [PubMed] [Google Scholar]
- 46.Biemans VB, van der Woude CJ, Dijkstra G, et al. Vedolizumab for inflammatory bowel disease: Two-year results of the Initiative on Crohn and Colitis (ICC) registry, a nationwide prospective observational cohort study: ICC registry – vedolizumab. Clin Pharmacol Ther. 2020;107(5):1189-99. doi:10.1002/cpt.1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Attauabi M, Vind I, Pedersen G, et al. Short and long-term effectiveness and safety of vedolizumab in treatment-refractory patients with ulcerative colitis and Crohn’s disease – A real-world two-center cohort study. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e709-18. [DOI] [PubMed] [Google Scholar]
- 48.Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. ; Observatory on Efficacy and of Vedolizumab in Patients With Inflammatory Bowel Disease Study Group; Groupe d’Etude Therapeutique des Affections Inflammatoires du tube Digestif . Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14(11):1593-601.e2. doi:10.1016/j.cgh.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 49.Khoo E, Lee A, Lo SW, et al. Real-world experience of tofacitinib in Australia for treatment of ulcerative colitis. J Gastroenterol Hepatol. 2020;35:124-5.31090096 [Google Scholar]
- 50.Honap S, Chee D, Chapman TP, et al. ; LEO [London, Exeter, Oxford] IBD Research Consortium . Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: A multicentre UK experience. J Crohns Colitis. 2020;14(10):1385-93. doi:10.1093/ecco-jcc/jjaa075 [DOI] [PubMed] [Google Scholar]
- 51.Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: Real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(1):35-42. doi:10.1093/ecco-jcc/jjaa145 [DOI] [PubMed] [Google Scholar]
- 52.Oh K, Hong SN, Kim ES, et al. Real-life effectiveness and safety of tofacitinib treatment in patients with ulcerative colitis: A KASID multicenter cohort study. J Crohns Colitis. 2022;16(suppl 1): S359-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kojima K, Yokoyama K, Kaku K, et al. Efficacy and safety of tofacitinib treatment for one year in Japanese patients with ulcerative colitis in a specialized inflammatory bowel disease center. Gastroenterology. 2021;160(suppl 6):S413. doi:10.1016/S0016-5085(21)01695-4 [Google Scholar]
- 54.Weisshof R, Aharoni Golan M, Sossenheimer PH, et al. Real-world experience with tofacitinib in IBD at a tertiary center. Dig Dis Sci. 2019;64(7):1945-51. doi:10.1007/s10620-019-05492-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong SJ, Krugliak Cleveland N, Akiyama S, et al. Real-world effectiveness and safety of ustekinumab for ulcerative colitis from 2 tertiary IBD centers in the United States. Crohns Colitis 360. 2021;3(1):otab002. doi:10.1093/crocol/otab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernández Martínez A, Navajas Hernández P, Martín Rodríguez MD, et al. Efficacy and safety of tofacitinib in the treatment of ulcerative colitis: real-life experience in Andalusia. Rev Esp Enferm Dig. 2022;114(9):516-21. doi:10.17235/reed.2022.8380/2021 [DOI] [PubMed] [Google Scholar]
- 57.Chaparro M, Garre A, Iborra M, et al. Effectiveness and safety of ustekinumab in ulcerative colitis: Real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(11):1846-51. doi:10.1093/ecco-jcc/jjab070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalal RS, Esckilsen S, Barnes EL, Pruce JC, Marcus J, Allegretti JR. Predictors and outcomes of ustekinumab dose intensification in ulcerative colitis: A multicenter cohort study. Clin Gastroenterol Hepatol. 2022;20(10):2399-401.e4. doi:10.1016/j.cgh.2021.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eriksson C, Visuri I, Vigren L, et al. ; GO SWIBREG study group . Clinical effectiveness of golimumab in ulcerative colitis: A prospective multicentre study based on the Swedish IBD Quality Register, SWIBREG. Scand J Gastroenterol. 2021;56(11):1304-11. doi:10.1080/00365521.2021.1963466 [DOI] [PubMed] [Google Scholar]
- 60.Farkas K, Rutka M, Ferenci T, et al. Infliximab biosimilar CT-P13 therapy is effective and safe in maintaining remission in Crohn’s disease and ulcerative colitis - Experiences from a single center. Expert Opin Biol Ther. 2017;17(11):1325-32. doi:10.1080/14712598.2017.1363885 [DOI] [PubMed] [Google Scholar]
- 61.Bálint A, Farkas K, Palatka K, et al. Efficacy and safety of adalimumab in ulcerative colitis refractory to conventional therapy in routine clinical practice. J Crohns Colitis. 2016;10(1):26-30. doi:10.1093/ecco-jcc/jjv169 [DOI] [PubMed] [Google Scholar]
