Abstract
Background
During pregnancy, a Rhesus‐negative (Rh‐negative) woman may develop antibodies if her fetus is Rh‐positive, which can cause fetal morbidity or mortality in following pregnancies, if untreated.
Objectives
To assess the effects of administering anti‐D immunoglobulin (Ig) after spontaneous miscarriage in a Rh‐negative woman, with no anti‐D antibodies.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 December 2012).
Selection criteria
Randomised controlled trials (RCT) in Rh‐negative women without antibodies who were given anti‐D Ig following spontaneous miscarriage compared with no treatment or placebo treatment following spontaneous miscarriage as control.
Data collection and analysis
Two review authors independently assessed trials for inclusion and trial quality. Two review authors extracted data and checked it for accuracy.
Main results
We included one RCT, involving 48 women who had a miscarriage between eight to 24 weeks of gestation. Of the 19 women in the treatment group, 14 had therapeutic dilatation & curettage (D&C) and five had spontaneous miscarriage; of the 29 women in the control group, 25 had therapeutic D&C and four had spontaneous miscarriage. The treatment group received 300 µg anti‐D Ig intramuscular injection and were compared with a control group who received 1 cc homogenous gamma globulin placebo.
This review's primary outcomes (development of a positive Kleihauer Betke test (a test that detects fetal cells in the maternal blood; and development of RhD alloimmunisation in a subsequent pregnancy) were not reported in the included study.
Similarly, none of the review's secondary outcomes were reported in the included study: the need for increased surveillance for suspected fetal blood sampling and fetal transfusions in subsequent pregnancies, neonatal morbidity such as neonatal anaemia, jaundice, bilirubin encephalopathy, erythroblastosis, prematurity, hypoglycaemia (low blood sugar) in subsequent pregnancies, maternal adverse events of anti‐D administration including anaphylactic reaction and blood‐borne infections.
The included study did report subsequent Rh‐positive pregnancies in three women in the treatment group and six women in the control group. However, due to the small sample size, the study failed to show any difference in maternal sensitisation or development of Rh alloimmunisation in the subsequent pregnancies.
Authors' conclusions
There are insufficient data available to evaluate the practice of anti‐D administration in an unsensitised Rh‐negative mother after spontaneous miscarriage. Thus, until high‐quality evidence becomes available, the practice of anti‐D Immunoglobulin prophylaxis after spontaneous miscarriage for preventing Rh alloimmunisation cannot be generalised and should be based on the standard practice guidelines of each country.
Keywords: Female; Humans; Pregnancy; Abortion, Spontaneous; Abortion, Spontaneous/immunology; Immunologic Factors; Immunologic Factors/administration & dosage; Randomized Controlled Trials as Topic; Rh Isoimmunization; Rh Isoimmunization/prevention & control; Rho(D) Immune Globulin; Rho(D) Immune Globulin/administration & dosage
Plain language summary
Anti‐D administration after spontaneous miscarriage for preventing Rhesus alloimmunisation
A Rhesus‐negative (Rh‐negative) pregnant woman might develop Rh antibodies in her blood stream when she carries a Rh‐positive baby. The subsequent antibody formation has the potential to attack the red blood cells of a Rh‐positive baby during pregnancy. This might make the baby anaemic and in severe cases, the baby might die. Other Cochrane reviews provide clear evidence that giving anti‐D immunoglobulin (anti‐D) within 72 hours of the birth to a Rh‐negative mother of a Rh‐positive baby and during the third trimester reduces Rh antibody formation in future pregnancies. The chances of developing Rh antibodies may also be reduced if anti‐D is given to Rh‐negative women following a spontaneous miscarriage or a dilatation & curettage (D&C) for incomplete miscarriage after 12 weeks. However, our review only identified one poor quality randomised controlled trial (involving 48 women) that considered anti‐D administration after therapeutic D&C or spontaneous miscarriage for preventing Rh alloimmunisation (development of antibodies in response to antigens from a non‐self protein). The included study did not report any data on the review's primary or secondary outcomes. More high‐quality research is needed in this field.
