Abstract
Background
Inhaled corticosteroids (ICS) are the cornerstone of asthma maintenance treatment in children. Particularly among parents, there is concern about the safety of ICS as studies in children have shown reduced growth. Small‐particle‐size ICS targeting the smaller airways have improved lung deposition and effective asthma control might be achieved at lower daily doses.
Ciclesonide is a relatively new ICS. This small‐particle ICS is a pro‐drug that is converted in the airways to an active metabolite and therefore with potentially less local (throat infection) and systemic (reduced growth) side effects. It can be inhaled once daily, thereby possibly improving adherence.
Objectives
To assess the efficacy and adverse effects of ciclesonide compared to other ICS in the management of chronic asthma in children.
Search methods
We searched the Cochrane Airways Group Register of trials with pre‐defined terms. Additional searches of MEDLINE (via PubMed), EMBASE and Clinicalstudyresults.org were undertaken. Searches are up to date to 7 November 2012.
Selection criteria
Randomised controlled parallel or cross‐over studies were eligible for the review. We included studies comparing ciclesonide with other corticosteroids both at nominally equivalent doses or lower doses of ciclesonide.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. Study authors were contacted for additional information. Adverse effects information was collected from the trials.
Main results
Six studies were included in this review (3256 children, 4 to 17 years of age). Two studies were published as conference abstracts only. Ciclesonide was compared to budesonide and fluticasone.
Ciclesonide compared to budesonide (dose ratio 1:2): asthma symptoms and adverse effect were similar in both groups. Pooled results showed no significant difference in children who experience an exacerbation (risk ratio (RR) 2.20, 95% confidence interval (CI) 0.75 to 6.43). Both studies reported that 24‐hour urine cortisol levels showed a statistically significant decrease in the budesonide group compared to the ciclesonide group.
Ciclesonide compared to fluticasone (dose ratio 1:1): no significant differences were found for the outcome asthma symptoms. Pooled results showed no significant differences in number of patients with exacerbations (RR 1.37, 95% CI 0.58 to 3.21) and data from a study that could not be pooled in the meta‐analysis reported similar numbers of patients with exacerbations in both groups. None of the studies found a difference in adverse effects. No significant difference was found for 24‐hour urine cortisol levels between the groups (mean difference 0.54 nmol/mmol, 95% CI ‐5.92 to 7.00).
Ciclesonide versus fluticasone (dose ratio 1:2) was assessed in one study and showed similar results between the two corticosteroids for asthma symptoms. The number of children with exacerbations was significantly higher in the ciclesonide group (RR 3.57, 95% CI 1.35 to 9.47). No significant differences were found in adverse effects (RR 0.98, 95% CI 0.81 to 1.14) and 24‐hour urine cortisol levels (mean difference 1.15 nmol/mmol, 95% CI 0.07 to 2.23).
The quality of evidence was judged 'low' for the outcomes asthma symptoms and adverse events and 'very low' for the outcome exacerbations for ciclesonide versus budesonide (dose ratio 1:1). The quality of evidence was graded 'moderate' for the outcome asthma symptoms, 'very low' for the outcome exacerbations and 'low' for the outcome adverse events for ciclesonide versus fluticasone (dose ratio 1:1). For ciclesonide versus fluticasone (dose ratio 1:2) the quality was rated 'low' for the outcome asthma symptoms and 'very low' for exacerbations and adverse events (dose ratio 1:2).
Authors' conclusions
An improvement in asthma symptoms, exacerbations and side effects of ciclesonide versus budesonide and fluticasone could be neither demonstrated nor refuted and the trade‐off between benefits and harms of using ciclesonide instead of budesonide or fluticasone is unclear. The resource use or costs of different ICS should therefore also be considered in final decision making.
Longer‐term superiority trials are needed to identify the usefulness and safety of ciclesonide compared to other ICS. Additionally these studies should be powered for patient relevant outcomes (exacerbations, asthma symptoms, quality of life and side effects). There is a need for studies comparing ciclesonide once daily with other ICS twice daily to assess the advantages of ciclesonide being a pro‐drug that can be administered once daily with possibly increased adherence leading to increased control of asthma and fewer side effects.
Keywords: Adolescent; Child; Child, Preschool; Humans; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Adrenal Cortex Hormones/adverse effects; Androstadienes; Androstadienes/administration & dosage; Androstadienes/adverse effects; Anti‐Asthmatic Agents; Anti‐Asthmatic Agents/administration & dosage; Anti‐Asthmatic Agents/adverse effects; Asthma; Asthma/drug therapy; Budesonide; Budesonide/administration & dosage; Budesonide/adverse effects; Fluticasone; Pregnenediones; Pregnenediones/administration & dosage; Pregnenediones/adverse effects; Randomized Controlled Trials as Topic
Plain language summary
Ciclesonide compared to budesonide and fluticasone in the treatment of asthma in children
Asthma is a common disease in childhood. Most children with chronic asthma are treated with inhaled corticosteroids (ICS) to control airway inflammation and reduce asthma symptoms. Although these drugs are considered to be very safe and effective, not all children achieve full asthma control and some parents are concerned about the possibility of reduced growth or local side effects such as hoarseness. The challenge for newer ICS is to achieve improved asthma control with fewer side effects. This could be achieved by small‐particle‐size ICS, leading to better lung deposition as they penetrate deeper into the small airways. Therefore, asthma control could be achieved with lower daily doses and with fewer side effects. In children, particle size of ICS might be even more important because of their smaller airways.
Ciclesonide is a new small‐particle‐size ICS. The smaller particle size may make the corticosteroid go deeper into the lungs. Potential advantages are a lower required dose to achieve asthma control, once daily instead of twice daily dosing, and reduced local (oral thrush) and systemic (growth suppression) side effects.
We found six studies comparing ciclesonide with either budesonide or fluticasone in 3256 children (aged four to 17 years) with chronic asthma. After three months of treatment with ciclesonide compared to budesonide or fluticasone, no relevant differences could be found on asthma symptoms, exacerbations or side effects. Ciclesonide compared to a double dose of fluticasone was assessed in one study and no differences were found in asthma symptoms, use of rescue medication and adverse effects. However, children receiving ciclesonide experienced more asthma exacerbations than children in the fluticasone group.
The results of this review regarding the efficacy and safety of ciclesonide compared to other ICS are not conclusive. Relatively few studies were found, different inhalers were compared and treatment and follow‐up time (12 weeks) was too short for the assessment of relevant outcomes such as exacerbations and growth retardation. Future studies should pay attention to those aspects.
Summary of findings
Summary of findings for the main comparison. Ciclesonide versus budesonide (dose ratio 1:2) for chronic asthma in children.
| Ciclesonide versus budesonide (dose ratio 1:2) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children Settings: all settings Intervention: ciclesonide Comparison: budesonide (dose ratio 1:2) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Budesonide (dose ratio 1:2) | Ciclesonide | |||||
| Asthma symptoms Asthma symptom score (scale 0 to 4) Follow‐up: 12 weeks | See comment | See comment | Not estimable | 1024 (2 studies) | ⊕⊕⊝⊝ low1,2 | Both studies used a 5‐point scale, but insufficient data were reported to allow meta‐analysis |
| Patients with exacerbations Number of patients with exacerbations Follow‐up: 12 weeks | 12 per 1000 | 26 per 1000 (9 to 77) | RR 2.2 (0.75 to 6.43) | 1024 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Adverse events Number of patients with adverse events Follow‐up: 12 weeks | See comment | See comment | Not estimable | 1024 (2 studies) | ⊕⊕⊝⊝ low1,2,3 | The data could not be meta‐analysed because the definitions of adverse events were too diverse |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In one study the dose of budesonide was much higher than what is commonly prescribed in clinical practice. 2 Both studies were sponsored by the manufacturer and at least one of the authors of each study was an employee of the manufacturer that sponsored the study. 3 The intervention period of 12 weeks was too short to expect any major changes in this outcome. 4 Confidence intervals of estimated effect include no effect and exceed a relative reduction or increase risk of 25%.
Summary of findings 2. Ciclesonide versus fluticasone (dose ratio 1:1) for chronic asthma in children.
| Ciclesonide versus fluticasone (dose ratio 1:1) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children Settings: all settings Intervention: ciclesonide Comparison: fluticasone (dose ratio 1:1) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluticasone (dose ratio 1:1) | Ciclesonide | |||||
| Asthma symptoms Asthma symptom score (scale 0 to 4) Follow‐up: 12 weeks | See comment | See comment | Not estimable | 1468 (3 studies) | ⊕⊕⊕⊝ moderate1 | 2 studies used a 5‐point scale and 1 study did not provide details how asthma symptoms were measured. Data could not be pooled due to diversity in scales |
| Patients with exacerbations Number of patients with exacerbations Follow‐up: 12 weeks | 18 per 1000 | 24 per 1000 (10 to 57) | RR 1.37 (0.58 to 3.21) | 1003 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Adverse events Number of patients with adverse events Follow‐up: 12 weeks | See comment | See comment | Not estimable | 1560 (6 studies) | ⊕⊕⊝⊝ low1,2 | Adverse events were defined differently across studies therefore results could not be pooled |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Two fully published studies were sponsored by the manufacturer and at least one of the authors of each study was an employee of the manufacturer that sponsored the study. 2 The intervention period of 12 weeks is too short to expect any major changes in this outcome. 3 Confidence intervals of estimated effect include no effect and exceed a relative reduction or increase risk of 25%.
Summary of findings 3. Ciclesonide versus fluticasone (dose ratio 1:2) for chronic asthma in children.
| Ciclesonide versus fluticasone (dose ratio 1:2) for chronic asthma in children | ||||||
| Patient or population: patients with chronic asthma in children Settings: all settings Intervention: ciclesonide Comparison: fluticasone (dose ratio 1:2) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluticasone (dose ratio 1:2) | Ciclesonide | |||||
| Asthma symptom Asthma symptom score (scale 0 to 4) Follow‐up: 12 weeks | The mean asthma symptom in the control groups was 1.33 | The mean asthma symptom in the intervention groups was 0.07 higher (0.14 to 0.29 higher) | 482 (1 study) | ⊕⊕⊝⊝ low1,2 | Estimates are medians indicating data was skewed | |
| Patients with exacerbations Number of patients with exacerbations Follow‐up: 12 weeks | 20 per 1000 | 70 per 1000 (27 to 174) | RR 3.48 (1.35 to 8.71) | 502 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| Adverse events Number of patients with adverse events Follow‐up: 12 weeks | 476 per 1000 | 471 per 1000 (424 to 514) | RR 0.99 (0.89 to 1.08) | 502 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Based on one study that was underpowered for a non‐inferiority trial. 2 The study was sponsored by the manufacturer and at least one author was an employee of the manufacturer that sponsored the study. 3 The intervention period of 12 weeks is too short to expect any major changes in this outcome.
Background
Description of the condition
Asthma is the most common chronic disease of childhood with a prevalence of 8% to 15% (Masoli 2004). It is a chronic inflammatory disease affecting the whole airway system, including the small airways (Hamid 1997). Daily inhaled corticosteroids (ICS) are the cornerstone of treatment of chronic asthma and in recent guidelines ICS are recommended for all patients except those with mild, intermittent symptoms (British Thoracic Society 2011; GINA 2011).
Description of the intervention
ICS reduce inflammation in the lungs by modulating the inflammatory response of the lung by binding to the glucocorticoid receptor and suppressing the expression of pro‐inflammatory genes. With asthmatic inflammation occurring in all airways including the small airways, the challenge of ICS treatment has now focused mainly on targeting the small airways (Gelfand 2009; Lahzami 2008). Small‐particle drugs (median diameter 1.5 µm) penetrate better in the small airways and improve total lung deposition in adults, more so when inhaled with slower inspiratory flows (Usmani 2005).
Potential adverse drug effects of ICS can be divided into local (such as oral candidiasis and hoarseness) and systemic (adrenal and growth suppression) effects. Particularly in children growth is still a major concern for parents and clinicians. Several longitudinal studies evaluating the effect of ICS on growth have shown a small decrease in growth velocity (approximately 1 to 2 cm) during the first year of treatment (Peters 2006). However, long‐term follow‐up studies show no change in final adult height (Brand 2001). A chronic disease such as asthma may lead to suppressed growth as children with asthma enter puberty at a later age (Brand 2001).
How the intervention might work
The most widely available ICS are beclomethasone dipropionate (BDP), budesonide and fluticasone propionate. Chlorofluorocarbon‐BDP and budesonide are considered equipotent; fluticasone is considered twice as potent compared to chlorofluorocarbon‐BDP and budesonide. Additionally fluticasone and hydrofluoroalkane‐BDP are regarded as equipotent to ciclesonide and the recommended dosage of the Global Initiative for Asthma (GINA), Global Strategy for Asthma Management and Prevention 2011 guideline, is based on this equipotency (GINA 2011). Almost all ICS are registered for twice‐daily use, except for budesonide, which is also registered for once‐daily use. No consistent significant or clinically relevant differences in effectiveness among available ICS have been identified (Adams 2007). One systematic review comparing ICS with small particles (HFA‐BDP) with fluticasone showed no significant difference on forced expiratory volume in one second (FEV1) and peak expiratory flow (PEF) at a dose ratio of 1:1 (Lasserson 2006). There are concerns about adrenal suppression with fluticasone given to children at doses greater than 400 μg/day (Adams 2007; Masoli 2004). Studies reporting on cases of acute adrenal insufficiency in children are almost invariably in children receiving fluticasone and not beclomethasone or budesonide (Eijkemans 2011; Todd 2002). In addition, children receiving fluticasone at half the daily dose of budesonide or beclomethasone appear to have a higher risk of pharyngitis (Adams 2007).