- 62.Armuzzi A, Saibeni S, Gasbarrini A. et al., Real-life evaluation of golimumab effectiveness and quality of life in patients with moderate-to-severe ulcerative colitis: The prospective go-care study. United European Gastroenterol J. 2021;9 (Supplement 8). [Google Scholar]
- 63.Lehtola E, Haapamäki J, Färkkilä MA. Outcome of inflammatory bowel disease patients treated with TNF-α inhibitors: Two-year follow-up. Scand J Gastroenterol. 2016;51(12):1476-81. doi:10.1080/00365521.2016.1218539 [DOI] [PubMed] [Google Scholar]
- 64.Tursi A, Mocci G, Allegretta L, et al. Comparison of performances of infliximab biosimilars CT-P13 versus SB2 in the treatment of inflammatory bowel diseases: A real-life multicenter, observational study in Italy. Expert Opin Biol Ther. 2022;22(2): 313-20. doi:10.1080/14712598.2022.2007881 [DOI] [PubMed] [Google Scholar]
- 65.Cohen RD, Bhayat F, Blake A, Travis S. The safety profile of vedolizumab in ulcerative colitis and Crohn’s disease: 4 years of global post-marketing data. J Crohns Colitis. 2020;14(2):192-204. doi:10.1093/ecco-jcc/jjz137 [DOI] [PubMed] [Google Scholar]
- 66.Judge C, McGettigan N, Ryan T, et al. Irish data on the safety and efficacy of vedolizumab in the treatment of inflammatory bowel disease. Scand J Gastroenterol. 2020;55(7):786-94. doi:10.1080/00365521.2020.1779340 [DOI] [PubMed] [Google Scholar]
- 67.Stallmach A, Langbein C, Atreya R, et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease - A prospective multicenter observational study. Aliment Pharmacol Ther. 2016;44(11-12):1199-212. doi:10.1111/apt.13813 [DOI] [PubMed] [Google Scholar]
- 68.Cummings F, Gaya DR, Levison S, et al. A retrospective observational study of early experiences of vedolizumab treatment for inflammatory bowel disease in the UK: The REVIVE study. Medicine (Baltimore). 2019;98(9):e14681. doi:10.1097/MD.0000000000014681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J, Yoon H, Kim N, et al. Clinical outcomes and response predictors of vedolizumab induction treatment for Korean patients with inflammatory bowel diseases who failed anti-TNF therapy: A KASID prospective multicenter cohort study. Inflamm Bowel Dis. 2021;27(12):1931-41. doi:10.1093/ibd/izaa361 [DOI] [PubMed] [Google Scholar]
- 70.Kotze PG, Ma C, Almutairdi A, et al. Real-world clinical, endoscopic and radiographic efficacy of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48(6):626-37. doi:10.1111/apt.14919 [DOI] [PubMed] [Google Scholar]
- 71.Tursi A, Mocci G, Faggiani R, et al. Vedolizumab is effective and safe in real-life treatment of inflammatory bowel diseases outpatients: A multicenter, observational study in primary inflammatory bowel disease centers. Eur J Intern Med. 2019;66:85-91. doi:10.1016/j.ejim.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 72.Eriksson C, Rundquist S, Lykiardopoulos V, et al. Real-world effectiveness of vedolizumab in inflammatory bowel disease: Week 52 results from the Swedish prospective multicentre SVEAH study. Ther Adv Gastroenterol. 2021;14:17562848211023386. doi:10.1177/17562848211023386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kochar B, Jiang Y, Winn A, et al. The early experience with vedolizumab in the United States. Crohns Colitis 360. 2019;1:3. doi:10.1093/crocol/otz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verstockt B, Mertens E, Dreesen E, et al. Influence of drug exposure on vedolizumab-induced endoscopic remission in anti-tumour necrosis factor [TNF] naïve and anti-TNF exposed IBD patients. J Crohns Colitis. 2020;14(3):332-41. doi:10.1093/ecco-jcc/jjz151 [DOI] [PubMed] [Google Scholar]
- 75.Kuo CJ, Le PH, Tai WC, et al. The effectiveness and safety of vedolizumab induction for moderate to severe ulcerative colitis for Asia patient: A real practice observational study. J Formos Med Assoc. 2022;121(9):1689-95. doi:10.1016/j.jfma.2021.11.012 [DOI] [PubMed] [Google Scholar]
- 76.Rubin DT, Modesto I, Vermeire S, et al. Worldwide post-marketing safety surveillance experience with tofacitinib in ulcerative colitis. Aliment Pharmacol Ther. 2022;55(3):302-10. doi:10.1111/apt.16619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiles CA, Shah N, Bell J, et al. Tofacitinib adherence and outcomes in patients with refractory ulcerative colitis. Am J Gastroenterol. 2020;115(1):S411. doi:10.14309/01.ajg.0000705252.01639.bf [Google Scholar]
- 78.Straatmijer T, van Gennep S, Duijvestein M, et al. Real-world clinical and endoscopic outcomes after one year tofacitinib treatment in ulcerative colitis. Eur J Gastroenterol Hepatol. 2021;33(10):1288-97. doi:10.1097/MEG.0000000000002028 [DOI] [PubMed] [Google Scholar]