Background
Description of the condition
Haemolytic disease of the fetus and newborn due to Rhesus D (RhD) alloimmunisation (development of antibodies in response to antigens from a non‐self protein) was a major cause of perinatal mortality and morbidity before the development and utilisation of Rhesus immunoglobulin (Ig) prior to the 1970s. The incidence of RhD haemolytic disease of the newborn has since dropped dramatically to 10.6 per 10,000 total births (Chevaz 1991) following the standard practice of post‐delivery anti‐D administration with further anti‐D administration for events known to result in fetomaternal haemorrhage (FMH) during pregnancy. From an observational study, the incidence during early pregnancy was reported as 0.1% to 0.2% (Contreras 1998) and the risk increases following induced abortion and diagnostic or therapeutic intervention during pregnancy to 4% to 5% (ACOG 2006)). Residual alloimmunisation occurs mainly for two reasons, i.e. failure to administer sufficient anti‐D at the correct time after the known risk events and alloimmunisation during pregnancy as a result of 'silent' FMH. There is clear evidence from Cochrane systematic reviews that anti‐D administration within 72 hours after delivery (Crowther 1997) and during third trimester of pregnancy at 28 weeks and 32 weeks (Crowther 1999) significantly reduces the risk of Rhesus alloimmunisation in the subsequent pregnancy. However, good‐quality evidence to justify the routine practice of anti‐D administration after spontaneous miscarriage before 20 weeks or ectopic pregnancy for preventing Rhesus alloimmunisation is lacking and debatable.
Description of the intervention
Studies suggest that anti‐D given to all non sensitised Rhesus‐negative (Rh‐negative) women having spontaneous or incomplete miscarriage after 12 weeks of pregnancy especially after curettage to remove products of conception prevents Rhesus alloimmunisation in the subsequent pregnancy (Ghosh 1994; Matthews 1969) The RhD antigen is well developed by six weeks' gestation and the fetoplacental blood volume increases during pregnancy (Gollin 1995; Urbaniak 1998). The Rh‐negative mother is at risk of Rh alloimmunisation upon exposure to RhD antigens from her Rhesus positive (Rh‐positive) fetus through a fetomaternal haemorrhage event. The sensitising event can occur during the antenatal period and childbirth as well as during spontaneous miscarriage. When women are exposed to Rh‐positive fetal red blood cells for the second time in a subsequent pregnancy, they produce a rapid generation of Ig antibodies, which cross the placenta causing fetal anaemia, erythroblastosis fetalis (haemolytic disease of the newborn), and intrauterine fetal death (Bowman 1997; Martin 2003). Ten per cent of infants who survive may develop spastic choreoathetosis (rapid, uncontrolled, involuntary, excessive movements), deafness, and mental retardation (Cosmi 1979). Fetomaternal haemorrhages are found to occur in as many as 75% of pregnancies, with the incidence increasing as gestation advances and with most cases occurring at delivery and obstetric events such as placenta abruption, placenta previa, external cephalic version, and operative delivery (Hughes 1994). While fetomaternal haemorrhage after a spontaneous miscarriage before 12 weeks' gestation is negligible (Ghosh 1994), evidence has shown that spontaneous miscarriage after 12 weeks' gestation onwards, especially after a curettage procedure to remove the products of conception, may cause significant transplacental haemorrhage to sensitise the Rh‐negative mother (Matthews 1969). However, some studies (Ghosh 1994) recommend against anti‐D administration after a spontaneous miscarriage as it is not cost‐effective due to low risk of alloimmunisation and thus a low incidence of haemolytic disease in the subsequent pregnancy.
How the intervention might work
The severity of haemolytic disease and the degree of sensitisation of maternal blood causing fetal red cell destruction in the next pregnancy is dependent on the volume of fetal blood presenting to the maternal circulation, the degree of maternal immune response, and concurrent ABO (blood group system) incompatibility. The Kleihauer‐Betke test can be used to quantify the volume of fetal blood in the maternal circulation after a fetomaternal haemorrhage event to determine the volume of fetal blood escaping into the maternal circulation and for an appropriate dose of anti‐D Immunoglobulin G (IgG) to be administered to the mother. The volume of fetal blood escaping into the maternal system can be as small as 0.1 mL but in rare cases can exceed 30 mL, following a spontaneous miscarriage after the first trimester. The overall risk of alloimmunisation with an ABO compatible fetus, if not treated with anti‐D IgG, is approximately 16% and with an ABO‐incompatible fetus, the risk is only 1.5% to 2% (Bowman 1985). The protective effect conferred by ABO incompatibility is believed to be due to maternal destruction and subsequent clearance of the ABO‐incompatible fetal erythrocytes (red blood cells) before Rh sensitisation can occur. If anti‐D is given after a first‐trimester event between 12 to 24 weeks of gestation, the recommended dose for anti‐D is 50 µg which protects against sensitisation from up to 2.5 mL of Rh‐positive red blood cells (ACOG 1999). A dose of 100 µg given intramuscularly is sufficient to treat fetomaternal haemorrhage up to 4 mL
Why it is important to do this review
Rhesus alloimmunisation is a preventable disease. The purpose of this review is to assess whether routine administration or non administration of anti‐D prophylaxis to Rh‐negative women following spontaneous miscarriage up to 24 weeks' gestation may prove beneficial in reducing the risk of alloimmunisation and thus eliminating risk of haemolytic disease of the newborn.