Ciclesonide is a relatively new drug with several potential advantages over the currently used ICS. It is inhaled as a pro‐drug, which is converted in the airways to an active metabolite (des‐ciclesonide) and therefore with potentially less local and systemic side effects. As both ciclesonide and its active metabolite des‐ciclesonide are highly protein bound (˜ 99%), this results in a low proportion of free, unbound drug in the circulation. The 100‐fold greater glucocorticoid receptor binding affinity of des‐ciclesonide compared to ciclesonide may be the explanation for the prolonged local anti‐inflammatory action in the lung and its clinical efficacy with once‐daily dosing. Because of extensive first‐pass metabolism, the systemic availability of des‐ciclesonide is less than 1%. For a detailed overview we refer to paper published by Dahl (Dahl 2006). Furthermore, from a pressurised metered dose inhaler (pMDI), ciclesonide consists of small particles with a volume median diameter of 1.9 µm (compared to a volume median diameter of 3.5 µm for fluticasone, 2.8 µm for budesonide and 1.9 µm for HFA‐BDP) (De Vries 2009). Because of smaller particle size and lower plume velocity, ciclesonide has a better delivery to the small airways and consequently, effective asthma control could be achieved at lower daily doses.
Ciclesonide is registered for once‐daily use. Mean adherence rates may decline with increased frequency of dosing and therefore a once‐daily use could lead to better compliance compared to twice‐daily use (Guest 2005; Osterberg 2005; Price 2010). Particularly in adolescents, adherence to treatment is a major problem. Ciclesonide has been approved in Europe for children 12 years of age and older. The drug is delivered by a metered dose inhaler (MDI) and registered for use with the AeroChamber Plus® spacer.
Why it is important to do this review
The Cochrane Airways Group decided to split the existing review entitled "Ciclesonide versus other inhaled steroids for chronic asthma" (Manning 2009) into a review restricted to children and one restricted to adults. The effect of ciclesonide compared to placebo is subject of another Cochrane review (Manning 2008).
Objectives
To assess the efficacy and adverse effects of ciclesonide compared to other ICS in the management of chronic asthma in children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCT) comparing ciclesonide with another ICS. We included trials of parallel group design and cross‐over trials with a wash‐out period of two weeks or more. Available unpublished data were considered.
Types of participants
Children (younger than 18 years) with physician‐diagnosed chronic asthma in all settings (general practice, outpatient departments, emergency departments and hospitalised) were eligible for inclusion. Trials that included children as well as adults (aged 18 years and older) were included provided that the data on children were reported separately.
Studies with participants with pulmonary diagnosis other than asthma were excluded.
Types of interventions
This review includes studies that have compared ciclesonide with other ICS at equivalent and lower doses of ciclesonide. The intervention period had to be at least four weeks. Concomitant therapies for asthma, such as short‐acting beta2‐agonists (rescue therapy), theophyllines, long‐acting beta2‐agonists (salmeterol or formoterol), and inhaled anticholinergics were permitted provided that the dose and type of drug remained stable and were the same in both groups and was not introduced at the start of the trial as part of the study protocol. Studies involving anti‐leukotrienes (e.g. singular, accolate), combination inhalers (fluticasone‐salmeterol and budesonide‐formoterol) or other airway anti‐inflammatory asthma therapy (e.g. cromones) were excluded.
Types of outcome measures
Primary outcomes
Asthma symptoms: asthma symptom score and number of days without symptoms and use of rescue medication.
-
(Severe) asthma exacerbations defined as:
hospital admission;
visit to emergency department;
need for additional course of corticosteroids;
a combination of the above.
Adverse effects: oropharyngeal candidiasis, sore throat, symptoms of hoarseness, growth, lower‐leg growth, adrenal insufficiency, plasma cortisol, urinary cortisol excretion.
Secondary outcomes
Quality of life.
Compliance.
Change in lung function (FEV1, Mid expiratory flow 25‐75%)
Airway inflammation assessed by biopsy, lavage or exhaled nitric oxide (fraction of nitric oxide in exhaled air (FeNO))
Search methods for identification of studies
Electronic searches
We identified from the Cochrane Airways Group (CAG) Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched records in the Specialised Register coded as 'asthma' using the following terms:
ciclesonide* or Alveso* or pregnenedione* or CIC
We searched the CAG trials register from June 2007 up to November 2012. Additional searches in MEDLINE and EMBASE were undertaken using the strategies in Appendix 2 for articles published more recently (2007 to 2012).
Searching other resources
Included and excluded studies of the earlier review that included adults as well as children (Manning 2009) were checked if data concerning children were reported separately. Reference lists of all primary studies and review articles were reviewed for additional references. The manufacturer of ciclesonide (ALTANA Pharma and Nycomed) and authors of identified trials were contacted and asked to identify other published and unpublished studies.
We searched www.clinicalstudyresults.org for trial reports of CIC (December 2011).
Data collection and analysis
Selection of studies
Two review authors (NB and BLR) screened the title and abstract of each citation identified for eligibility. Articles that appeared to meet the inclusion criteria were retrieved in full text. Published abstracts of trials and trials published in a language other than English were also included. Then, based on the full text of the articles, NB and BLR independently established whether each study met the inclusion criteria of the review. Disagreement was solved by discussion.
Data extraction and management
Two review authors extracted the data from the included studies independently of each other. We attempted to contact study authors to identify additional papers, confirm data for accuracy and completeness.
We extracted data concerning the following characteristics of the included studies: study design; patient characteristics such as age, gender, asthma severity, inclusion and exclusion criteria, setting; diagnosis and diagnostic criteria used; characteristics of the interventions such as ICS type, dose, duration of study, method of delivery (MDI with or without spacer, breath actuated inhaler (BAI) or dry powder inhaler (DPI)); inhalation technique (breath hold after inhalation from DPI or BAI, inhalation from spacer with single breath followed by breath hold or tidal breathing) and reported outcome measures.
Assessment of risk of bias in included studies
Risk of bias of the included studies was independently assessed by two review authors according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreement was solved by discussion. The following items were assessed:
adequate sequence generation;
allocation concealment;
blinding (patient reported/subjective outcomes: asthma symptoms, adverse effects, quality of life, compliance);
blinding (other outcomes);
incomplete outcome data addressed (patient reported/subjective outcomes: asthma symptoms, adverse effects, quality of life, compliance);
incomplete outcome data addressed (other outcomes);
free of selective reporting;
free of other bias? (e.g. baseline differences).
Measures of treatment effect
A mean difference (MD) and 95% confidence interval (CI) was calculated for continuous variables measured on identical metrics. A standardised mean difference (SMD) was used for the continuous variables that addressed the same type of outcome, but were measured on different scales.
For dichotomous outcomes, we calculated a risk ratio (RR).
Unit of analysis issues
The unit of analysis was the patient.
Dealing with missing data
We contacted the authors of trials in which relevant data or information was missing that was needed for data synthesis and analyses.
Assessment of heterogeneity
Heterogeneity was assessed by comparing clinical characteristics of the included studies such as type of patients (age, gender, asthma severity, etc.), intervention (dose, inhalation technique, duration, etc.), comparison and outcome measures. Clinical homogeneity was discussed by the authors of this review and included experts in the field. Based on this discussion we decided whether pooling of results was sensible. Statistical heterogeneity was first assessed by visual inspection of the forest plots. We also applied the Chi2 test for homogeneity and we calculated the I2 statistic. To increase the power of the test for homogeneity we used a P < 0.1 for rejecting the null‐hypothesis of homogeneity. Interpretation of the statistical heterogeneity was according to the recommendation of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) and was as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
When interpreting the results of the test for homogeneity and the I2 statistic, we took into account the size of the studies that were included in the meta‐analysis.
Assessment of reporting biases
We planned to visually inspect funnel plots to assess reporting bias if we had been able to combine 10 or more trials in a forest plot.
Data synthesis
We only considered data of clinically homogeneous studies eligible to be combined. We hypothesised that the individual studies that evaluated the effect of ciclesonide estimated a common effect and therefore we chose to combine the results using a fixed‐effect model. If statistical heterogeneity was observed (Chi2: P < 0.1 and I2 > 30%) a sensitivity analysis using a random‐effects model was applied, to determine whether variation between the studies affected the pooled estimate. Furthermore, evidence of statistical heterogeneity prompted exploration of factors that can explain heterogeneity such as clinical or methodological characteristics of studies.
Summary of findings table
We created 'Summary of findings' (SoF) tables for each comparison and primary outcomes. We used GRADE‐profiler software to generate SoF tables that included the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the overall quality of evidence on relevant (primary) outcomes (asthma symptoms, exacerbations and adverse effects) (Schunemann 2011a; Schunemann 2011b). Two review authors (SK and NB) independently graded the body of evidence. According to GRADE, RCTs start as high‐quality evidence. There are five reasons for downgrading the quality of a body of evidence for a specific outcome: limitations in design, indirectness of evidence, inconsistency, imprecision of results and high probability of publication bias. All these items were scored and reasons for downgrading were explicitly stated. Overall quality of evidence was graded 'high', 'moderate' or 'low' based on the likelihood of further research changing our confidence in the estimate of effect. We resolved discrepancies by consensus among two review authors (SK and NB).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses, provided we had sufficient data, according to age (< six years and ≥ six years), asthma severity, dose of ciclesonide and delivery device (identical or different devices used for ciclesonide and BDP/budesonide/fluticasone) as well as inhalation manoeuvre.
Sensitivity analysis
Sensitivity analyses were planned to test the robustness of the results based on the results of the 'Risk of bias' assessment, provided we had sufficient data. We planned to repeat analyses with studies that scored a low risk of bias for allocation concealment, blinding (outcome: asthma symptoms, adverse effects, quality of life, compliance) or incomplete follow‐up (outcome: asthma symptoms, adverse effects, quality of life, compliance).
Results
Description of studies
Results of the search
Of the included and excluded studies of the existing Cochrane review of Manning 2009 three studies met our inclusion criteria (Pedersen 2006; Vermeulen 2007; von Berg 2007). The updated search yielded 296 citations and an additional search of the website www.clinicalstudyresults.org yielded 22 references. After screening of title and abstracts, the full text of 24 studies was assessed. Two reports identified by the search of www.clinicalstudy.org were reports of studies identified in the search of the databases (Agertoft 2010; Pedersen 2009). Of all identified studies, one study published in full text (Pedersen 2009) and two studies published as abstracts (Hiremath 2006; Paunovic 2010) met our inclusion criteria. Therefore, a total of six studies were included into this review. An overview of the selection process is shown in Figure 1.
1.
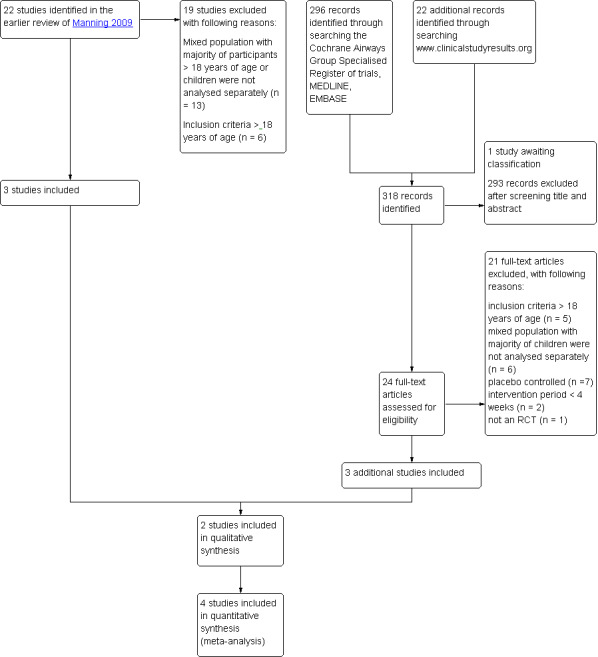
Study flow diagram.
In the previous review, four of the 13 ongoing studies were identified that potentially met our inclusion criteria. To date, three studies have been completed. One study was published but did not separately describe the data of children younger than 18 years of age (Postma 2011) and one study only including adults was excluded (van den Berge 2009). One study is awaiting classification since no full reports were available of the study data (see Characteristics of studies awaiting classification table). One study originally found in the National Research Register record could not be found in the registers archives, and contact details of the author were no longer up to date (GIWA 2003). No references were found to published data of this study and therefore this study is regarded as obsolete.
To retrieve additional data we contacted all contact authors of the included studies. Two of them replied and re‐directed us to the pharmaceutical companies involved. We did not get a reply from the companies on our request for additional data.
Included studies
The characteristics of the six included studies are presented in the Characteristics of included studies table.
Two studies were described as randomised double‐blind parallel group designs (Hiremath 2006; Paunovic 2010) and four studies as randomised double‐blind double‐dummy parallel group designs (Pedersen 2006; Pedersen 2009; Vermeulen 2007; von Berg 2007). All were designed as non‐inferiority studies on lung function.
The six studies randomised 3256 children with asthma and included children between the age of 4 and 17 years. One study did not specify how asthma was diagnosed (von Berg 2007), whereas the other studies diagnosed asthma according to either the guidelines of the American Thoracic Society (ATS) (American Thoracic Society 1987) or the GINA 2003 classification (Pedersen 2006; Pedersen 2009; Vermeulen 2007). There was insufficient information on how asthma was diagnosed in two studies (Hiremath 2006; Paunovic 2010). The children in the fully published studies had suffered from asthma for at least six months.
In the six included studies, two different comparisons were assessed. Ciclesonide was compared to budesonide (Vermeulen 2007; von Berg 2007) or fluticasone (Hiremath 2006; Paunovic 2010; Pedersen 2006; Pedersen 2009) (see Table 4). All treatment periods were 12 weeks and outcomes were measured before and after the intervention period. The dose and delivery of the interventions varied between studies (see Table 4). Ciclesonide was delivered via MDIs in all studies.
