Abstract
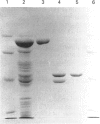
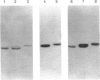
Recombinant human phenylalanine hydroxylase (hPAH) was produced in high yields in Escherichia coli using the pET and pMAL expression vectors. In the pMAL system, hPAH was fused through the target sequences of the restriction protease factor Xa (IEGR) or enterokinase (D4K) to the C-terminal end of the highly expressed E. coli maltose-binding protein (MBP). The recombinant hPAH, recovered in soluble forms, revealed a high specific activity even in crude extracts and was detected as a homogeneous band by Western-blot analysis using affinity-purified polyclonal rabbit anti-(rat PAH) antibodies. The enzyme expressed in the pET system was subject to limited proteolysis by host cell proteases and was difficult to purify with a satisfactory yield. By contrast, when expressed as a fusion protein in the pMAL system, hPAH was resistant to cleavage by host cell proteases and was conveniently purified by affinity chromatography on an amylose resin. Catalytically active tetramer-dimer (in equilibrium) forms of the fusion protein were separated from inactive, aggregated forms by size-exclusion h.p.l.c. After cleavage by restriction protease, factor Xa or enterokinase, hPAH was separated from uncleaved fusion protein, MBP and restriction proteases by hydroxylapatite or ion-exchange (DEAE) chromatography. The yield of highly purified hPAH was approx. 10 mg/l of culture. The specific activity of the isolated recombinant enzyme was high (i.e. 1440 nmol of tyrosine.min-1.mg-1 with tetrahydrobiopterin as the cofactor) and its catalytic and physicochemical properties are essentially the same as those reported for the enzyme isolated from human liver. The recombinant enzyme, both as a fusion protein and as purified full-length hPAH, was phosphorylated in vitro by the catalytic subunit of cyclic AMP-dependent protein kinase. The phosphorylated from of hPAH electrophoretically displayed an apparently higher molecular mass (approximately 51 kDa) than the non-phosphorylated (approximately 50 kDa) form.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abita J. P., Blandin-Savoja F., Rey F. Phenylalanine hydroxylase. Evidence that the enzyme from human liver might not be a phosphoprotein. Biochem Int. 1983 Dec;7(6):727–737. [PubMed] [Google Scholar]
- Abita J. P., Milstien S., Chang N., Kaufman S. In vitro activation of rat liver phenylalanine hydroxylase by phosphorylation. J Biol Chem. 1976 Sep 10;251(17):5310–5314. [PubMed] [Google Scholar]
- Boyd V. L., Bozzini M., Zon G., Noble R. L., Mattaliano R. J. Sequencing of peptides and proteins from the carboxy terminus. Anal Biochem. 1992 Nov 1;206(2):344–352. doi: 10.1016/0003-2697(92)90376-i. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chestkov V. V., Laptev A. V., Shishkin S. S. Membrane-bound phenylalanine hydroxylase of human liver. J Hepatol. 1992 Sep;16(1-2):11–15. doi: 10.1016/s0168-8278(05)80088-1. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Cotton R. G., Danks D. M., Jennings I. G. Genetics of the mammalian phenylalanine hydroxylase system. Studies of human liver phenylalanine hydroxylase subunit structure and of mutations in phenylketonuria. Biochem J. 1979 Aug 1;181(2):285–294. doi: 10.1042/bj1810285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., McAdam W., Jennings I., Morgan F. J. A monoclonal antibody to aromatic amino acid hydroxylases. Identification of the epitope. Biochem J. 1988 Oct 1;255(1):193–196. doi: 10.1042/bj2550193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplay P., Bedouelle H., Fowler A., Zabin I., Saurin W., Hofnung M. Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10606–10613. [PubMed] [Google Scholar]
- Døskeland A. P., Døskeland S. O., Ogreid D., Flatmark T. The effect of ligands of phenylalanine 4-monooxygenase on the cAMP-dependent phosphorylation of the enzyme. J Biol Chem. 1984 Sep 25;259(18):11242–11248. [PubMed] [Google Scholar]
- Døskeland A., Ljones T., Skotland T., Flatmark T. Phenylalanine 4-monooxygenase from bovine and rat liver: some physical and chemical properties. Neurochem Res. 1982 Apr;7(4):407–421. doi: 10.1007/BF00965494. [DOI] [PubMed] [Google Scholar]
- Eisensmith R. C., Woo S. L. Molecular basis of phenylketonuria and related hyperphenylalaninemias: mutations and polymorphisms in the human phenylalanine hydroxylase gene. Hum Mutat. 1992;1(1):13–23. doi: 10.1002/humu.1380010104. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Kaufman S. Some characteristics of partially purified human liver phenylalanine hydroxylase. Biochim Biophys Acta. 1973 Jan 12;293(1):56–61. doi: 10.1016/0005-2744(73)90375-6. [DOI] [PubMed] [Google Scholar]