Objectives
To assess the effects of anti‐D administration to Rh‐negative women with no anti‐D antibodies after a spontaneous miscarriage.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised trials, quasi‐randomised trials and cluster‐randomised trials from full‐text journals and abstracts.
Types of participants
Rh‐negative mothers without anti‐D antibodies who have had spontaneous miscarriage before 24 weeks of gestation, irrespective of parity (number of pregnancies), including those who had medical evacuation of the uterus and early pregnancy complications, e.g. ectopic and molar pregnancy.
Types of interventions
Intervention
Administration of anti‐D Ig after spontaneous miscarriage, therapeutic evacuation of the uterus, early pregnancy complications up to 24 weeks' gestation irrespective of parity, ABO compatibility, size of fetomaternal haemorrhage, timing or dosage of anti‐D given.
Comparison
Rh‐negative mothers who have not received anti‐D or who have received a placebo after spontaneous miscarriage, therapeutic termination of pregnancy or early pregnancy complication up to 24 weeks of gestation.
Types of outcome measures
Primary outcomes
Development of a positive Kleihauer Betke test (a test that detects fetal cells in the maternal blood).
Development of RhD alloimmunisation in a subsequent pregnancy.
Secondary outcomes
Detection of atypical blood group antibodies by positive indirect Coombs test after six months of exposure (non‐prespecified outcome).
Need for increased surveillance for suspected fetal blood sampling and fetal transfusions in subsequent pregnancies.
Neonatal morbidity such as neonatal anaemia, jaundice, bilirubin encephalopathy, erythroblastosis, prematurity, hypoglycaemia (low blood sugar) in subsequent pregnancies.
Maternal adverse events of anti‐D administration including anaphylactic reaction.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 December 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors (Laxminarayan Karanth (KLK) and Ankur Barua (AB)) independently assessed for inclusion all the potential studies we identified from a result of the search strategy. We resolved any disagreement through discussion and if issues were not resolved, we sought opinion of the third author Sharifah Halimah Jaafar (SHJ) and clarified them.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (KLK and AB) extracted the data using the agreed form. We resolved discrepancies through discussion and when required, we consulted SHJ (third review author). We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
Assessment of risk of bias in included studies
Two review authors (KLK and AB) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion by consulting SHJ.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias;
high risk of bias;
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias;
high risk of bias;
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis.
We assessed methods as:
low risk of bias;
high risk of bias;
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias;
high risk of bias;
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
In future updates of this review, for continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
In future updates of this review, if identified, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample size using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Multi‐armed trials
In future updates, if multi‐arm studies are identified, we will include the intervention groups of relevance in a pair‐wise comparison of intervention groups that would meet the criteria for including studies in the review. All intervention groups of a multi‐intervention study will be mentioned in the Characteristics of included studies table ln either the 'intervention' or 'notes' section.
Dealing with missing data
For the included study, we noted levels of attrition. In future updates, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
In future updates, we will assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We will regard heterogeneity as substantial if I² is greater than 30% and either T² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we will use the test proposed by Egger 1997 and for dichotomous outcomes we will use the test proposed by Harbord 2006. If asymmetry is detected in any of these tests or is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
As more data become available we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was not possible in this review as only one study with a small sample size was included. However in future updates of the review, if more studies are available and we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
Dosage of anti‐D administration during early (less than 14 weeks of gestation) and late miscarriage (14 to 20 weeks of gestation).
Amount of fetomaternal haemorrhage during early (less than 14 weeks of gestation) and late miscarriage (14 to 20 weeks of gestation) compared with Rh‐negative women without antibodies not given anti‐D after spontaneous miscarriage.
Development of Rhesus D alloimmunisation in any subsequent pregnancy due to alloimmunisation occurred during early (less than 14 weeks of gestation) and late miscarriage (14 to 20 weeks of gestation) compared with Rh‐negative women without antibodies not given anti‐D after spontaneous miscarriage.