1. Characteristics of the interventions.
| Study ID | Ciclesonide dose | Comparator ICS | Application | Inhalation technique | Treatment period |
| Ciclesonide versus budesonide | |||||
| von Berg 2007 | 160 μg OD (ex‐actuator; equivalent to 200 μg ex‐valve) 2 x 80 μg puffs in the evening | Budesonide 400 μg OD 2 x 200 μg puffs | Ciclesonide: HFA‐MDI with an AeroChamber®; Budesonide: Turbohaler® |
Not described | 12 weeks |
| Vermeulen 2007 | 320 μg OD (ex‐actuator; equivalent to 2 puffs of 200 μg ex‐valve) 2 x 160 μg puffs administered in the evening | Budesonide 800 μg OD (4 inhalations of 200 μg from the Turbohaler® device), administered in the evening | Ciclesonide: HFA‐MDI without spacer Budesonide: Turbohaler® | Not described | 12 weeks |
| Ciclesonide versus fluticasone | |||||
| Hiremath 2006 | 160 μg OD | Fluticasone 88 μg BID | MDI with spacer, AeroChamber Plus® | Not described | 12 weeks |
| Paunovic 2010 | 160 μg OD | Fluticasone 88 μg BID | No information provided | Not described | 12 weeks |
| Pedersen 2006 | 80 μg BID (ex‐actuator; equivalent to 100 μg BID ex‐valve) | Fluticasone 88 μg BID (ex‐actuator dose, equivalent to 100 μg BID ex‐valve) | HFA‐MDI without spacer | Adequate inhalation technique no details described | 12 weeks |
| Pedersen 2009 | 80 or 160 μg OD (ex‐actuator; equivalent to 100 and 200 μg ex‐valve) administered in the evening | Fluticasone 88 μg BID (176 ex‐actuator; equivalent to 100 μg BID ex‐valve) in the morning and evening | HFA 134‐MDI without spacer | Good inhalation technique, no details described | 12 weeks |
BID: twice daily; ex‐actuator: drugs that leaves the inhaler; ex‐valve: drugs that leaves the metering chamber valve; HFA‐MDI: hydrofluoroalkane‐propelled metered dose inhaler; ICS: inhaled corticosteroid; MDI: metered dose inhaler; OD: once daily.
All studies assessed our primary outcome asthma symptoms. Five studies assessed exacerbations and one study did not address this outcome at all (Hiremath 2006). None of the studies specifically defined asthma exacerbations that conformed to our definition as hospital admissions or visits to an emergency department or additional course of corticosteroids and the description of exacerbation varied between studies. Four studies defined asthma exacerbations as increasing asthma symptoms requiring change or addition of patient's medication other than increasing rescue medication (Pedersen 2006; Pedersen 2009; Vermeulen 2007; von Berg 2007) and in one of these studies the patients with exacerbations were withdrawn (Vermeulen 2007) and in two studies no definitions of exacerbations were described. One study did not report adverse events (Paunovic 2010). None of the studies reported on compliance.
The four fully published studies were all supported or sponsored by the manufacturer of ciclesonide. In all studies at least one of the authors was an employee of the manufacturer that sponsored the study.
Excluded studies
We excluded 21 records (see Characteristics of excluded studies). Two studies were excluded because the intervention period in these studies was two weeks (Agertoft 2010; Matsunaga 2009) and, therefore, did not met our criteria of at least four weeks.
Risk of bias in included studies
The risk of bias of the included studies is summarised in Figure 2. The risk of bias was unclear for the two studies that were published as conference abstracts as no information was available to make a definite judgement on the different items (Hiremath 2006; Paunovic 2010). Our judgements for the remaining four studies that were published in full text are discussed below per item.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The randomisation method was clearly described and adequate in three studies (Pedersen 2006; Pedersen 2009; Vermeulen 2007). One study did not provide sufficient information (von Berg 2007). No study described allocation concealment and therefore the risk of bias for this item was deemed unclear.
Blinding
The four fully published studies were described as double‐blind and double‐dummy. Therefore, risk of bias was assessed as low for both subjective outcomes and other outcomes (Pedersen 2006; Pedersen 2009; Vermeulen 2007; von Berg 2007).
Incomplete outcome data
Loss to follow‐up was reported in all four studies. In three studies, 4% of the randomised patients did not complete the study (Pedersen 2009; Vermeulen 2007; von Berg 2007), only one study gave a clear description of the number of patients per group and the reasons for loss to follow‐up (von Berg 2007). In one study, 8% of the patients randomised terminated the study prematurely. The number of patients per group was described; however, no reasons for loss to follow‐up were reported (Pedersen 2006). All studies described that an intention‐to‐treat (ITT) analysis was performed but no details of the analyses were provided and it was not specified which values were imputed in the analyses. Therefore, risk of bias for the items regarding incomplete outcome data were deemed unclear. Two of the four studies reported the number of patients that violated the study protocol (Pedersen 2009; von Berg 2007). The percentage of patients that violated the study protocol was similar in the three different groups in the study of Pedersen 2009 (ciclesonide 80 μg = 6%; 160 μg = 7%; fluticasone 88 μg = 6%). In the study of von Berg 2007 the percentage of patients that violated the study protocol was also similar, 14% of the ITT population in both groups. Two studies did not provide detailed information on study protocol violations (Pedersen 2006; Vermeulen 2007).
Selective reporting
The four fully published studies all reported the outcomes that were specified in their method section (Pedersen 2006; Pedersen 2009; Vermeulen 2007; von Berg 2007).
Other potential sources of bias
None of the four studies showed any obvious baseline differences, therefore for all studies risk of other biases were rated low.
Effects of interventions
See: Table 1; Table 2; Table 3
Ciclesonide versus budesonide
Two studies assessed the effect of ciclesonide compared to budesonide both administered once daily at dose ratios of 1:2. The dose of both ciclesonide and budesonide in one study (Vermeulen 2007) was twice the dose of the other study (von Berg 2007). Ciclesonide was delivered using a hydrofluoroalkane‐propelled metered dose inhaler (HFA‐MDI) with the AeroChamber® spacer in one study (von Berg 2007) and without a spacer in the other study (Vermeulen 2007). In both studies, the comparator drug, budesonide, was deliver using a Turbohaler®.
Both studies were designed to assess non‐inferiority of ciclesonide versus budesonide. One study used the per protocol (PP) population to test for non‐inferiority (Vermeulen 2007) and one study based the primary analysis on the PP population and used the ITT population to confirm the results (von Berg 2007). One study set non‐inferiority limits for lung function outcomes (FEV1: ‐150 mL; forced vital capacity (FVC): ‐150 mL and PEF: ‐20 L/minute), percentage of days without asthma symptoms and rescue medication (‐8%) and quality of life measured with Standardized Pediatric Asthma Quality of Life Questionnaire (PAQLQ(S)) scores (‐0.5%) (Vermeulen 2007). One study set the non‐inferiority acceptance limit for the outcome FEV1 at ‐100 mL, using the lower limit of 95% CI for differences between treatment groups (von Berg 2007). The studies were considered to be clinically similar and therefore data were pooled when possible. The results are shown in Table 5 (see Table 1).
2. Effect of the intervention: ciclesonide versus budesonide.
| Dose | CIC 160 μg OD versus BUD 400 μg OD | CIC 320 μg OD versus BUD 800 μg OD |
| Dose ratio | 1:2 | 1:2 |
| Study | von Berg 2007 | Vermeulen 2007 |
| Primary outcomes | ||
| Asthma symptoms: asthma symptom score (sum score) | ITT: MD 0.01, 95% CI ‐0.14 to 0.16 PP: MD 0.03, 95% CI ‐0.20 to 0.25 Non‐inferiority acceptance limit = 0.3 |
Median change from baseline (no CIs reported) ITT: CIC: ‐0.07; BUD: ‐0.14 PP: CIC: ‐0.07; BUD: ‐0.14 |
| Asthma symptoms: use of rescue medication (puff/day) | ITT: MD 0.06 puffs/day, 95% CI ‐0.26 to 0.38 | Not assessed |
| Asthma symptoms: % of asthma symptom and rescue medication‐free days | ITT: CIC: mean 73%; BUD: mean 70% No difference between groups |
ITT and PP: CIC: median 84%; BUD: median 85% Lower limit of the between difference was ‐1.4% and above non‐inferiority limit of ‐8% |
| Exacerbations: patients with exacerbations* | ITT: RR 2.71, 95% CI 0.61 to 12.11; Analysis 1.1 | ITT: RR 1.69, 95% CI 0.36 to 8.00; Analysis 1.1 |
| Adverse events: patients with adverse events | Adverse events were reported in 38% of patients in both groups | ITT: RR** 1.44, 95% CI 0.96 to 2.18 |
| Adverse events: change in body height | Mean change from baseline (least square mean) CIC: 1.18 cm; BUD: 0.70 cm |
Not assessed |
| Adverse events: 24‐hour urine cortisol adjusted for creatinine | ITT: 2.99 nmol/mmol creatinine; P < 0.0001, one‐sided (decrease greater in the BUD group) | ITT: significant difference between groups (lower level in BUD group) |
| Secondary outcomes | ||
| Quality of life: PAQLQ(S) | ITT: MD ‐0.11, 95% CI ‐0.12 to 0.10, one‐sided superiority; Analysis 1.2 Non‐inferiority acceptance limits = not provided PP not reported |
ITT: MD (least square mean) 0.01, 95% CI ‐0.14 to 0.16; Analysis 1.2 Non‐inferiority acceptance limit = ‐0.5% PP results were similar |
| Quality of life: PACQLQ | ITT: MD ‐0.08, 95% CI ‐0.27 to 0.11, one‐sided superiority Non‐inferiority acceptance limit not provided PP not reported |
Not assessed |
| Compliance | Not assessed | Not assessed |
| Lung function: FEV1 (L) | ITT: MD (least square means) ‐0.019 L, 95% CI ‐0.059 to 0.022; Analysis 1.3 PP: MD (least square means) ‐0.034 L, 95% CI ‐75 to 10 Non‐inferiority acceptance limit = ‐100 mL |
ITT: MD (least square means) ‐0.03 L, 95% ‐0.14 to 0.8; Analysis 1.3 PP: MD (least square means) ‐0.02 L, 95% CI ‐0.13 to 0.1 Non‐inferiority acceptance limit = ‐150 mL |
| Airway inflammation | Not assessed | Not assessed |
BUD: budesonide; CI: confidence interval; CIC: ciclesonide; ITT: intention to treat analysis; MD: mean difference; OD: once daily; PACQLQ: Pediatric Asthma Caregiver Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PP: per protocol; RR: risk ratio.
* Exacerbations were defined as an increasing asthma symptoms requiring change or addition of patient's medication other than increasing rescue medication.
** Adverse events that needed treatment, reported in over 2% of patients in CIC or BUD group of safety population (N = 403).
Primary outcomes
Two studies on 1024 children found no significant differences between the groups regarding the outcome asthma symptoms (symptom scores, asthma symptom and rescue medication‐free days) (see Table 5). Asthma symptom scores were assessed using 5‐point scales where a score of 0 represented no asthma symptoms and a score of 4 very bad symptoms, unable to carry out daily activities. One study reported asthma symptom scores as a median change from baseline, which indicates skewed data and therefore we did not perform a meta‐analysis for this outcome.
Pooled data for exacerbations (as defined in the original studies) showed no significant difference between ciclesonide versus budesonide (RR 2.20, 95% CI 0.75 to 6.43; two studies; 1024 children) (Analysis 1.1).
1.1. Analysis.

Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 1 Patients with exacerbations.
The occurrence of adverse effects was similar in both treatment groups in one study on 621 children. Pharyngitis was one of the most reported adverse effect (ciclesonide: 6.0%; budesonide: 6.8%) in this study (von Berg 2007). Adverse effects likely to be related to treatment were low in the study comparing ciclesonide 320 μg versus budesonide 800 μg; 0.7% and 0.8% for ciclesonide and budesonide, respectively. This study also reported treatment emergent adverse effects (including pharyngitis, asthma aggravated, nasopharyngitis, upper respiratory tract infections) that were reported in more than 2% of the patients per group in a safety population (N = 403) and found no difference between ciclesonide and budesonide (RR 1.44, 95% CI 0.96 to 2.18) (Vermeulen 2007). Pooling of data was not possible because definition of adverse effects were very different between the studies.
One study reported the outcome changes in body height after 12 weeks of intervention. The measurements were taken only in some centres and selection criteria and procedures of the subgroup of patients was not described. Height was measured by stadiometry in 58 patients of the ciclesonide 160 μg group and 26 in the budesonide 400 μg group. The study reported that the increase in height was significantly bigger in the ciclesonide compared to the budesonide group (1.18 cm versus 0.70 cm, respectively) (von Berg 2007).
In the study that compared ciclesonide 160 μg once daily versus budesonide 400 μg once daily, one patient in each treatment group terminated participation due to serious adverse effects, but the author did not specify the nature of these effects (von Berg 2007).
Both studies (1024 children) reported that 24‐hour urine cortisol adjusted for creatinine levels showed a significant decrease in the budesonide group compared to the ciclesonide group, but no numerical data were reported.
Secondary outcomes
Both studies measured quality of life on the PAQLQ(S). One study used the interview version (von Berg 2007) and in the other study the PAQLQ(S) was self‐administered (Vermeulen 2007). Patients answered questions using a 7‐point scale where a score of 1 indicated maximum impairment and 7 indicated no impairment. Pooled results showed no significant differences between the groups (RR ‐0.00, 95% CI ‐0.09 to 0.09; two studies; 1010 children) (Analysis 1.2). One study on 621 children also assessed quality of life using the self‐administered Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ). Carers answered questions using a 7‐point scale, where a score of 1 indicated maximum impairment and 7 indicated no impairment, and reported one‐sided superiority of ciclesonide but did not provide acceptance limits (von Berg 2007).
1.2. Analysis.
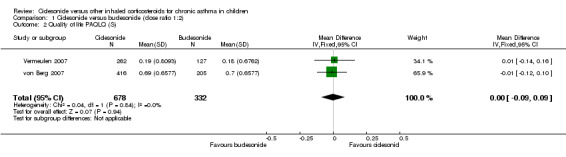
Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 2 Quality of life PAQLQ (S).
Pooled result of FEV1 (higher scores indicates better lung function) showed no significant MD between groups (RR ‐0.02, 95% CI ‐0.10 to 0.05; two studies; 1021 children) (Analysis 1.3).