- 79.Lair-Mehiri L, Stefanescu C, Vaysse T, et al. Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Dig Liver Dis. 2020;52(3):268-73. doi:10.1016/j.dld.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 80.Deepak P, Alayo QA, Khatiwada A, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(8):1592-601.e3. doi:10.1016/j.cgh.2020.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biemans VB, Sleutjes JA, de Vries AC, et al. ; Dutch Initiative on Crohn and Colitis (ICC) . Tofacitinib for ulcerative colitis: Results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51(9):880-8. doi:10.1111/apt.15689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoffmann P, Globig AM, Thomann AK, et al. Tofacitinib in treatment-refractory moderate to severe ulcerative colitis: Real-world experience from a retrospective multicenter observational study. J Clin Med. 2020;9(7):2177. doi:10.3390/jcm9072177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verstockt B, Alsoud D, Outtier A, et al. Su462 one year endoscopic and histologic outcomes to tofacitinib therapy in refractory ulcerative colitis. Gastroenterology. 2021;160(6):S-702. doi:10.1016/S0016-5085(21)02384-2 [Google Scholar]
- 84.Chiappetta MF, Viola A, Mastronardi M, et al. One-year effectiveness and safety of ustekinumab in ulcerative colitis: A multicenter real-world study from Italy. Expert Opin Biol Ther. 2021;21(11):1483-9. doi:10.1080/14712598.2021.1981855 [DOI] [PubMed] [Google Scholar]
- 85.Amiot A, Filippi J, Abitbol V, et al. ; UC-USK-GETAID Study Group . Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: A GETAID multicentre real-world cohort study. Aliment Pharmacol Ther. 2020;51(11):1039-46. doi:10.1111/apt.15717 [DOI] [PubMed] [Google Scholar]
- 86.Hussey M, Mc Garrigle R, Kennedy U, et al. Long-term assessment of clinical response to adalimumab therapy in refractory ulcerative colitis. Eur J Gastroenterol Hepatol. 2016;28(2):217-21. doi:10.1097/MEG.0000000000000515 [DOI] [PubMed] [Google Scholar]
- 87.Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther. 2014;40(11-12):1324-32. doi:10.1111/apt.12968 [DOI] [PubMed] [Google Scholar]
- 88.Yu J, Park SJ, Kim HW, et al. Effectiveness and safety of golimumab in patients with ulcerative colitis: A multicenter, prospective, post-marketing surveillance study. Gut Liver. 2022;16(5):764-74. doi:10.5009/gnl210335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee SJ, Baek K, Lee YJ, et al. A large pooled safety analysis of 3 post-marketing studies conducted in inflammatory bowel disease patients treated with biosimilar infliximab (CT-P13). United European Gastroenterol J. 2019;7(suppl 8):363. [Google Scholar]
- 90.Petitdidier N, Beaugerie L, Carbonnel F, et al. Real-world use of therapeutic drug monitoring of CT-P13 in patients with inflammatory bowel disease: A 12-month prospective observational cohort study. Clin Res Hepatol Gastroenterol. 2020;44(4):609-18. doi:10.1016/j.clinre.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 91.Sakemi R, Miyakawa M, Tanaka H, et al. Predicting a rapid response to adalimumab treatment and favorable short-term outcomes through the high platelet count in patients with ulcerative colitis: A multicenter retrospective cohort study. Medicine (Baltimore). 2020;99(47):e23344. doi:10.1097/MD.0000000000023344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ogata H, Hagiwara T, Kawaberi T, et al. Safety and effectiveness of adalimumab in the treatment of ulcerative colitis: Results from a large-scale, prospective, multicenter, observational study. Intest Res. 2021;19(4):419-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin SY, Park SJ, Kim Y, et al. ; IBD Research Group of the Korean Association for the Study of Intestinal Diseases . Clinical outcomes and predictors of response for adalimumab in patients with moderately to severely active ulcerative colitis: A KASID prospective multicenter cohort study. Intest Res. 2022;20(3): 350-60. doi:10.5217/ir.2021.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tursi A, Elisei W, Faggiani R, et al. Effectiveness and safety of adalimumab to treat outpatient ulcerative colitis: A real-life multicenter, observational study in primary inflammatory bowel disease centers. Medicine (Baltimore). 2018;97(34):e11897. doi:10.1097/MD.0000000000011897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orlandini B, Dragoni G, Variola A, et al. Clinical efficacy and safety of golimumab in biologically experienced and naive patients with active ulcerative colitis: A real-life experience from two Italian IBD centers. J Dig Dis. 2018;19(8):468-74. doi:10.1111/1751-2980.12648 [DOI] [PubMed] [Google Scholar]