Anti‐D administration intravenous versus intramuscular.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2011). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
If more studies become available in future updates of the review, we will repeat the primary analysis or meta‐analysis, substituting alternative decisions or ranges of values for decisions that were arbitrary or unclear. Some issues suitable for sensitivity analysis may be identified during the review process. When sensitivity analyses show that the overall result and conclusions are not affected by the different decisions that could be made during the review process, the results of the review can be regarded to be more certain.
Results
Description of studies
Results of the search
The search retrieved two trial reports.(Figure 1) One study (Visscher 1972) was included and the other (Keith 1977) was excluded from the review.
1.
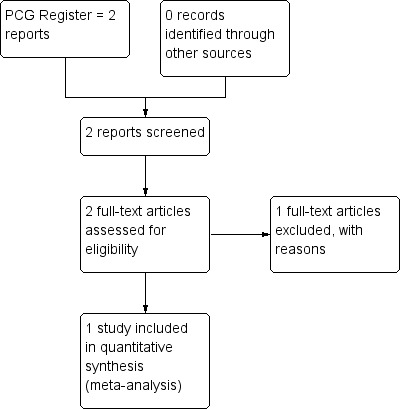
Study flow diagram.
Included studies
Visscher 1972 In this study, out of the 57 Rh‐negative mothers who had a spontaneous miscarriage between eight to 24 weeks' gestation and who gave consent, 48 participated in the double‐blind study. The coded ampules containing 300 µg (1500 IU) Rh immune globulin and 1 mL of homologous gamma globulin placebo were randomly allocated to the participants within 72 hours after a spontaneous complete miscarriage or operative termination of an incomplete miscarriage. Both the participants and the clinicians were blinded. For further details, see Characteristics of included studies table.
Excluded studies
(Keith 1977) This excluded study was a randomised controlled trial of a micro dose 50 µg (250IU) anti‐D versus 300 µg (1500 IU) anti‐D given within 72 hours after spontaneous or induced first trimester miscarriage. A total of 400 women from a private abortion clinic who consented to participate in the study were randomised to receive the reduced dose of 50 µg (250 IU) anti‐D immunoglobulin or standard dose of 300 µg (1500 IU) anti‐D. Allocation was predetermined by the numerical code, however, it was not clear how the numerical code was generated. The numerical code was revealed six months later after the blood samples were collected for serological studies to detect atypical blood group antibodies in the maternal blood. At six months 85 participants were lost from the study as they did not turn up for antibody assessment. After the code was opened, it was noted that 298/400 (74.5%) women received micro dose 50 µg anti‐D and 17/400 (4.3%) received a standard dose anti‐D. In both groups there were no atypical blood group antibodies detected after six months. The outcome of a subsequent pregnancy and evidence of alloimmunisation was not measured in this study. As all participants in this study received anti‐D immunoglobulin, there is no comparison with non‐intervention or control group as prespecified in the methodology section of this review. See Characteristics of excluded studies table for further details.
Risk of bias in included studies
See Figure 2 for a graphical summary of the 'Risk of bias' assessment.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
The Visscher 1972 study was stated to be a double‐blinded study in which coded ampules containing 300 µg Rh anti‐D and 1 mL of homogenous gamma globulin placebo were randomly allocated to eligible consenting participants within 72 hours of spontaneous miscarriage. We graded the risk of selection bias as high because it is not clear how the sequence of the code was generated and how randomisation of the participants was done. The allocation concealment was probably adequate as the coded ampules are reported as homogenous. The clinicians and the participants were both blinded until the code was broken and revealed six months later after blood samples were taken for serology assessment. The detection bias is rated as unclear as the first outcome assessment, i.e. presence of atypical antibody in the serum by indirect Coombs test was blinded but the outcome assessment of subsequent Rh‐positive pregnancies with regards to development of Rh alloimmunisation from both groups was anticipated. On record there was about 18/57 subsequent pregnancies, 9/57 of which were Rh‐positive pregnancies, but there was no record on the other 39/57 participants, whether or not they had subsequent Rh‐positive pregnancies beyond the follow‐up period. The attrition and reporting bias is thus rated high due to incomplete outcome reporting.