1.3. Analysis.
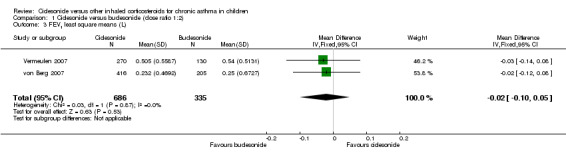
Comparison 1 Ciclesonide versus budesonide (dose ratio 1:2), Outcome 3 FEV1 least square means (L).
Compliance and airway inflammation were not formally assessed in either of the studies comparing ciclesonide versus budesonide.
Ciclesonide versus fluticasone propionate
Four studies assessed the effect of ciclesonide versus fluticasone (Hiremath 2006; Paunovic 2010; Pedersen 2006; Pedersen 2009) at a dose ratio of 1:1 and one study also assessed a dose ratio of 1:2 (ciclesonide 80 μg once daily compared to the fluticasone 88 μg twice daily; Pedersen 2009). Ciclesonide was administered once daily in all but one study that administered ciclesonide 80 μg twice a day (Pedersen 2006). One study did not report how either of the study drugs were delivered (Paunovic 2010). In one study both ciclesonide and fluticasone were delivered using an MDI with the AeroChamber Plus® spacer (Hiremath 2006) and in the other two studies both drugs were delivered using an HFA‐MDI without a spacer (Pedersen 2006; Pedersen 2009). Two studies were designed to assess non‐inferiority of ciclesonide. Both studies performed a PP analysis and used an ITT analysis to test for robustness of the results. In both studies, the non‐inferiority limits were set for the primary endpoint FEV1 at ‐0.100 L (Pedersen 2006; Pedersen 2009). In the study by Pedersen 2009, non‐inferiority limits were also set at 0.5 for PAQLQ(S) and PACQLQ scores; and +0.30 scores for asthma symptom score sum.
Of the four studies that assessed a dose ratio of 1:1, the study that administered ciclesonide 80 μg twice daily (Pedersen 2006) was considered to be clinically similar to the studies that administered ciclesonide 160 μg once daily (Hiremath 2006; Paunovic 2010; Pedersen 2009). Therefore, we pooled the data of these studies where possible. The results are shown in Table 6.
3. Effects of the intervention: ciclesonide versus fluticasone.
| Dose | CIC 80 μg BID vs. FP 88 μg BID | CIC 160 μg OD vs. FP 88 μg BID | CIC 80 μg BID vs. FP 88 μg BID | CIC 160 μg OD vs. FP 88 μg BID | CIC 80 μg OD vs. FP 88 μg BID |
| Dose ratio | 1:1 | 1:1 | 1:1 | 1:1 | 1:2 |
| Study | Pedersen 2006 | Pedersen 2009 | Hiremath 2006 | Paunovic 2010 | Pedersen 2009 |
| Primary outcomes | |||||
| Asthma symptoms: asthma symptom score | Median difference (Hodges Lehmann point estimate) ITT and PP: 0.00, 95% CI ‐0.29 to 0.14 |
Median difference (Hodges Lehmann point estimate) Unclear if ITT or PP *: 0.07, 95% CI ‐0.14 to 0.28 Non‐inferiority acceptance limit = 0.30 sum score |
Not assessed | Asthma symptom score decreased and was similar in both groups | Median difference (Hodges Lehmann point estimate) Unclear if ITT or PP **: 0.07, 95% CI ‐0.14 to 0.28 Non‐inferiority acceptance limit = 0.30 sum score |
| Asthma symptoms: use of rescue medication | Median difference (Hodges Lehmann point estimate) ITT and PP: 0.00, 95% CI ‐1.23 to 2.12 |
Median change from baseline (Hodges Lehmann point estimate) ITT: CIC: ‐1.13; FP: ‐1.29 PP: CIC: ‐1.14; FP: ‐1.29 All P < 0.0001 |
Not assessed | Use of rescue medication decreased and was similar in both groups | Median change from baseline (Hodges Lehmann point estimate) ITT: CIC: ‐1.20; FP: ‐1.29 PP: CIC: ‐1.21; FP: ‐1.29 All P < 0.0001 |
| Asthma symptoms: a sthma symptom‐free days | Median difference (Hodges Lehmann point estimate) ITT: ‐1.01, 95% CI ‐4.60 to 2.46 PP: ‐1.01, 95% CI ‐4.82 to 2.51 |
Not assessed | Not assessed | Not assessed | Not assessed |
| Asthma symptoms: % of asthma symptom and rescue medication‐free days combined | Not assessed | Mean percentage was high and did not differ significantly between the treatment groups (PP) | Median CIC: 91.5%; FP: 94% P = 0.1320 (2‐sided between treatments) |
Not assessed | PP: mean percentage was high and did not differ between the treatment groups |
| Exacerbations: number of patients with exacerbations | RR 1.26, 95% CI 0.34 to 4.66; Analysis 2.1 | RR 1.45, 95% CI 0.47 to 4.49; Analysis 2.1 | Not assessed | CIC: 2.3%; FP: 2.2% | RR 3.57, 95% CI 1.35 to 9.47; Analysis 2.1 |
| Adverse events: % of patients with adverse events | A similar percentage of patients reported adverse events | RR 0.88, 95% CI 0.72 to 1.07; Analysis 2.2 | The incidence of adverse events was similar in both groups | Not assessed | RR 0.98, 95% CI 0.81 to 1.17; Analysis 2.2 |
| Adverse events: cortisol 24‐hour urine sample (nmol/mmol) | ITT: difference between 2 groups was not statistically significant ITT and restricted ITT (which included only those urine cortisol measurements with a corresponding urine creatinine value within the normal range) A statistically significant difference in favour of CIC was seen in the restricted ITT analysis (P = 0.006). The findings were similar for patients who were ICS‐naive and patients who had received ICS prior to study entry although the differences were numerically greater in previously ICS‐naive patients |
Safety analysis**: MD 0.54 nmol/mmol, 95% CI ‐5.92 to 7.00; Analysis 2.3 |
Not assessed | Not assessed | Safety analysis**: MD 1.15 nmol/mmol, 95% CI 0.07 to 2.23; Analysis 2.3 |
| Secondary outcomes | |||||
| Quality of life: PAQLQ | Not assessed | ITT and PP: Non‐inferiority was confirmed CIC 160 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = ‐0.5 |
Not assessed | Not assessed | ITT and PP: Non‐inferiority was confirmed for CIC80 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = ‐0.5 |
| Quality of life: PACQLQ | Not assessed | ITT and PP: Non‐inferiority was confirmed CIC 160 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = 15 |
Not assessed | Not assessed | ITT and PP: Non‐inferiority was confirmed for CIC80 compared to FP (P < 0.0001, one‐sided) Non‐inferiority limit = 15 |
| Compliance | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
|
Change in lung function: FEV1 (L) |
ITT: MD (least square means) 0.0 L, 95% CI ‐0.042 to 0.042; Analysis 2.4 PP: MD (least square means) 0.001, 95% ‐0.044 to 0.046 |
ITT: MD (least square means) ‐0.02 L, 95% CI ‐0.07 to 0.04; Analysis 2.4 PP: MD (least square means) ‐0.026, 95% CI ‐0.086 to 0.34 |
Improvement similar between groups no point estimates | Improvement similar between groups no point estimates | ITT: MD (least square means) ‐0.05 L, 95% CI ‐0.11 to 0.01; Analysis 2.4 PP: MD (least square means) ‐0.056, 95% CI ‐0.12 to ‐0.004 |
| Airway inflammation | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
BID: twice daily; CI: confidence interval; CIC: ciclesonide; FP: fluticasone; ICS: inhaled corticosteroid; ITT: intention to treat analysis; OD: once daily; PACQLQ: Pediatric Asthma Caregiver Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; PP: per protocol analysis.
* = In this study analyses were based on PP population and analysis of ITT population was used to confirm results, description of the results are unclear but we assumed it to be based on analysis of PP population.
** = safety analysis excluded patients with concurrent nasal, ophthalmological or dermatological corticosteroid treatment.
Primary outcomes
Dose ratio 1:1
In two studies on 1048 children asthma symptom scores were assessed using a 5‐point scale where a score of 0 represented no asthma symptoms and a score of 4 represented very bad symptoms, unable to carry out daily activities (Pedersen 2006; Pedersen 2009). The results could not be pooled since data were reported as medians and this indicates skewed data. The other two studies on 932 children did not provide information on how asthma symptoms were measured (Hiremath 2006; Paunovic 2010) (see: Table 2).
No significant differences were found in asthma symptoms and rescue medication‐free days (four studies; 1934 children) (Hiremath 2006; Paunovic 2010; Pedersen 2006; Pedersen 2009) and non‐inferiority of ciclesonide was confirmed (limit was set at 0.3) for asthma symptom scores in one study on 492 children (Pedersen 2009) (see Table 6).
Pooled data of two studies comparing ciclesonide 160 μg versus fluticasone 88 μg twice daily showed no significant difference in number of patients with exacerbations (RR 1.37, 95% CI 0.58 to 3.21; two studies; 1003 children) (Analysis 2.1) (Pedersen 2006; Pedersen 2009). One study on 420 children reported that the number of patients with exacerbations was similar in both the ciclesonide and fluticasone groups (2.3% and 2.2%, respectively) (Paunovic 2010).
2.1. Analysis.

Comparison 2 Ciclesonide versus fluticasone, Outcome 1 Patients with exacerbations.
One study on 492 children reported that five (2.1%) children treated with ciclesonide 160 μg and two (0.8%) children treated with fluticasone 88 μg twice daily discontinued the study prematurely due to asthma exacerbation (Pedersen 2009).
No significant difference in number of patients with adverse events were found between ciclesonide 160 μg and fluticasone 88 μg twice daily (RR 0.88, 95% CI 0.72 to 1.07; one study; 492 children) (Analysis 2.2) (Pedersen 2009). The other two studies on 1023 children reported that adverse effects were similar in both groups (Hiremath 2006; Pedersen 2006) and one study did not assess adverse effects (Paunovic 2010).
2.2. Analysis.
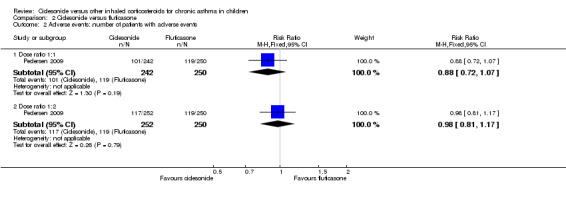
Comparison 2 Ciclesonide versus fluticasone, Outcome 2 Adverse events: number of patients with adverse events.
The outcome 24‐hour urine cortisol adjusted for creatinine levels was reported in one study. No significant differences were found for ciclesonide compared to fluticasone (MD 0.54 nmol/mmol, 95% CI ‐5.92 to 7.00; one study; 492 children) (Analysis 2.3).
2.3. Analysis.
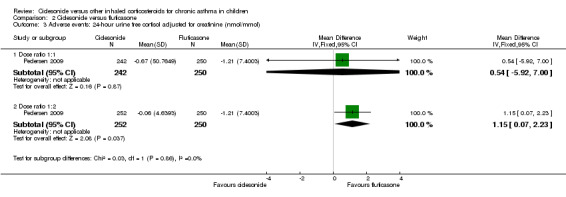
Comparison 2 Ciclesonide versus fluticasone, Outcome 3 Adverse events: 24‐ hour urine free cortisol adjusted for creatinine (nmol/mmol).
Dose ratio 1:2
In one study on 502 children, no significant differences were found in asthma symptoms and rescue medication‐free days. For asthma symptom sum scores non‐inferiority (limit was set at 0.3) was confirmed (Pedersen 2009)
The number of exacerbations was significantly higher in the ciclesonide 80 μg once‐daily group compared to the fluticasone 88 μg twice‐daily group (RR 3.57, 95% CI 1.35 to 9.47; one study; 502 children) (Analysis 2.1) (Pedersen 2009).
Thirteen (5.2%) participants treated with ciclesonide 80 μg and two (0.8%) treated with fluticasone 88 μg discontinued the study prematurely due to asthma exacerbation (Pedersen 2009).
No significant differences in number of patients with adverse effects were found between ciclesonide 80 μg once daily and fluticasone 88 μg twice daily (RR 0.98, 95% CI 0.81 to 1.1; one study; 502 children) (Analysis 2.2) (Pedersen 2009).
No significant difference was found for 24‐hour urine cortisol adjusted for creatinine levels in ciclesonide 80 μg once daily versus fluticasone 88 μg twice daily (MD 1.15 nmol/mmol, 95% CI 0.07 to 2.23; one study; 502 children) (Analysis 2.3).
Secondary outcomes
Dose ratio 1:1
Quality of life measured by the PAQLQ and the PACQLQ was reported in one study on 492 children (Pedersen 2009). Patients and carers answered questions using a 7‐point scale where a score of 1 indicated maximum impairment and 7 indicated no impairment.
Non‐inferiority was confirmed for both measurements for ciclesonide compared to fluticasone (P < 0.0001, one‐sided) (Pedersen 2009). Non‐inferiority limits were set at ‐0.5 for the PAQLQ scores and 15 for the PACQLQ scores. The other studies did not formally assess quality of life.
Pooled data of two studies showed no significant difference in FEV1 between ciclesonide 160 μg and fluticasone 88 μg (‐0.01 L, 95% CI ‐0.04 to 0.02; two studies; 1000 children) (Analysis 2.4).
2.4. Analysis.
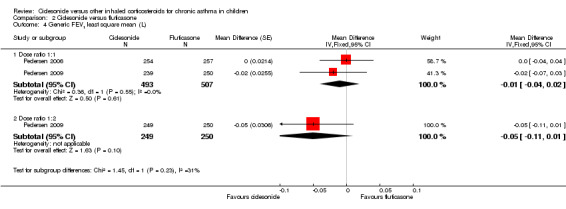
Comparison 2 Ciclesonide versus fluticasone, Outcome 4 Generic FEV1 least square mean (L).