- Kaufman S. The phenylalanine hydroxylating system. Adv Enzymol Relat Areas Mol Biol. 1993;67:77–264. doi: 10.1002/9780470123133.ch2. [DOI] [PubMed] [Google Scholar]
- Knappskog M., Eiken H. G., Martinez A., Olafsdotti S., Haavik J., Flatmark T., Apold J. Expression of wild type and mutant forms of human phenylalanine hydroxylase in E. coli. Adv Exp Med Biol. 1993;338:59–62. doi: 10.1007/978-1-4615-2960-6_11. [DOI] [PubMed] [Google Scholar]
- Kwok S. C., Ledley F. D., DiLella A. G., Robson K. J., Woo S. L. Nucleotide sequence of a full-length complementary DNA clone and amino acid sequence of human phenylalanine hydroxylase. Biochemistry. 1985 Jan 29;24(3):556–561. doi: 10.1021/bi00324a002. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauritzen C., Tüchsen E., Hansen P. E., Skovgaard O. BPTI and N-terminal extended analogues generated by factor Xa cleavage and cathepsin C trimming of a fusion protein expressed in Escherichia coli. Protein Expr Purif. 1991 Oct-Dec;2(5-6):372–378. doi: 10.1016/1046-5928(91)90096-2. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., DiLella A. G., Kwok S. C., Woo S. L. Homology between phenylalanine and tyrosine hydroxylases reveals common structural and functional domains. Biochemistry. 1985 Jul 2;24(14):3389–3394. doi: 10.1021/bi00335a001. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Grenett H. E., DiLella A. G., Kwok S. C., Woo S. L. Gene transfer and expression of human phenylalanine hydroxylase. Science. 1985 Apr 5;228(4695):77–79. doi: 10.1126/science.3856322. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Grenett H. E., Woo S. L. Biochemical characterization of recombinant human phenylalanine hydroxylase produced in Escherichia coli. J Biol Chem. 1987 Feb 15;262(5):2228–2233. [PubMed] [Google Scholar]
- Maina C. V., Riggs P. D., Grandea A. G., 3rd, Slatko B. E., Moran L. S., Tagliamonte J. A., McReynolds L. A., Guan C. D. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene. 1988 Dec 30;74(2):365–373. doi: 10.1016/0378-1119(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Okano Y., Eisensmith R. C., Güttler F., Lichter-Konecki U., Konecki D. S., Trefz F. K., Dasovich M., Wang T., Henriksen K., Lou H. Molecular basis of phenotypic heterogeneity in phenylketonuria. N Engl J Med. 1991 May 2;324(18):1232–1238. doi: 10.1056/NEJM199105023241802. [DOI] [PubMed] [Google Scholar]
- Petruschka L., Rebrin I., Grimm U., Herrmann F. H. The immunological evidence for a phenylalanine hydroxylase like immunoreactive protein in different human cells and tissues. Clin Chim Acta. 1990 Dec 13;193(1-2):65–77. doi: 10.1016/0009-8981(90)90008-g. [DOI] [PubMed] [Google Scholar]
- Shiman R., Gray D. W., Pater A. A simple purification of phenylalanine hydroxylase by substrate-induced hydrophobic chromatography. J Biol Chem. 1979 Nov 25;254(22):11300–11306. [PubMed] [Google Scholar]
- Shiman R. Relationship between the substrate activation site and catalytic site of phenylalanine hydroxylase. J Biol Chem. 1980 Nov 10;255(21):10029–10032. [PubMed] [Google Scholar]
- Smith S. C., Kemp B. E., McAdam W. J., Mercer J. F., Cotton R. G. Two apparent molecular weight forms of human and monkey phenylalanine hydroxylase are due to phosphorylation. J Biol Chem. 1984 Sep 25;259(18):11284–11289. [PubMed] [Google Scholar]
- Woo S. L., Gillam S. S., Woolf L. I. The isolation and properties of phenylalanine hydroxylase from human liver. Biochem J. 1974 Jun;139(3):741–749. doi: 10.1042/bj1390741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. L., Lidsky A. S., Güttler F., Chandra T., Robson K. J. Cloned human phenylalanine hydroxylase gene allows prenatal diagnosis and carrier detection of classical phenylketonuria. Nature. 1983 Nov 10;306(5939):151–155. doi: 10.1038/306151a0. [DOI] [PubMed] [Google Scholar]
- Woolf L. I. The isolation, properties, and assay of phenylalanine hydroxylase from human and rat liver. Biochem Med. 1976 Dec;16(3):284–291. doi: 10.1016/0006-2944(76)90034-x. [DOI] [PubMed] [Google Scholar]
- Wretborn M., Humble E., Ragnarsson U., Engström L. Amino acid sequence at the phosphorylated site of rat liver phenylalanine hydroxylase and phosphorylation of a corresponding synthetic peptide. Biochem Biophys Res Commun. 1980 Mar 28;93(2):403–408. doi: 10.1016/0006-291x(80)91091-8. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Minato S., Arai M., Kishida Y., Nagatsu T., Umezawa H. Purification of phenylalanine hydroxylase from human adult and foetal livers with a monoclonal antibody. Biochem Biophys Res Commun. 1985 Nov 27;133(1):202–207. doi: 10.1016/0006-291x(85)91861-3. [DOI] [PubMed] [Google Scholar]
- di Guan C., Li P., Riggs P. D., Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1988 Jul 15;67(1):21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]