Effects of interventions
After the code was broken, it was noted that a total of 19 mothers received 300 µg (1500 IU) anti‐D whilst 29 mothers received gamma globulin placebo. Of the mothers who received anti‐D Rh immunoglobulin, 14/19 (74%) mothers received it after a therapeutic D&C and 5/19 (26%) after spontaneous miscarriage. On the other hand, of mothers who received placebo, 25/29 (86%) had a therapeutic D&C and 4/29 (14%) had a spontaneous miscarriage. Serologic studies by indirect Coombs test to detect atypical blood group antibodies in both intervention and control group after six months revealed negative results. Subsequent Rh‐positive pregnancies, 3/19 mothers from the intervention group and 6/29 mothers from the control group have shown no evidence of sensitisation. As both groups recorded no maternal sensitisation no prespecified secondary outcomes could be measured. The study was terminated at two years.
Primary outcomes
The primary outcomes such as development of a positive Kleihauer Betke test and development of RhD alloimmunisation in a subsequent pregnancy detailed in the protocol could not be evaluated since no maternal sensitisation was observed in the study.
Secondary outcomes
Secondary outcome surveillance for suspected fetal blood sampling and fetal transfusions in subsequent pregnancies and neonatal morbidity such as neonatal anaemia, jaundice, bilirubin encephalopathy, erythroblastosis, prematurity, hypoglycaemia (low blood sugar) in subsequent pregnancies as specified in the protocol could not be evaluated since no maternal sensitisation has been observed in the study. The non prespecified secondary outcome of detection of atypical blood group antibodies by positive indirect Coombs test after six months of exposure was evaluated (see Differences between protocol and review).
Serology study after six months of intervention showed a null effect, i.e. negative indirect Coombs test indicating no evidence of atypical blood group in maternal serum when the participants were given a standard dose 300 µg Rh anti‐D or given a placebo. The subsequent nine Rh‐positive pregnancies (6/19 from the intervention group and 3/29 from placebo group) showed no evidence of Rh alloimmunisation. It is not clear whether the other 39 participants from both groups had any subsequent Rh‐positive pregnancies beyond the follow‐up period. There are no secondary outcomes reported which could be measured in this review as both groups showed no evidence of maternal sensitisation.
Discussion
The practice of administering Rh immune globulin to Rh‐negative women with a spontaneous miscarriage at early pregnancy is based on expert opinion and extrapolation from experience with fetomaternal haemorrhage in late pregnancy.
This systematic review found insufficient evidence to draw any conclusions about whether or not the practice of administration of anti‐D prophylaxis to Rh‐negative women following spontaneous miscarriage up to 24 weeks' gestation is beneficial in reducing the risk of alloimmunisation in a subsequent pregnancy and thus eliminating the risk of haemolytic disease of the newborn. This review found no evidence to assess adverse events or neonatal morbidity.
The review is based on only one methodologically weak study (Visscher 1972), involving 48 women. The study did not have statistical power to show any difference between the intervention and non intervention groups with regards to maternal sensitisation during a miscarriage event, with or without surgical evacuation of the products of conception. This is likely to be due to the sample size being too small to detect any incident of significant fetomaternal haemorrhage to result in maternal sensitisation from fetal Rh antigen exposure in early pregnancy. The primary outcomes were not reported in the included study. In the presence of ABO incompatibility between mother and fetus, the risk of alloimmunisation is reported to be low or absent (Bowman 1985). The likelihood is that mother‐fetus blood group incompatibility would be determined early in pregnancy. The amount of transplacental haemorrhage and subsequent risk of alloimmunisation occurring after a miscarriage event, and the doses of anti‐D Ig that should be given in first and second trimester is also debatable.
There is a direct, proportional relationship between the incidence of anti‐Rh alloimmunisation and the volume of RhD‐positive red blood cells to which the Rh(D)‐negative woman has been exposed. Studies show that 3% of pregnant women had fetomaternal haemorrhage in the first trimester and 12% in the second trimester, but failed to look for subsequent maternal sensitisation or morbidity in the newborn due to alloimmunisation (ACOG 2006; Zpipursky 1963). The risk of alloimmunisation by spontaneous complete or incomplete miscarriage before 12 weeks of gestation is negligible when there has been no instrumentation to evacuate the products of conception.
There are differences in practice among countries on the dosage of anti‐D Ig to be administered to the unsensitised Rh D‐negative mother with early pregnancy complications. Canadian guidelines recommend that after spontaneous miscarriage or induced abortion during the first 12 weeks of gestation, non‐sensitised D‐negative women should be given a minimum anti‐D of 120 µg (SOGC 2003). Some guidelines advocate microdose 50 µg anti‐D Ig (Lubusky 2010; NHMRC 2003) instead of a standard dose 300 µg. In the practice guidelines for some countries (UK) (RCOG 2011), anti‐D Ig is not routinely administered in spontaneous complete or incomplete miscarriage before 12 weeks of gestation. The reason for not administering anti‐D Ig routinely being lack of evidence, cost of the treatment and limited supply of anti‐D Ig. On the contrary, despite the lack of quality evidence, most experts recommend administering anti‐D Ig due to the potential serious risks of maternal sensitisation that have been experienced with late pregnancy bleeding and subsequent sensitisation.