None of the studies formally assessed outcomes on compliance or airway inflammation.
Dose ratio 1:2
Quality of life was measured by the PAQLQ(S) and the PACQLQ. Patients and carers answered questions using a 7‐point scale where a score of 1 indicated maximum impairment and 7 indicated no impairment. Non‐inferiority of ciclesonide versus fluticasone was confirmed for both measurements (P < 0.0001, one‐sided) (Pedersen 2009).
Results were similar in both groups and non‐significant for FEV1 (higher FEV1 indicates better lung function) and non‐inferiority was confirmed (MD ‐0.05 L, 95% CI ‐0.11 to 0.01; one study; 499 children) (Analysis 2.4) (limits set at ‐100 L) (Pedersen 2009).
The outcomes compliance or airway inflammation were not formally assessed.
It was not possible to conduct subgroup or sensitivity analyses due to lack of sufficient data.
Discussion
Summary of main results
In this review we assessed the efficacy and safety of ciclesonide compared to other ICS (budesonide and fluticasone) at a dose ratio 1:1 and 1:2 in the treatment of children younger than 18 years of age with chronic asthma. We found six studies including 3256 children that met our inclusion criteria.
We found no significant differences in efficacy between ciclesonide and fluticasone or budesonide for asthma symptoms and exacerbations after 12 weeks of treatment, except for one study comparing ciclesonide versus fluticasone (1:2) that found significantly more exacerbations in the ciclesonide group. Adherence was not assessed in the studies.
With regards to safety, local side effects such as pharyngitis were seen in both treatment groups with no significant differences, even in the study using a very high dose of budesonide (800 μg) administered once daily. Looking at systemic side effects, one study showed a significant improvement in height in the ciclesonide group compared to the budesonide group after 12 weeks of intervention, but measurements were only performed in a subset of patients. Studies assessing 24‐hour urinary cortisol levels showed either less suppression (ciclesonide versus budesonide) or no significant difference (ciclesonide versus fluticasone).
Overall completeness and applicability of evidence
Only six studies fulfilled our inclusion criteria, of which two studies were only published in abstract form, with limited details available concerning the participants enrolled, definition of outcome measures and trial methodology. The studies were mainly performed in Eastern European countries and South‐Africa, where fewer children might have received ICS treatment before enrolment in the studies than in western European countries. Patients included in the published studies were aged four to 15 years and diagnosed with chronic moderate‐to‐severe asthma according to ATS/GINA criteria, with a relatively poor FEV1 as a requirement at study entry in most of the included studies. No studies comparing ciclesonide versus HFA‐BDP were found. Different doses of both ciclesonide and comparator ICS were used; ciclesonide 80 to 320 μg, budesonide 400 to 800 μg, and fluticasone 88 to 176 μg in a pMDI‐AeroChamber Plus® combination (fluticasone, ciclesonide), as a pMDI without a spacer (fluticasone, ciclesonide) or DPI (budesonide). Current evidence is insufficient to recommend the optimal doses of ICS. Studies comparing different ICS doses could not reveal a clear dose‐response relationship in terms of efficacy and safety in children with mild‐to‐moderate asthma (Zhang 2011). However, all ICS doses in the studies were within accepted ranges for children. Fluticasone and ciclesonide are not registered with the AeroChamber Plus®; further, the use of a pMDI without a spacer is discouraged with children. Because all these different combinations were used, it is not known which part of the effect can be attributed to the ICS used and which part to the inhaler used and the conclusions are only valid for the chosen comparisons.
In all studies for the outcome adverse effects, 24‐hour urinary cortisol levels was measured. The clinical relevance of lower 24‐hour urinary cortisol levels for patients and practitioners is unclear and more important is the ability of the adrenal cortex to be able to respond to stressful circumstances, such as an infection, fever, etc. The most appropriate test would then be the more invasive low‐dose adrenocorticotropic hormone (ACTH) (Synacthen) stimulation test, which is more sensitive in detecting adrenal impairment (Crowley 1991; Lipworth 1999). However, for relevant systemic adverse effects, such as growth and adrenal insufficiency, a follow‐up period of 12 weeks is too short.
One study was published assessing the long‐term safety of ciclesonide (Skoner 2008). This RCT was not included in this review, because it compared ciclesonide to placebo. Mean linear growth velocity and 24‐hour urinary cortisol levels were similar in the three groups after one year. However, this study could not provide enough reassurance about safety, as considerable concern was expressed about compliance of the children as their asthma was very mild and the study failed to show any benefit of ciclesonide in terms of lung function or asthma control (Chapman 2008; Malozowski 2008).
All studies included in this review were designed as non‐inferiority trials. The allowance of setting pre‐defined non‐inferiority acceptance limits the concern is that drugs that are less effective will be classified as non‐inferior or as effective as the control drug. A trial showing non‐inferiority of the experimental drug suggests that the experimental drug is as good as the standard treatment. However, the width of the pre‐defined margins of inferiority has to be taken into account when interpreting the results of these trials individually. Wide margins can result in concluding that the experimental treatment is equally beneficial when it is really less beneficial. Additionally, non‐inferiority should be assessed for relevant outcomes, with a sufficiently long treatment and follow‐up period.
Not all of the included studies in our review provided non‐inferiority acceptance limits for our primary outcomes. Additionally most of the non‐inferiority limits were hard to interpret for reasons such as unclear description of the outcome measure (asthma symptom scores) and no information available on clinical important difference of the questionnaire (PACQLQ). To help readers of this review interpret data of individual studies we provided pre‐defined non‐inferiority limits where possible.
The results of the primary studies are focused on non‐inferiority of ciclesonide versus another ICS. However, when data could be pooled non‐inferiority was not a concern anymore since the point estimate and CI are not influenced by the acceptance limits set in the individual studies. In addition, a meta‐analysis of non‐inferiority studies showed that drugs that were found non‐inferior in published RCTs were not shown to be systematically less effective than standard treatments (Soonawala 2010).
Quality of the evidence
Using recommendations in the Cochrane Handbook and from the GRADE working group, we judged that the quality of the evidence was 'low' for the outcomes asthma symptoms and adverse events and 'very low' for the outcome exacerbations for ciclesonide versus budesonide (dose ratio 1:1; Summary of findings table 1). The quality of evidence was graded 'moderate for the outcome asthma symptoms, 'very low' for the outcome exacerbations and 'low' for the outcome adverse events for ciclesonide versus fluticasone (dose ratio 1:1; Summary of findings table 2). For ciclesonide versus fluticasone (dose ratio 1:2) the quality was rated 'low' for the outcome asthma symptoms and 'very low' for exacerbations and adverse events (dose ratio 1:2; Summary of findings table 3).
The evidence was regarded TO BE indirect due to the fact that in all studies the outcomes were measured after a 12‐week intervention period, which was regarded as an insufficient period to expect an effect on the outcomes adverse events and exacerbations.
Potential biases in the review process
We used the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b) to prevent or restrict the risk of bias in our review process. A comprehensive search of the literature searching several databases was conducted. We are confident that all relevant published studies for this review were found. We did attempt to find study protocols by searching www.clinicalstudyresults.org. We included six studies and therefore we could not generate funnel plots to identify publication bias. We contacted study authors in an attempt to find additional data, but did not receive any. Two review authors independently performed study selection, data collection, risk of bias and GRADE assessment to minimise bias. We did not write a protocol for this review but used the protocol of the review of Manning 2009. Any changes to this protocol are listed in the following section of this review (Differences between protocol and review).
Agreements and disagreements with other studies or reviews
Our findings are largely in keeping with other reviews on small particle size and reviews in adult patients. The systematic review by Manning 2009 comparing ciclesonide to other ICS in adults reached the same conclusions on efficacy outcomes; ciclesonide is equal to budesonide/fluticasone in terms of lung function end points, but for our primary outcomes this could not be established due to wide CIs (Manning 2009). The results of this review are also similar to the reported results on the outcomes FEV1 and quality of life in a narrative review that discusses ciclesonide as a treatment for asthma in adults and children (Dahl 2006). In this narrative review, the authors reported that in children the efficacy of ciclesonide was equivalent to fluticasone for the outcomes FEV1 and quality of life (Dahl 2006). A contrast with Manning 2009 and Dahl 2006 was the lower oral candidiasis with ciclesonide compared to fluticasone in adults, which we did not find in children. Other reviews comparing small‐particle‐size ICS with normal‐particle‐size ICS so far could not identify improved efficacy or safety on relevant end points compared to normal‐particle‐size ICS (Adams 2007).
Authors' conclusions
Implications for practice.
A beneficial effect on asthma symptoms, exacerbations and side effects of ciclesonide versus budesonide or fluticasone could be neither demonstrated nor refuted. Ciclesonide was non‐inferior compared to budesonide or fluticasone in terms of lung function end points and in some studies less suppression on cortisol outcomes was demonstrated.
New medications should either be more effective, safer or cheaper before they can be recommended for clinical practice. Because older medications have been used for longer periods of time, more knowledge is available on their long‐term safety and they are usually cheaper than new drugs (resource use). As far as we are aware there were few data available for the cost‐effectiveness of ciclesonide compared to other ICS.
Several other considerations must be taken into account before making clinical decisions, such as the trade‐off between benefits and harms, patient preferences and values, and resource use. The importance of these considerations can differ among different countries and cultures, leading to different recommendations for practice. For patient and parents, long‐term safety of ICS is an important issue. Well‐designed long‐term safety studies for ciclesonide are lacking. We cannot exclude that children receiving ciclesonide experience more exacerbations, as the CIs included potential harm as well as benefit. Therefore, the trade‐off between benefits and harms of using ciclesonide instead of budesonide or fluticasone is unclear.
An advantage of ciclesonide over other ICS is that it is licensed for once‐daily use, which could enhance compliance (Osterberg 2005), particularly in patients where compliance is a problem. Although ciclesonide is not registered for use with an AeroChamber® in paediatric practice, it is common to use a spacer device and AeroChamber Plus® is an adequate choice based on the low plume velocity of the ciclesonide‐pMDI. In addition, several studies have also used an AeroChamber® to deliver ciclesonide (Hiremath 2006; Pedersen 2010; von Berg 2007). In the end, resource use or costs of different ICS should be considered in final decision making.
Implications for research.
Based on this review a number of recommendations can be made for future trials. First, instead of non‐inferiority studies, superiority trials are needed to identify the efficacy and safety of ciclesonide compared to other ICS. In addition, these studies should be powered for patient‐relevant outcomes (exacerbations, asthma symptoms, quality of life and side effects) and not only on surrogate endpoints such as lung function and cortisol.
Studies comparing ciclesonide once daily with other ICS twice daily should be conducted, to test the advantages of ciclesonide being a pro‐drug that can be administered once daily. Once daily administration versus twice daily may result in increased adherence and to increased control of asthma and fewer side effects. In general, studies of at least six to 12 months' duration are needed to compare the relative benefits and side effects of the various ICS and their ways of administration on the longer term. Finally, inhaler devices and inhaler techniques needs to be taken into consideration in designing future trials and ideally, two doses of each drug‐device combination should be compared to two doses of the comparator drug‐device combination.
Acknowledgements
We are grateful to the staff of the editorial base of the Cochrane Airways Group for providing assistance in running searches and obtaining papers.
We would like to acknowledge the authors of the previous review: P. Manning (initiation of protocol; assessment of studies, data extraction, write up), P.G. Gibson (protocol development, write up, contact editor) and T. Lasserson (assessment of studies, data extraction, data entry and analysis, write up) for their excellent work. The results of their efforts formed the basis of this review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Database search strategies
PubMed search
#11 search #9 and #10
#10 Search ("2007"[Entrez Date] : "2011"[Entrez Date])
#9 Search #5 and #8
#8 Search #6 or #7
#7 Search (((((randomised[Title/Abstract]) OR randomized[Title/Abstract]) OR placebo[Title/Abstract]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract]
#6 Search "Randomized Controlled Trials"[MESH] OR "Clinical Trials"[MESH] OR "Controlled Clinical Trials"[MESH] OR "Cross‐Over Studies"[MESH] OR "Multicenter Studies"[MESH]
#5 Search #3 and #4
#4 Search ciclesonide[Text Word] OR CIC[Text Word] OR Alvesco[Text Word]
#3 Search #1 or #2
#2 Search "asthma*"[tw] or "wheez*"[tw]
#1 Search "asthma"[MESH]
EMBASE (Ovid) search
1. exp Asthma/
2. (asthma$ or wheez$).mp.
3. 1 or 2
4. ciclesonide/
5. (ciclesonide or alvesco or CIC).mp.
6. 4 or 5
7. 3 and 6
8. Randomized Controlled Trial/
9. randomization/
10. Controlled Study/
11. Clinical Trial/
12. controlled clinical trial/
13. Double Blind Procedure/
14. Single Blind Procedure/
15. Crossover Procedure/
16. exp Placebo/
17. or/8‐16
18. (randomized or randomised).ot,ab.
19. placebo.ot,ab.
20. randomly.ot,ab.
21. trial.ot,ab.
22. groups.ot,ab.
23. or/18‐22
24. 17 or 23
25. exp ANIMAL/
26. Nonhuman/
27. Human/
28. 25 or 26
29. 28 not 27
30. 24 not 29
31. 7 and 30
32. (2007$ or 2008$ or 2009$ or 2010$ or 2011$).em.