- 96.Zacharias P, Damião AOMC, Moraes AC, et al. Adalimumab for ulcerative colitis: Results of a Brazilian multicenter observational study. Arq Gastroenterol. 2017;54(4):321-7. doi:10.1590/s0004-2803.201700000-51 [DOI] [PubMed] [Google Scholar]
- 97.Nakamura S, Asano T, Tsuchiya H, et al. Real-world data for golimumab treatment in patients with ulcerative colitis in Japan: Interim analysis in post-marketing surveillance. Intest Res. 2022;20(3):329-41. doi:10.5217/ir.2021.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Armuzzi A, Biancone L, Daperno M, et al. ; Italian Group for the Study of Inflammatory Bowel Disease . Adalimumab in active ulcerative colitis: A “real-life” observational study. Dig Liver Dis. 2013;45(9):738-43. doi:10.1016/j.dld.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 99.Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10(9):1002-7. doi:10.1016/j.cgh.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 100.Schreiber S, Dignass A, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: Real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 2018;53(9):1048-64. doi:10.1007/s00535-018-1480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: Systematic review with meta-analysis. Inflamm Bowel Dis. 2022;28(1):32-40. doi:10.1093/ibd/izab011 [DOI] [PubMed] [Google Scholar]
- 102.Lucaciu LA, Constantine-Cooke N, Plevris N, et al. Real-world experience with tofacitinib in ulcerative colitis: A systematic review and meta-analysis. Therap Adv Gastroenterol. 2021;14:17562848211064004. doi:10.1177/17562848211064004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taxonera C, Oliveras D, Lopez-Garcia ON, et al. Meta-analysis: Real-world effectiveness and safety of ustekinumab in patients with ulcerative colitis. Aliment Pharmacol Therap. 2023;57(6):610-9. doi:10.1111/apt.17386 [DOI] [PubMed] [Google Scholar]
- 104.European Medicines Agency. Guideline on the development of new medicinal products for the treatment of Ulcerative Colitis. 2018. Accessed January 04, 2023. Guideline on the development of new medicinal products for the treatment of ulcerative colitis (europa.eu). [Google Scholar]
- 105.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384-413. doi:10.14309/ajg.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 106.Magro F, Doherty G, Peyrin-Biroulet L, et al. ECCO position paper: Harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis. 2020;14(11):1503-11. doi:10.1093/ecco-jcc/jjaa110 [DOI] [PubMed] [Google Scholar]
- 107.Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD . STRIDE-II: An update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-83. doi:10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 108.Van Gennep S, De Vries L, De Jonge WJ, et al. Impact of 8 weeks tofacitinib treatment on histological inflammation in patients with moderate to severe ulcerative colitis. United European Gastroenterol J. 2020;8(suppl 8):452-3. [Google Scholar]
- 109.Maruszczyk K, Aiyegbusi OL, Cardoso VR, et al. Implementation of patient-reported outcome measures in real-world evidence studies: Analysis of ClinicalTrials.gov records (1999-2021). Contemp Clin Trials. 2022;120:106882. doi:10.1016/j.cct.2022.106882 [DOI] [PubMed] [Google Scholar]
- 110.Vodicka E, Kim K, Devine EB, Gnanasakthy A, Scoggins JF, Patrick DL. Inclusion of patient-reported outcome measures in registered clinical trials: Evidence from ClinicalTrials.gov (2007-2013). Contemp Clin Trials. 2015;43:1-9. doi:10.1016/j.cct.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 111.Calvert MJ, O’Connor DJ, Basch EM. Harnessing the patient voice in real-world evidence: the essential role of patient-reported outcomes. Nat Rev Drug Discov. 2019;18(10):731-2. doi:10.1038/d41573-019-00088-7 [DOI] [PubMed] [Google Scholar]
- 112.Maruszczyk K, Aiyegbusi OL, Torlinska B, Collis P, Keeley T, Calvert MJ. Systematic review of guidance for the collection and use of patient-reported outcomes in real-world evidence generation to support regulation, reimbursement and health policy. J Patient Rep Outcomes. 2022;6(1):57. doi:10.1186/s41687-022-00466-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cohen AT, Goto S, Schreiber K, Torp-Pedersen C. Why do we need observational studies of everyday patients in the real-life setting? Eur Heart J Suppl. 2015;17(suppl D):D2-8. doi:10.1093/eurheartj/suv035 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data underlying this article are available in the article and in its online Supplementary Materials.