In the event where fetomaternal haemorrhage is possible due to breech of choriodecidual space, e.g. surgical evacuation of the uterus, medically‐induced abortion, hydatidiform mole, and ectopic pregnancy, anti‐D Ig should be administered within 72 hours of the event, regardless of the gestational age. There are also differences in practice among countries on the dosage of anti‐D Ig to be administered to the unsensitised Rh D‐negative mother with early pregnancy complications, which are influenced by availability, cost of anti‐D Ig and cost of laboratory assessments of the volume of feto‐maternal haemorrhage.
Published data on which to base the recommendations are scanty. A randomised study by Keith 1977 has proven that a micro dose of 50 µg anti‐D Ig instead of 300 µg is adequate to protect women undergoing early pregnancy miscarriages and thus is cost effective. It is estimated that the total fetal blood volume at 12 weeks is approximately 4.2 mL and 50 µg anti‐D will neutralise 5 mL of Rh D blood, hence 50 µg dose should be adequate in all cases of first trimester miscarriage. However, this study had too small a sample size to show a difference in the detection rate of atypical blood group antibodies in the maternal serum.
The dosage requirement of anti‐D Ig following a second trimester miscarriage, either spontaneous or induced, in an unsensitised Rh D‐negative mother, is again debatable. The recommended dosage of anti‐D Ig in guidelines are varied. In the UK, the recommendation is 50 µg (250 IU), in Australia 125 µg (625 IU) (NHMRC 2003) and in the USA and parts of Europe 300 µg (1500 IU) (ACOG 2006; Lubusky 2010). The optimum dosage of anti‐D Ig administration in unsensitised Rh‐negative mothers with second trimester miscarriage either due to spontaneous or induced can be calculated by estimating FMH by Kleihauer‐Betke acid elution assay (KB test). If fetomaternal haemorrhage is in excess of the amount covered by the dose given, 10 microgram additional anti‐D should be given for every additional 0.5 mL fetal red blood cells (1 mL of fetal blood). Supportive evidence to the recommendations on the optimal amount of anti‐D are scanty and weak.
Until more data and evidence become available, the practice of anti‐D Ig prophylaxis after spontaneous miscarriage for preventing Rh alloimmunisation should be based on the standard practice guidelines of each country.
Authors' conclusions
Implications for practice.
There are no high‐quality data available to evaluate the practice of anti‐D administration in a unsensitised Rh‐negative mother after spontaneous miscarriage, with or without dilatation and curettage. Thus, until high‐quality evidence becomes available, the practice of anti‐D administration after spontaneous miscarriage should follow the standard practice guidelines of each country.
Implications for research.
Further randomised controlled trials are warranted to determine the optimal timing, number of treatments, and effective dosage of anti‐D administration in unsensitised Rh‐negative mothers after spontaneous miscarriage with or without dilatation and curettage. Trials should be adequately powered and include clinically relevant outcomes such as those described in this review. The cost effectiveness of the drug and effect of antenatal anti‐D on subsequent pregnancies also requires further study.
History
Protocol first published: Issue 2, 2012 Review first published: Issue 3, 2013
| Date | Event | Description |
|---|---|---|
| 11 September 2012 | Amended | Author contact details updated. |
Acknowledgements
We thank the management of Melaka Manipal Medical College Melaka, Manipal University for giving us the opportunity to be involved in the completion of this review. We also thank Ms Sonja Henderson and Ms Denise Atherton for their help with this review.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Adverse pregnancy outcome following anti‐D administration following spontaneous miscarriage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse reaction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Antibody D titre at 6 months following administration (non‐prespecified outcome) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Rh isoimmunisation in subsequent pregnancies following anti‐D administration | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Health of infant in subsequent pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Positive Kleihauer test after miscarriage before 14 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Positive Kleihauer test after miscarriage following 14 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Need for increased fetal surveillance for suspected isoimmunisation in subsequent pregnancies | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.2. Analysis.
Comparison 1 Adverse pregnancy outcome following anti‐D administration following spontaneous miscarriage, Outcome 2 Antibody D titre at 6 months following administration (non‐prespecified outcome).