33. 31 and 32
Data and analyses
Comparison 1. Ciclesonide versus budesonide (dose ratio 1:2).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with exacerbations | 2 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.75, 6.43] |
| 2 Quality of life PAQLQ (S) | 2 | 1010 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 3 FEV1 least square means (L) | 2 | 1021 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.05] |
Comparison 2. Ciclesonide versus fluticasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with exacerbations | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Dose ratio 1:1 | 2 | 1003 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.58, 3.21] |
| 1.2 Dose ratio 1:2 | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.35, 9.47] |
| 2 Adverse events: number of patients with adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Dose ratio 1:1 | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.07] |
| 2.2 Dose ratio 1:2 | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.17] |
| 3 Adverse events: 24‐ hour urine free cortisol adjusted for creatinine (nmol/mmol) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Dose ratio 1:1 | 1 | 492 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐5.92, 7.00] |
| 3.2 Dose ratio 1:2 | 1 | 502 | Mean Difference (IV, Fixed, 95% CI) | 1.15 [0.07, 2.23] |
| 4 Generic FEV1 least square mean (L) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 Dose ratio 1:1 | 2 | 1000 | Mean Difference (Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 4.2 Dose ratio 1:2 | 1 | 499 | Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.11, 0.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hiremath 2006.
| Methods | Design: randomised controlled trial following a baseline period of 2 to 4 weeks (rescue medication only) and an intervention period of 12 weeks Location and number of centres: not reported |
|
| Participants | Number screened: not reported Number randomised: 512 Number completed: not reported Age: children and adolescents (4 to 15 years) with predominantly moderate‐to‐severe asthma Gender: not reported Asthma severity: forced expiratory volume in 1 second (FEV1) 50‐90% of predicted Inclusion/exclusion criteria: not reported |
|
| Interventions | Ciclesonide 160 μg (ex‐actuator; N = 254) once daily in the evening Fluticasone 88 μg twice daily (176 μg/day, ex‐actuator; N = 258) Delivery: both medications were administered via a metered‐dose inhaler with spacer (AeroChamber Plus®) Inhalation technique: not reported Treatment period: 12 weeks (following 2 to 4 weeks' baseline period rescue medication only) Allowed asthma medication: not reported |
|
| Outcomes | FEV1 from baseline to the end of the treatment period, morning peak expiratory flow, median percentage of asthma symptom‐ and rescue medication‐free days and incidence of adverse events | |
| Notes | Incomplete data since this study was only published as an abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Outcomes 1, 3, 4, 5 | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Outcomes 1, 3, 4, 5 | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Other outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not enough information |
| Other bias | Unclear risk | Not enough information |
Paunovic 2010.
| Methods | Design: randomised, double‐blind, 2 parallel‐group study Location and number of centres: not reported |
|
| Participants | Number screened: not reported Number randomised: 420 Number completed: not reported Age: 7 to 12 years Gender: not reported Asthma severity: FEV1 50‐90% of predicted Inclusion/exclusion criteria: not described |
|
| Interventions | 1. Ciclesonide once daily (160 µg/day) 2. Fluticasone twice daily (176 µg/day) Delivery: not reported Inhalation technique: not reported Treatment period: 12 weeks (following 2 to 4 weeks baseline period rescue medication only) Allowed asthma medication: not reported |
|
| Outcomes | Forced expiratory volume in 1 second (FEV1) (mL), peak expiratory flow (PEF) (L/minute), asthma symptom scores, rescue medication use, asthma exacerbation | |
| Notes | Incomplete data since this study was only published as an abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Outcomes 1, 3, 4, 5 | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Outcomes 1, 3, 4, 5 | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Other outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not enough information |
| Other bias | Unclear risk | Not enough information |
Pedersen 2006.
| Methods | Design: 12‐week, randomised, multicentre, double‐blind, double‐dummy, 2‐arm, parallel group study, with a 2‐ to 4‐week baseline period Location and number of centres: 51 centres in Europe, South Africa and Canada |
|
| Participants | Number screened: 728 enrolled
Number randomised: 556 (baseline details given for per‐protocol set. Ciclesonide: N = 277; fluticasone: N = 279)
Number completed: not reported. Age: median 10 years Gender: 331 boys; 180 girls Baseline details: add‐on therapy prior to baseline: ciclesonide N = 80, 64%; fluticasone N = 170, 66%; inhaled corticosteroid (ICS) therapy prior to baseline: ciclesonide N = 162, 31%; fluticasone N = 67, 27%; mean ICS dose: 390 μg/day overall; mean forced expiratory volume in 1 second (FEV1): 1.7 L overall; mean FEV1 % predicted: 80% overall; mean reversibility change in FEV1: 20% Inclusion criteria: aged 6 to 15 years; persistent asthma for at least 6 months (American Thoracic Society criteria); clinically stable for 4 weeks prior to study entry; FEV1 predicted: 50‐90% rescue medication only, 80‐100% in patients treated with ICS only; symptom score > 1 on 6 of last 10 days of run‐in; adequate metered dose inhaler (MDI) device technique without spacer Exclusion criteria: history of life‐threatening asthma; 2 or more inpatient hospitalisations in previous year; > 60 days of systemic corticosteroids in past year; > 400 budesonide or equivalent/day in 30 days prior to baseline; > 8 puffs short‐acting beta2‐agonist/day for 3 consecutive days during run‐in |
|
| Interventions | 1. Ciclesonide 100 μg twice daily
2. Fluticasone 100 μg twice daily Delivery: hydro‐fluoroalkane metered dose inhaler Inhalation technique: adequate inhalation technique no details described Treatment period: 12 weeks (following 2‐ to 4‐week baseline period with rescue medication (beta2 agonist only) Allowed asthma medication: not reported |
|
| Outcomes | FEV1, clinic peak expiratory flow (PEF), a.m. PEF, p.m. PEF, symptoms, rescue medication usage, adverse events | |
| Notes | Analysis of co‐variance included age and randomisation values as co‐variates and sex, treatment, and region/country as fixed factors Funding: Grant sponsor: ALTANA Pharma AG, Konstanz, Germany. This study was supported by ALTANA Pharma, Konstanz, Germany. The authors would like to thank Pro Ed Communications, Inc., Beachwood, also all Medicus International, London, UK for their editorial assistance. Editorial support was funded by ALTANA Pharma. Dr. Søren Pedersen has received remuneration for lectures from AstraZeneca and GlaxoSmithKline and served as a paid consultant for ALTANA Pharma and AstraZeneca. Ilse Theron is an employee of ALTANA Madaus Ltd, Woodmead, South Africa. Dr. Renate Engelstatter is an employee of ALTANA Pharma AG, Konstanz, Germany |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a computer‐generated list (Program RANDOM) provided to the study centres by ALTANA Pharma AG (Konstanz, Germany)" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Outcomes 1, 3, 4, 5 | Low risk | Quote: "Neither the investigator nor anyone at the study centre knew whether ciclesonide or fluticasone was administered" |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Quote: "Neither the investigator nor anyone at the study centre knew whether ciclesonide or fluticasone was administered" |
| Incomplete outcome data (attrition bias) Outcomes 1, 3, 4, 5 | Unclear risk | Not described which values used in intention‐to‐treat (ITT) analysis |
| Incomplete outcome data (attrition bias) Other outcomes | Unclear risk | Not described which values used in ITT analysis |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes described in methods were reported |
| Other bias | Low risk | Small differences in baseline characteristics |
Pedersen 2009.
| Methods | Design: 12‐week, randomised, double‐blind, double‐dummy, 3‐arm, parallel‐group study, following a 2– to 4‐week run‐in period Location and number of centres: 50 centres in Brazil, Germany, Hungary, Poland, Portugal and South Africa |
|
| Participants | Number screened: 904 enrolled Number randomised: 744 randomised and entered treatment period Number completed: 33 patients terminated study, 711 completed (of the 744, 50 violated protocol leaving 694 in per protocol population) Age: 6 to 11 years; median age in each group 9 years (range: 6 to 11). Gender: 170 boys; 161 girls Inclusion criteria: outpatients aged 6 to 11 years with a history of persistent bronchial asthma, for ≥ 6 months were eligible for participation. To be entered into the treatment period, patients were required to have a forced expiratory volume in 1 second (FEV1) 50–90% of predicted and a FEV1 reversibility of ≥ 12% after inhalation of salbutamol 200 to 400 mg at the end of the run‐in period. In addition, patients had to present asthma symptoms on at least 6 of the last 10 consecutive days of the baseline period, or to use at least 8 puffs of rescue medication within the last 10 consecutive days of the baseline period. Furthermore, patients had to demonstrate a good inhalation technique when using a metered dose inhaler (MDI) without a spacer Exclusion criteria: a history of near‐fatal asthma that required intubation; a respiratory tract infection or asthma exacerbation within the last 30 days prior to study entry; more than 2 inpatient hospitalisations for asthma in the previous year; use of systemic corticosteroids during the study, within the last 30 days prior to study entry or for more than 60 days in the previous 2 years |
|
| Interventions | 1. Ciclesonide MDI (80 μg once daily) (N = 252) 2. Ciclesonide 160 MDI (160 μg once daily) (N = 242) Both: in the evening (ex‐actuator; equivalent to 100 and 200 μg ex‐valve) 3. Fluticasone MDI (88 μg twice daily) (N = 250) ‐ fluticasone 176 (ex‐actuator; equivalent to 100 μg twice daily ex‐valve) in the morning and evening without a spacer Delivery: administered via HFA134‐a MDIs Inhalation technique: good inhalation technique, no details described Treatment period: a run‐in period (of at least 2 weeks and up to 4 weeks), in which eligible patients discontinued previous inhaled corticosteroids (ICS) and other controller medications followed by a 12‐week treatment period Allowed asthma medication: rescue medication salbutamol, patients were allowed to continue regular nasal corticosteroids at a constant dose |
|
| Outcomes | Change in FEV1 (L), peak expiratory flow (PEF) (L/minute), PD20FEV1 to methacholine (bronchial provocation test with methacholine to assess the provocative dose producing a 20% fall of FEV1) was performed at a subgroup of sites, Pediatric Asthma Quality of Life Questionnaire (PAQLQ) and Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ), asthma symptom scores and use of rescue medication (salbutamol), safety was assessed by adverse effect reporting, physical examination, vital signs and laboratory investigations, including haematology, urinalysis and biochemistry | |
| Notes | Analysis of co‐variance included treatment, gender and centre pool as fixed factors and baseline value and age as co‐variates Funding: Professor S. Pedersen has received consultancy fees and lecture honoraria from Nycomed and GlaxoSmithKline, and has worked on research projects supported by Nycomed, GlaxoSmithKline and AstraZeneca. Dr R. Engelstatter and Dr S. Hirsch are employees of Nycomed. Dr H.‐J. Weber was an employee of Nycomed at the time of writing of the manuscript. Professor A. Emeryk has received consultancy fees from Nycomed and lecture honoraria from Nycomed, GlaxoSmithKline and AstraZeneca, and has worked on research projects supported by Nycomed, Thorax‐Chisei and Pierre Fabre Medicament. Dr J. Vermeulen has worked on research projects supported by Nycomed. Professor L. Barkai and Dr H. Weber have nothing to disclose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "…a 1:1:1 randomisation scheme by means of a computer generated randomisation list.…." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Outcomes 1, 3, 4, 5 | Low risk | Double‐blind and double‐dummy design |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Ciclesonide provided in the evening 1 or 2 puffs and fluticasone was administered in the morning and evening |
| Incomplete outcome data (attrition bias) Outcomes 1, 3, 4, 5 | Unclear risk | Not described which values used in intention‐to‐treat (ITT) analysis |
| Incomplete outcome data (attrition bias) Other outcomes | Unclear risk | Not described which values used in ITT analysis |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes described in methods were reported |
| Other bias | Low risk | No obvious baseline differences |
Vermeulen 2007.
| Methods | Design: 12‐week, randomised, double‐blind, double‐dummy, parallel‐group study, following a 2‐week run‐in period Location and number of centres: 31 centres in Europe and South Africa |
|
| Participants | Number screened: 431
Number randomised: 403 (ciclesonide: 272; budesonide: 131)
Number completed: 384
Age: median 14 years Gender: 272 boys; 131 girls Astma severity: forced expiratory volume in 1 second (FEV1) 73% predicted Inclusion criteria: 12 to 17 years old; FEV1 50‐80% predicted; severe asthma (GINA 2003 definition); not well controlled after constant treatment with fixed‐dose budesonide 400 mg/day (or equivalent) 4 weeks prior to study entry with FEV1 45‐80% predicted; Alternatively constant treatment with fixed‐dose budesonide 400 to 800 mg/day (or equivalent) 4 weeks prior to study entry, with FEV1 46‐85% predicted; entry into treatment period at randomisation (baseline), FEV1 50‐80% predicted, FEV1 reversibility > 15% salbutamol. Exclusion criteria: oral corticosteroids within 4 weeks of study entry; concomitant severe diseases; relevant lung diseases or clinically relevant abnormal laboratory values; > 10 cigarette pack‐year smoking history; females of child‐bearing potential without contraception |
|
| Interventions | 1. Ciclesonide 400 μg once daily
2. Budesonide 800 μg once daily Delivery: HFA‐MDI (ciclesonide); Turbohaler® dry powder inhaler (DPI) (budesonide) Inhalation technique: not described Treatment period: 12 weeks Allowed asthma medication: not reported % on inhaled corticosteroids (ICS): 100 |
|
| Outcomes | FEV1; peak expiratory flow (PEF); 24‐hour urinary free cortisol concentrations | |
| Notes | Analysis of co‐variance included baseline value, treatment, age, sex and country pool as co‐variates or factors (not specified) Funding: this study (EudraCT No: 2004‐ 001233‐41) was sponsored by ALTANA Pharma. ALTANA Pharma had a role in the study design, the collection, analysis and interpretation of the data and was involved in the writing of the report and the decision to submit the manuscript. The co‐authors H. Rauerc and R. Engelstatter were both employees of ALTANA Pharma |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation list was generated by the sponsor using a multiplicative congruential pseudo‐random number generator with modulus 231‐1 (Program RANDOM based on Fishman and Moore" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Outcomes 1, 3, 4, 5 | Low risk | Double‐blind and double‐dummy design |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Double‐dummy but ciclesonide was administered in 2 puffs with metered dose inhaler (MDI) and budesonide with Turbohaler® device 4 inhalations |
| Incomplete outcome data (attrition bias) Outcomes 1, 3, 4, 5 | Unclear risk | Not described which values used in intention‐to‐treat (ITT) analysis |
| Incomplete outcome data (attrition bias) Other outcomes | Unclear risk | Not described which values used in ITT analysis |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes described in methods were reported |
| Other bias | Low risk | No obvious baseline differences |
von Berg 2007.
| Methods | Design: 12‐week, randomised, double‐blind, double‐dummy, 2‐arm, parallel‐group study, following a 2‐ to 4‐week run‐in period Location and number of centres: 59 centres in Europe and South Africa |
|
| Participants | Number screened: 774
Number randomised: 621 (ciclesonide: 416; budesonide: 205)
Number completed: 594
Age: mean 9 years Gender: 395 boys; 226 girls Astma severity: forced expiratory volume in 1 second (FEV1) 78% predicted; inhaled corticosteroid (ICS) treatment: 51% Inclusion criteria: aged 6 to 11 years; diagnosis of persistent asthma for 6 months; FEV1 > 50‐90% predicted if rescue medication only, > 50‐100% predicted if using constant dose of controller medication other than corticosteroids for 1 month; FEV1 80%‐105% predicted if using ≤ 400 μg/day beclomethasone dipropionate equivalent for 1 month before inclusion. Post‐run‐in: FEV1 50‐90% predicted after withholding short‐acting beta2‐agonist (SABA) for at least 4 hours; reversibility of FEV1 > 12% of initial post‐SABA; asthma symptom scores > 1 on at least 6 of previous 10 days or use of > 8 puffs of rescue medication during the previous 10 days Exclusion criteria: history of life‐threatening asthma, concomitant severe diseases; 2 or more hospitalisations for asthma within previous 12 months; asthma exacerbation during 4 weeks before baseline; systemic corticosteroids during 30 days before baseline; use of systemic corticosteroids for more than 60 days within the previous 2 years; participation in another study within 30 days before baseline. No other asthma medication permitted during study |
|
| Interventions | 1. Ciclesonide 200 μg once daily
2. Budesonide 400 μg once daily Delivery: ciclesonide: hydro‐fluoroalkane metered dose inhaler (HFA‐MDI) (+ AeroChamber®); budesonide: Pulmicort Turbohaler® Inhalation technique: not described Treatment period: 12 weeks Allowed co‐medication: none % on ICS: not reported |
|
| Outcomes | FEV1, peak expiratory flow, asthma symptoms, rescue medication, bone growth, 24‐hour urinary cortisol, adverse events | |
| Notes | Analysis of co‐variance included baseline value at randomisations visit and age as co‐variates Funding: this study was funded and sponsored by ALTANA Pharma. The authors would like to thank ProEd Communications, Inc., Beachwood Ohio and Medicus International, London, UK, for their editorial assistance. Editorial support was funded by ALTANA Pharma. The co‐authors Renate Engelstatter Stefan Leichtl, Stefan Hellbardt and Thomas D. Bethke were employees of ALTANA Pharma |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomised at a ratio of 2:1…" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Outcomes 1, 3, 4, 5 | Low risk | Double‐blind and double‐dummy design |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Double‐blind, double‐dummy, not specified who was blinded Ciclesonide and budesonide were administered in the evening via an HFA‐MDI with an AeroChamber Plus® spacer and Pulmicort Turbohaler®, respectively |
| Incomplete outcome data (attrition bias) Outcomes 1, 3, 4, 5 | Unclear risk | Not described which values used in intention‐to‐treat (ITT) analysis |
| Incomplete outcome data (attrition bias) Other outcomes | Unclear risk | Not described which values used in ITT analysis |
| Selective reporting (reporting bias) | Low risk | The results of all outcomes described in methods were reported |
| Other bias | Low risk | No obvious baseline differences |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adachi 2006 | Children not analysed separately |
| Agertoft 2010 | Treatment < 4 weeks |
| Bateman 2008 | Children not analysed separately |
| Berger 2009 | Placebo controlled |
| BY9010/M1‐207 | Children not analysed separately |
| Cohen 2011 | Placebo controlled |
| Dahl 2010 | Children not analysed separately |
| Derom 2009 | Included patients > 18 years of age |
| Dusser 2007 | Included patients > 18 years of age |
| Erin 2008 | Included patients > 18 years of age |
| Gelfand 2006 | Placebo controlled |
| Hoshino 2010 | Included patients > 18 years of age |
| Knox 2007 | Children not analysed separately |
| Kosztyla‐Hojna 2007 | Included patients > 18 years of age |
| Malozowski 2008 | Not a randomised controlled trial |
| Matsunaga 2009 | Treatment < 4 weeks |
| Meltzer 2009 | Placebo controlled |
| Molen 2010 | Children not analysed separately |
| Pedersen 2010 | Placebo controlled |
| Postma 2011 | Children not analysed separately |
| Skoner 2006 | Placebo controlled |
| Stoica 2010 | Children not analysed separately |
| van den Berge 2009 | Included patients > 18 years of age |
Characteristics of studies awaiting assessment [author‐defined order]
BY9010/M1‐205.
| Methods | Randomised controlled trial, double‐blind, study duration consists of a baseline period (2 to 4 weeks) and a treatment period (12 weeks) |
| Participants | Children aged 4 to 15 years Main inclusion criteria: history of persistent bronchial asthma for at least 6 months, forced expiratory volume in one second (FEV1) 50‐90% of predicted Main exclusion criteria: concomitant severe diseases or diseases which are contraindications for the use of inhaled corticosteroids; chronic obstructive pulmonary disease (chronic bronchitis or emphysema), other relevant lung diseases causing alternating impairment in lung function, or a combination; respiratory tract infection or asthma exacerbation within the last 30 days prior to entry into the study; history of life‐threatening asthma; premature birth; current smoking; smoking history with either ≥ 10 pack‐years; pregnancy; intention to become pregnant during the course of the study; breast feeding; lack of safe contraception |
| Interventions | Ciclesonide 200 μg/day Fluticasone propionate 200 μg/day |
| Outcomes | Primary outcome measures: FEV1 absolute values Secondary outcome measures: FEV1 as % of predicted, peak expiratory flow (PEF) from spirometry, diary‐based morning and evening PEF, diary‐based symptom score, diary‐based salbutamol metered dose inhaler (MDI) use, diurnal PEF fluctuation, drop‐out rate due to asthma exacerbations, time until asthma exacerbation, number of symptom‐free and rescue medication‐free days, number of days with asthma control, physical examination, vital signs, laboratory work‐up, adverse events |
| Notes |
Differences between protocol and review
This review is a result of an amendment of a review published in 2009 by Manning assessing the effect of ciclesonide compared to other ICS in adults and children. In this review we only focused on a population younger than 18 years of age. We based the methods of this review on the methods of the review of Manning 2009 and apart from the change of population of interest we made some additional changes. Our primary outcomes were asthma symptoms, exacerbations and adverse effect. We did not include surrogate measures of lung function as our primary outcome since this is not an outcome that is regarded as relevant to patients, but this outcome was included as one of our secondary outcomes.
In the earlier review, study quality was assessed using the Jadad scale. We assessed risk of bias using The Cochrane Collaboration's 'Risk of bias' tool. Studies identified in the earlier review were re‐assessed. Furthermore, we provide 'Summary of findings' tables according to GRADE for primary outcomes.
For the planning of exploring heterogeneity we did not use any cut of points based on values of the I2 statistics, but decisions would have been based on combined information of I2, Chi2, study characteristics and sample size of individual studies. Additional subgroups were pre‐defined to explore heterogeneity including subgroups according to age (< six years and ≥ six years), asthma severity, dose of ciclesonide and delivery device (identical or different devices used for ciclesonide and BDP/budesonide/fluticasone) as well as inhalation manoeuvre.
Contributions of authors
SFK: conception and design of study, and analysis and the interpretation of data. Drafting the review. Final approval of the document to be published. BLR: drafting the review and commenting on it critically for intellectual content. Final approval of the document to be published.
RPJMS: drafting the review and commenting on it critically for intellectual content. Final approval of the document to be published.
NB: drafting the review and commenting on it critically for intellectual content. Final approval of the document to be published.
Sources of support
Internal sources
-
SFK used to be a staff member of the Dutch Cochrane Centre and is now a staff member of the Australasian Cochrane Centre, Australia.
Received salary
External sources
NB Dutch Quality Fund for Medical Specialists (SKMS), Netherlands.
Declarations of interest
None declared.
New
References
References to studies included in this review
Hiremath 2006 {published data only}
- Hiremath L, Mohan‐Kumar T, Singh V, Raman P, Ramsperger U, Engelstätter R. Comparison of once daily ciclesonide 160 µg versus twice daily fluticasone propionate 88 µg in children with moderate to severe asthma. European Respiratory Journal 2006;28(Suppl 50):711s. [Google Scholar]
Paunovic 2010 {published data only}
- Paunovic Z. Comparison of ciclesonide and fluticasone propionate in the treatment of children with persistent asthma. Proceedings of the 29th Congress of the European Academy of Allergy and Clinical Immunology; 2010 June 5‐9; London.
Pedersen 2006 {published data only}
- Pedersen S, Garcia‐Garcia ML, Manjra A, Theron I, Engelstätter R. A comparative study of inhaled ciclesonide 160 microg/day and fluticasone propionate 176 microg/day in children with asthma. Pediatric Pulmonology 2006;41(10):954‐61. [DOI] [PubMed] [Google Scholar]
- Pedersen S, Garcia‐Garcia ML, Manjra I, Vermeulen H, Venter L, Engelstätter R. Ciclesonide and fluticasone propionate show comparable efficacy in children and adolescents with asthma. European Respiratory Journal 2004;24(Suppl 48):346s. [Google Scholar]
- Pedersen S, Gyurkovits K, Delft KHE, Boss H, Engelstätter R. Safety profile of ciclesonide as compared with fluticasone propionate in treatment of children and adolescents with asthma. European Respiratory Journal 2004;24(Suppl 48):346s. [Google Scholar]
- Pedersen S, Theron I, Engelstätter R. Ciclesonide is as effective as fluticasone propionate for treatment of children and adolescents with asthma. Respirology 2005;10(Suppl):A25. [Google Scholar]
Pedersen 2009 {published data only}
- Pedersen S, Engelstätter R, Weber HJ, Hirsch S, Barkai L, Emeryk A, et al. Efficacy and safety of ciclesonide once daily and fluticasone propionate twice daily in children with asthma. Pulmonary Pharmacology and Therapeutics 2009;22:214‐20. [DOI] [PubMed] [Google Scholar]
Vermeulen 2007 {published data only}
- Vermeulen JH, Gyurkovits K, Rauer H, Engelstätter R. Randomized comparison of the efficacy and safety of ciclesonide and budesonide in adolescents with severe asthma. Respiratory Medicine 2007;101(10):2182‐91. [DOI] [PubMed] [Google Scholar]
- Vermeulen JH, Kósa L, Villa JR, Rauer H, Wurst W, Engelstätter R. Effects of ciclesonide on lung function and cortisol excretion in adolescent asthma patients – a comparative study with budesonide. European Respiratory Journal 2006;28(Suppl 50):206s. [Google Scholar]
- Vermeulen JH, Minic P, Barkai L, Sreckovic MD, Gyurkovitis K, Rauer H, et al. Ciclesonide 320 mcg once daily is as effective as budesonide 800 mcg once daily and has a favourable safety profile in adolescent patients with persistent asthma. Proceedings of the American Thoracic Society International Conference; 2006 May 19‐24; San Diego.
von Berg 2007 {published data only}
- Berg A, Engelstatter R, Minic P, Sreckovic M, Garcia‐Garcia ML, Latos T, et al. Comparison of the efficacy and safety of ciclesonide 160 microg once daily versus budesonide 400 microg once daily in children with asthma. Pediatric Allergy and Immunology 2007;18(5):391‐400. [DOI] [PubMed] [Google Scholar]
- Berg A, Garcia‐Garcia ML, Herllbardt S, Bethke TD. Ciclesonide 160 ug once daily is as effective as budesonide 400 ug once daily in pediatric patients with persistent asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):s11. [Google Scholar]
- Berg A, Minic P, Sreckovic M, Bollroth C, Hellbardt S, Engelstätter R. Efficacy and safety of once‐daily ciclesonide 160 µg as compared with once‐daily budesonide 400 µg in paediatric asthma patients. Thorax 2005;2(Suppl II):ii43. [Google Scholar]
- Berg A, Vermeulen J, Manjra A, Weber H, Hellbardt S, Engelstätter R. Once‐daily ciclesonide 160 mcg is as effective as once‐daily budesonide 400 mcg in improving lung function in children with asthma and demonstrates a favorable safety profile. Paediatric Respiratory Reviews 2006;7:s283‐4. [Google Scholar]
- Berg A, Vermeulen JH, Zachgo W, Hellbardt S, Bethke TD, Engelstätter R. Comparison of ciclesonide 160 mcg/d with budesonide 400 mcg/d in a randomised, double‐blind study in children with moderate to severe asthma. European Respiratory Journal 2006;28(Suppl 50):711s. [Google Scholar]
References to studies excluded from this review
Adachi 2006 {published data only}
- Adachi M, Miyamoto T, Masuda K, Sakai T. Equal or superior efficacy of ciclesonide 320 μg or 640 μg as compared with CFC beclomethasone dipropionate 800 μg in the treatment of patients with moderate to severe asthma. Proceedings of the American Thoracic Society International Conference; 2006 May 19‐24; San Diego; A73.