1.3. Analysis.

Comparison 1 Adverse pregnancy outcome following anti‐D administration following spontaneous miscarriage, Outcome 3 Rh isoimmunisation in subsequent pregnancies following anti‐D administration.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Visscher 1972.
| Methods | Randomised control trial. | |
| Participants | 57 eligible Rh‐negative women who had had spontaneous miscarriage at gestation 8‐24 weeks consented to participate in the study. 9 dropped out pre intervention and no reason was stated. 48 participants randomly received injection of coded ampules within 72 hours of spontaneous miscarriage. 19/57 treatment group: 14/19 had dilatation & curettage (therapeutic dilatation & curettage), 5/19 had spontaneous miscarriage. 29/57 control group: 25/29 had dilatation & curettage (therapeutic dilatation & curettage), 4/29 had spontaneous miscarriage. |
|
| Interventions | Intervention: IM 300 µg Rhesus anti‐D immunoglobulin in a coded ampule. Control: IM 1 mL of homogenous gamma globulin placebo in a coded ampule. The coded ampoules were randomly assigned to the eligible participants and both the participants and the clinicians were blinded. Blood was collected after 6 months and the code was revealed. About 19/57 participants received 300 µg of Rhogam and 29/57 participants received 1 mL of homologous gamma globulin placebo. |
|
| Outcomes | At 6 months follow‐up, in the treatment group all 19 were non‐sensitised as evidenced by indirect Coombs' test. In subsequent follow‐up, 3 Rh +ve non‐sensitised pregnancies were observed. On 6 months follow‐up in control group, all 29 were non‐sensitised evidenced by indirect Coombs' test. In subsequent follow‐up, 6 Rh +ve non‐sensitised pregnancies were observed. |
|
| Notes | ABO incompatibility and parity were not mentioned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Double blind study with coded ampules. The ampules were randomly assigned to the eligible participants. However, it is not clear how the sequence of the code were generated and how randomisation of the participants was done. |
| Allocation concealment (selection bias) | Low risk | The ampules containing 300 µg Rh immunoglobulin and gamma globulin placebo were coded and all ampules were homogenous. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The clinician and the participants were both blinded from pre intervention up to 6 months post intervention when the codes were broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Serology tests were done for both groups to detect atypical blood group antibody by indirect Coombs test and the code was revealed. Subsequently onwards, having known who received anti‐D or placebo, the outcome of subsequent Rh‐positive pregnancy was anticipated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All participants were followed up post‐intervention. Of 18/57 subsequent pregnancies, 3/19 from the treatment group and 6/29 from the control group reported Rh‐positive pregnancies. |
| Selective reporting (reporting bias) | High risk | It is not known whether or not the other 39/57 participants ever got pregnant with a Rh‐postive pregnancy and what was the outcome after the follow‐up period was over. While it is clear that the recruitment was terminated at 2 years, it is not clear how long each of the participants from both groups were followed up in order to see the outcome of the subsequent Rh‐positive pregnancy. |
| Other bias | Unclear risk | Not noted. |
IM: intramuscular
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Keith 1977 | All participants in the trial received anti‐D. One study arm received micro dose 50 µg anti‐D compared with other arm that received a standard dose 300 µg anti‐D. Thus, there was no comparison with non‐intervention group as prespecified in the review. |
Differences between protocol and review
The secondary outcome, detection of atypical blood group antibodies by positive indirect Coombs test after six months of exposure (non‐prespecified outcome) was not mentioned in the protocol but was later added during the review as a paper under review had specifically analysed this outcome.
In our protocol we stated types of participants (and interventions) to include women who have had a spontaneous miscarriage before 20 weeks of gestation. We have changed this to 24 weeks of gestation for consistency with other reviews on miscarriage.
Contributions of authors
K Laxminarayan Karanth wrote the review together with Dr Sharifah Halimah Jaafar. Dr Ankur Barua helped with selection of the studies for inclusion and extracted the data. Prof Sachchithanantham Kanagasabai and Prof Sreekumaran Nair helped in the development of the proposal and provided expert clinical opinion and input into current practice of anti‐D administration.
Sources of support
Internal sources
Melaka Manipal Medical College,Manipal University, Malaysia.
KPJ Ipoh Specialist Hospital, Malaysia.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Visscher 1972 {published data only}
- Visscher RD, Visscher HC. Do Rh‐negative women with an early spontaneous abortion need Rh immuno prophylaxis?. American Journal of Obstetrics and Gynecology 1972;113:158‐65. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Keith 1977 {published data only}
- Keith L, Bozorgi N. Small dose Anti‐Rh therapy after first trimester abortion. International Journal of Gynecology and Obstetrics 1977;15:235‐7. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2006
- American College of Obstetricians and Gynecologists. Prevention of RhD alloimmunization. ACOG Practice Bulletin no. 4. Washington, DC: American College of Obstetricians and Gynecologists, 2006. [Google Scholar]
Bowman 1985
- Bowman JM. Controversies in Rh prophylaxis: who needs Rh immune globulin and when should it be given?. American Journal of Obstetrics and Gynecology 1985;151:289‐94. [DOI] [PubMed] [Google Scholar]
Bowman 1997
- Bowman J. The management of hemolytic disease in the fetus and newborn. Seminars in Perinatology 1997;21(1):39‐44. [DOI] [PubMed] [Google Scholar]
Chevaz 1991
- Chavez GF, Mulinare J, Edmonds LD. Epidemiology of Rh hemolytic disease of the newborn in the United States. JAMA 1991;265:3270‐4. [DOI] [PubMed] [Google Scholar]
Contreras 1998
- Contreras M. The prevention of Rh haemolytic disease of the fetus and newborn‐general background. British Journal of Obstetrics and Gynaecology 1998;105:7‐10. [DOI] [PubMed] [Google Scholar]
Cosmi 1979
- Cosmi EV. Exchange transfusion of the newborn infant. Clinical Management of Mother and Newborn(Marx GF Ed.). New York: Springer‐Verlag, 1979:199. [Google Scholar]
Crowther 1997
- Crowther CA, Middleton P. Anti‐D administration after childbirth for preventing Rhesus alloimmunisation. Cochrane Database of Systematic Reviews 1997, Issue 2. [DOI: 10.1002/14651858.CD000021] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 1999
- Crowther CA, Middleton P. Anti‐D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000020] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghosh 1994
- Ghosh, Murphy WG. Implementation of the rhesus prevention programme: a prospective study. Scottish Medical Journal 1994;39:147‐9. [DOI] [PubMed] [Google Scholar]
Gollin 1995
- Gollin YG, Copel JA. Management of the Rh‐sensitized mother. Clinics in Perinatology 1995;22(3):545‐59. [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1994
- Hughes RG, Craig JI, Murphy WG, Greer IA. Causes and clinical consequences of Rhesus (D) haemolytic disease of the newborn: a study of a Scottish population, 1985‐1990. British Journal of Obstetrics and Gynaecology 1994;101(4):297‐300. [DOI] [PubMed] [Google Scholar]
Lubusky 2010
- Lubusky M. Prevention of RhD alloimmunization in Rh D negative women. Biomedical papers of the Medical Faculty of the University Palacký, Olomouc, Czechoslovakia 2010;154(1):3‐7. [DOI] [PubMed] [Google Scholar]
Martin 2003
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2002. National Vital Statistics Reports 2003;52(10):1‐113. [PubMed] [Google Scholar]
Matthews 1969
- Matthews CD, Matthew AE. Transplacental haemorrhage in spontaneous and induced abortion. Lancet 1969;1(7597):694‐5. [DOI] [PubMed] [Google Scholar]
NHMRC 2003
- Guidelines on the prophylactic use of Rh D immunoglobulin (anti‐D) in obstetrics. National Health and Medical Research Council 2003.
RCOG 2011
- Royal College of Obstetricians and Gynaecologists. The Use of Anti‐D Immunoglobulin for Rhesus D Prophylaxis. RCOG Green‐top Guideline 2011;(Green‐top 22):1‐14. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
SOGC 2003
- SOGC Clinical Practice Guidelines. Prevention of Rh alloimmunisation. Journal of Obstetrics and Gynaecology Canada 2003;25(9)(No 133):765–73. [Google Scholar]
Urbaniak 1998
- Urbaniak SJ. The scientific basis of antenatal prophylaxis. British Journal of Obstetrics and Gynaecology 1998;105(18):11‐8. [DOI] [PubMed] [Google Scholar]
Zpipursky 1963
- Zpipursky A, Pollock J, Neelands P, Chown B, Israels LG. The transplacental passage of fetal red blood cells and the pathogenesis of Rh immunization during pregnancy. Lancet 1963;2(7306):489‐93. [DOI] [PubMed] [Google Scholar]