Agertoft 2010 {published data only}
- Agertoft L, Pedersen S. Inhaled ciclesonide does not affect lower leg growth rate or HPA‐axis function in children with mild asthma. European Respiratory Journal 2004;24(Suppl 48):377s. [Google Scholar]
- Agertoft L, Pedersen S. Lower‐leg growth rates in children with asthma during treatment with ciclesonide and fluticasone propionate. Pediatric Allergy and Immunology 2010;21(1 pt 2):e199‐e205. [DOI] [PubMed] [Google Scholar]
- Agertoft L, Pedersen S. Short‐term lower‐leg growth rate and urine cortisol excretion in children treated with ciclesonide. Journal of Allergy and Clinical Immunology 2005;115(5):940‐5. [DOI] [PubMed] [Google Scholar]
- Agertorft L, Pedersen S. Lower‐leg growth rate and APA‐axis function in children with asthma during treatment with inhaled ciclesonide. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S119. [Google Scholar]
Bateman 2008 {published data only}
- Bateman D, Ketterer P, Pieters R, Dutchman D, Smau L, Engelstätter R. Efficacy and safety of ciclesonide compared with fluticasone propionate in patients with moderate to severe asthma. European Respiratory Journal 2006;28(Suppl 50):206. [Google Scholar]
- Bateman ED, Langan J, Vereecken G, Smau L, Engelstätter R. Efficacy and tolerability of ciclesonide compared with fluticasone propionate in patients with moderate‐to‐severe asthma. Proceedings of the American Thoracic Society International Conference; 2006 May 19‐24; San Diego; A103.
- Bateman ED, Linnhof AE, Homik L, Freudensprung U, Smau L, Engelstätter R. Comparison of twice‐daily inhaled ciclesonide and fluticasone propionate in patients with moderate‐to‐severe persistent asthma. Pulmonary Pharmacology and Therapeutics 2008;21(2):264‐75. [doi:10.1016/j.pupt.2007.05.002] [DOI] [PubMed] [Google Scholar]
Berger 2009 {published data only}
- Berger WE, Kerwin E, Bernstein DI, Pedinoff A, Bensch G, Karafilidis J. Efficacy and safety evaluation of ciclesonide in subjects with mild‐to‐moderate asthma not currently using inhaled corticosteroids. Allergy and Asthma Proceedings 2009;30(3):304‐14. [DOI] [PubMed] [Google Scholar]
BY9010/M1‐207 {unpublished data only}
- Alvesco. Efficacy and Safety – Study by ALTANA on Ciclesonide in Pre‐school Asthma Patients. www.clinicalstudyresults.org. (accessed 14 February 2011).
Cohen 2011 {published data only}
- Cohen J, Postma DS, Douma WR, Vonk JM, Boer AH, Hacken NH. Particle size matters: diagnostics and treatment of small airways involvement in asthma. European Respiratory Journal 2011;37:532‐40. [DOI] [PubMed] [Google Scholar]
Dahl 2010 {published data only}
- Dahl R, Trebas‐Pietras E, Kuna P, Engelstaetter R. Comparison of ciclesonide 80 μg and fluticasone propionate 176 μg in patients with mild to moderate asthma. Proceedings of the American Thoracic Society International Conference; 2008 May 16‐21; Toronto.
- Ronald Dahl R, Engelstätter R, Trębas‐Pietras E, Kuna P. A 24‐week comparison of low‐dose ciclesonide and fluticasone propionate in mild to moderate asthma. Respiratory Medicine 2010;104(8):1121‐30. [DOI] [PubMed] [Google Scholar]
Derom 2009 {published data only}
- Derom E, Louis R, Tiesler C, Engelstaetter R, Joos GF. Comparison of systemic and clinical effects of inhaled ciclesonide and fluticasone propionate in moderate to severe asthma. American Thoracic Society International Conference; 2008 May 16‐21; Toronto.
- Derom E, Louis R, Tiesler C, Engelstatter R, Kaufman JM, Joos GF. Effects of ciclesonide and fluticasone on cortisol secretion in patients with persistent asthma. European Respiratory Journal 2009;33(6):1277‐86. [DOI] [PubMed] [Google Scholar]
Dusser 2007 {published data only}
- Dusser D, McGoldrick H, Pieters WR, Luengo M, Hellwig M, Engelstatter R. Comparable efficacy of ciclesonide and fluticasone propionate and lower incidence of oropharyngeal candidiasis with ciclesonide in patients with well controlled moderate to severe persistent asthma. European Respiratory Journal 2007;30(Suppl 51):351s. [Google Scholar]
Erin 2008 {published data only}
- Erin EM, Zacharasiewicz AS, Nicholson GC, Tan AJ, Neighbour H, Engelstätter R, et al. Rapid effect of inhaled ciclesonide in asthma: a randomized, placebo‐controlled study. Chest 2008;134(4):740‐5. [DOI] [PubMed] [Google Scholar]
Gelfand 2006 {published data only}
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. Journal of Pediatrics 2006;148(3):377‐83. [DOI] [PubMed] [Google Scholar]
Hoshino 2010 {published data only}
- Hoshino M. Comparison of effectiveness in ciclesonide and fluticasone propionate on small airway function in mild asthma. Allergology International 2010;59(1):59‐66. [DOI] [PubMed] [Google Scholar]
Knox 2007 {published data only}
- Knox A, Langan J, Martinot JB, Gruss C, Höfner D. Comparison of a step‐down dose of once‐daily ciclesonide with a continued dose of twice‐daily fluticasone propionate in maintaining control of asthma. Current Medical Research and Opinion 2007;23(10):2387‐94. [DOI] [PubMed] [Google Scholar]
Kosztyla‐Hojna 2007 {published data only}
- Kosztyla‐Hojna B, Rutkowski R, Popko M. Vocal function of larynx in bronchial asthma patients treated with fluticasone propionate (FP) or ciclesonide (CIC). International Review of Allergology and Clinical Immunology 2007;13(2‐3):61‐8. [Google Scholar]
Malozowski 2008 {published data only}
- Malozowski S. Assessment of the long‐term safety of inhaled ciclesonide on growth in children with asthma. Pediatrics 2008;122(1):213. [DOI] [PubMed] [Google Scholar]
Matsunaga 2009 {published data only}
- Matsunaga K, Kawayama T, Toda R, Imamura Y, Hoshino T, Aizawa H. Effects of fluticasone and ciclesonide on pulmonary function and airway inflammation in stable mild asthmatics. Proceedings of the European Respiratory Society Annual Congress; 2009 Sep 12‐16; Vienna; 1971.
Meltzer 2009 {published data only}
- Meltzer EO, Korenblat PE, Weinstein SF, Noonan M, Karafilidis J. Efficacy and safety evaluation of ciclesonide in mild‐to‐moderate persistent asthma previously treated with inhaled corticosteroids. Allergy and Asthma Proceedings 2009;30(3):293‐303. [DOI] [PubMed] [Google Scholar]
Molen 2010 {published data only}
- Molen TVD, Foster JM, Caeser M, Muller T, Postma DS. Difference between patient‐reported side effects of ciclesonide versus fluticasone propionate. Respiratory Medicine 2010;104(12):1825‐33. [DOI] [PubMed] [Google Scholar]
Pedersen 2010 {published data only}
- Pedersen S, Potter P, Dachev S, Bosheva M, Kaczmarek J, Springer E, et al. Efficacy and safety of three ciclesonide doses vs placebo in children with asthma: the Rainbow study. Respiratory Medicine 2010;104(11):1618‐28. [DOI] [PubMed] [Google Scholar]
Postma 2011 {published data only}
- Postma DS, O'Byrne PM, Pedersen S. Comparison of the effect of low‐dose ciclesonide and fixed‐dose fluticasone propionate and salmeterol combination on long‐term asthma control. Chest 2011;139(2):311‐8. [DOI] [PubMed] [Google Scholar]
Skoner 2006 {published data only}
- Skoner D, Baena‐Cagnani E, Maspero J. Long term treatment with ciclesonide once daily has no effect on growth velocity skeletal maturity and HPA axis function in children with mild persistent asthma. European Respiratory Journal 2006;28(Suppl 50):711s. [Google Scholar]
Stoica 2010 {published data only}
- Stoica IG, Archip M, Babu M. Ciclesonide once daily (80 mg/d, 160 mg/d) versus fluticasone propionate twice daily (250 mg/d) in the treatment of patients with persistent asthma. Proceedings of the European Respiratory Society Annual Congress; 2010 Sep 18‐22; Barcelona; 3988.
van den Berge 2009 {published data only}
- Berge M, Arshad SH, Ind PW, Magnussen H, Hamelmann E, Kanniess F, et al. Similar efficacy of ciclesonide versus prednisolone to treat asthma worsening after steroid tapering. Respiratory Medicine 2009;103:1216‐23. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
BY9010/M1‐205 {published data only}
Additional references
Adams 2007
- Adams N, Lasserson TJ, Cates CJ, Jones PW. Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002310.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
American Thoracic Society 1987
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. American Review of Respiratory Disease 1987;136(1):225‐44. [DOI] [PubMed] [Google Scholar]
Brand 2001
- Brand PL. Inhaled corticosteroids reduce growth. European Respiratory Journal 2001;17(2):287‐94. [DOI] [PubMed] [Google Scholar]
British Thoracic Society 2011
- British Thoracic Society. British Guideline on the Management of Asthma: A National Clinical Guideline, 2011. www.sign.ac.uk/guidelines/fulltext/101/index.html. (accessed 14 December 2012).
Chapman 2008
- Chapman KR. Safer inhaled corticosteroid therapy for asthma. Pediatrics 2008;121(1):179‐80. [DOI] [PubMed] [Google Scholar]
Crowley 1991
- Crowley S, Hindmarsh PC, Holownia P, Honour JW, Brook CG. The use of low doses of ACTH in the investigation of adrenal function in man. Journal of Endocrinology 1991;130(3):475‐9. [DOI] [PubMed] [Google Scholar]
Dahl 2006
- Dahl R. Ciclesonide for the treatment of asthma. Therapeutics and Clinical Risk Management 2006;2(1):25‐37. [PMC free article] [PubMed] [Google Scholar]
De Vries 2009
- Vries TW, Rottier BL, Gjaltema D, Hagedoorn P, Frijlink HW, Boer AH. Comparative in vitro evaluation of four corticosteroid metered dose inhalers: consistency of delivered dose and particle size distribution. Respiratory Medicine 2009;103(8):1167‐73. [DOI] [PubMed] [Google Scholar]
Eijkemans 2011
- Eijkemans M, Ottenen BJ, Yntema JB. Adrenal cortex insufficiency in children due to inhaled corticosteroids. Nederlands tijdschrift voor Geneeskunde 2011;155:A2862. [PubMed] [Google Scholar]
Gelfand 2009
- Gelfland EW, Kraft M. The importance of features of the distal airways in children and adults. Journal of Allergy and Clinical Immunology 2009;124(6 suppl):S84‐7. [DOI] [PubMed] [Google Scholar]
GINA 2003
- Global Initiative for Asthma (GINA) National Institutes of Health (NIH), National Heart, Lung and Blood Institute. Global strategy for asthma management and prevention, 2003. www.ginasthma.org/Guidelines/guidelines‐archived‐2003‐update‐workshop‐report.html. (accessed 14 December 2012).
GINA 2011
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2011. www.ginasthma.com. (accessed 14 December 2012).
Guest 2005
- Guest JF, Davie AM, Ruiz FJ, Greener MJ. Switching asthma patients to a once‐daily inhaled steroid improves compliance and reduces healthcare costs. Primary Care Respiratory Journal 2005;14(2):88‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamid 1997
- Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, Hegele RG, et al. Inflammation of small airways in asthma. Journal of Allergy and Clinical Immunology 1997;100(1):44‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011a
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lahzami 2008
- Lahzami S, King GG. Targeting small airways in asthma: the new challenge of inhaled corticosteroid treatment. European Respiratory Journal 2008;31(6):1145‐7. [DOI] [PubMed] [Google Scholar]
Lasserson 2006
- Lasserson TJ, Cates CJ, Jones AB, Lasserson EH, White J. Fluticasone versus HFA‐beclomethasone dipropionate for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005309.pub3] [DOI] [PubMed] [Google Scholar]
Lipworth 1999
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta‐analysis. Archives of Internal Medicine 1999;159(9):941‐55. [DOI] [PubMed] [Google Scholar]
Manning 2008
- Manning PJ, Gibson PG, Lasserson TJ. Ciclesonide versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manning 2009
- Manning P, Gibson PG, Lasserson TJ. Ciclesonide versus other inhaled steroids for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R. Developed for Global Initiative for Asthma (GINA). Allergy 2004;59:469‐78. [Full report available on www.ginasthma.com] [DOI] [PubMed] [Google Scholar]
Osterberg 2005
- Osterberg L, Blaschke T. Adherence to medication. New England Journal of Medicine 2005;353(5):487‐97. [DOI] [PubMed] [Google Scholar]
Peters 2006
- Peters SP. Safety of inhaled corticosteroids in the treatment of persistent asthma. Journal of the National Medical Association 2006;98(6):851‐61. [PMC free article] [PubMed] [Google Scholar]
Price 2010
- Price D, Robertson A, Bullen K, Rand C, Horne R, Staudinger H. Improved adherence with once‐daily versus twice‐daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open‐label study. BMC Pulmonary Medicine 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2011a
- Schunemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Skoner 2008
- Skoner DP, Maspero J, Banerji D. Assessment of the long‐term safety of inhaled ciclesonide on growth in children with asthma. Pediatrics 2008;121(1):e1‐14. [DOI] [PubMed] [Google Scholar]
Soonawala 2010
- Soonawala D, Middelburg RA, Egger M, Vandenbroucke JP, Dekkers OM. Efficacy of experimental treatments compared with standard treatments in non‐inferiority trials: a meta‐analysis of randomized controlled trials. International Journal of Epidemiology 2010;39:1567‐81. [DOI] [PubMed] [Google Scholar]
Todd 2002
- Todd GRG, Acerini CL, Ross‐Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Archives of Disease in Childhood 2002;87:457‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Usmani 2005
- Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2‐agonist particle size. American Journal of Respiratory Critical Care Medicine 2005;172(12):1497‐504. [DOI] [PubMed] [Google Scholar]
Zhang 2011
- Zhang L, Axelsson I, Chung M, Lau J. Dose response of inhaled corticosteroids in children with persistent asthma: a systematic review. Pediatrics 2011;127(1):129‐38. [DOI] [PubMed] [Google Scholar]


