Abstract
A central event in Alzheimer's disease is the conformational change from normally circulating soluble amyloid beta peptides (A beta) and tau proteins into amyloid fibrils, in the form of senile plaques and neurofibrillary tangles respectively. The apolipoprotein E (apoE) gene locus has recently been associated with late-onset Alzheimer's disease. It is not know whether apoE plays a direct role in the pathogenesis of the disease. In the present work we have investigated whether apoE can affect the known spontaneous in vitro formation of amyloid-like fibrils by synthetic A beta analogues using a thioflavine-T assay for fibril formation, electron microscopy and Congo Red staining. Our results show that, under the conditions used, apoE directly promotes amyloid fibril formation, increasing both the rate of fibrillogenesis and the total amount of amyloid formed. ApoE accelerated fibril formation of both wild-type A beta-(1-40) and A beta-(1-40A), an analogue created by the replacement of valine with alanine at residue 18, which alone produces few amyloid-like fibrils. However, apoE produced only a minimal effect on A beta-(1-40Q), found in the Dutch variant of Alzheimer's disease. When recombinant apoE isoforms were used, apoE4 was more efficient than apoE3 at enhancing amyloid formation. These in vitro observations support the hypothesis that apoE acts as a pathological chaperone, promoting the beta-pleated-sheet conformation of soluble A beta into amyloid fibres, and provide a possible explanation for the association of the apoE4 genetic isoform with Alzheimer's disease.
Full text
PDF
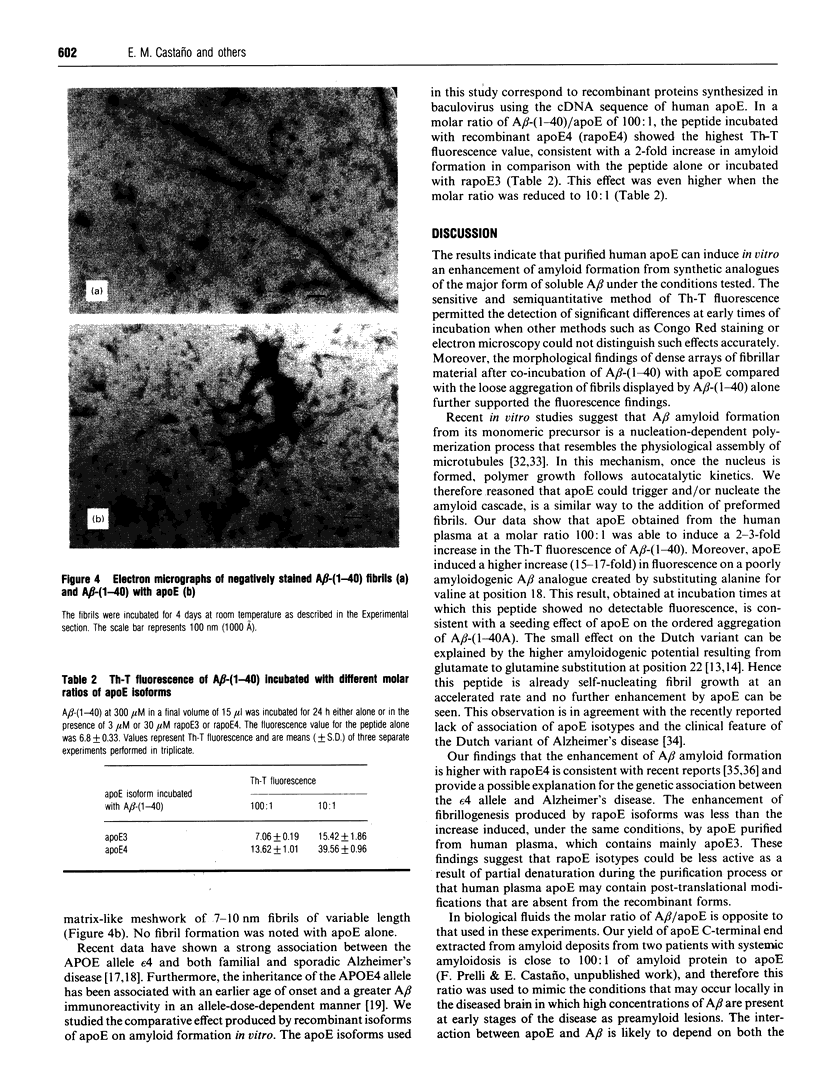


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busciglio J., Gabuzda D. H., Matsudaira P., Yankner B. A. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Castaño E. M., Ghiso J., Prelli F., Gorevic P. D., Migheli A., Frangione B. In vitro formation of amyloid fibrils from two synthetic peptides of different lengths homologous to Alzheimer's disease beta-protein. Biochem Biophys Res Commun. 1986 Dec 15;141(2):782–789. doi: 10.1016/s0006-291x(86)80241-8. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Clements A., Walsh D. M., Williams C. H., Allsop D. Effects of the mutations Glu22 to Gln and Ala21 to Gly on the aggregation of a synthetic fragment of the Alzheimer's amyloid beta/A4 peptide. Neurosci Lett. 1993 Oct 14;161(1):17–20. doi: 10.1016/0304-3940(93)90129-9. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Fabian H., Szendrei G. I., Mantsch H. H., Otvos L., Jr Comparative analysis of human and Dutch-type Alzheimer beta-amyloid peptides by infrared spectroscopy and circular dichroism. Biochem Biophys Res Commun. 1993 Feb 26;191(1):232–239. doi: 10.1006/bbrc.1993.1207. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Inouye H., Surewicz W. K., Selkoe D. J., Podlisny M. B., Kirschner D. A. Fibril formation by primate, rodent, and Dutch-hemorrhagic analogues of Alzheimer amyloid beta-protein. Biochemistry. 1992 Nov 10;31(44):10716–10723. doi: 10.1021/bi00159a011. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Wisniewski T., Vidal R., Rostagno A., Frangione B. Epitope map of two polyclonal antibodies that recognize amyloid lesions in patients with Alzheimer's disease. Biochem J. 1992 Mar 1;282(Pt 2):517–522. doi: 10.1042/bj2820517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Haan J., Van Broeckhoven C., van Duijn C. M., Voorhoeve E., van Harskamp F., van Swieten J. C., Maat-Schieman M. L., Roos R. A., Bakker E. The apolipoprotein E epsilon 4 allele does not influence the clinical expression of the amyloid precursor protein gene codon 693 or 692 mutations. Ann Neurol. 1994 Sep;36(3):434–437. doi: 10.1002/ana.410360315. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Levy E., Carman M. D., Fernandez-Madrid I. J., Power M. D., Lieberburg I., van Duinen S. G., Bots G. T., Luyendijk W., Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990 Jun 1;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Ma J., Yee A., Brewer H. B., Jr, Das S., Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994 Nov 3;372(6501):92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Papayannopoulos I. A., Styles J., Bobin S. A., Lin Y. Y., Biemann K., Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993 Feb 15;301(1):41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- Naiki H., Higuchi K., Nakakuki K., Takeda T. Kinetic analysis of amyloid fibril polymerization in vitro. Lab Invest. 1991 Jul;65(1):104–110. [PubMed] [Google Scholar]
- Namba Y., Tomonaga M., Kawasaki H., Otomo E., Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991 Feb 8;541(1):163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Nathan B. P., Bellosta S., Sanan D. A., Weisgraber K. H., Mahley R. W., Pitas R. E. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994 May 6;264(5160):850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991 Aug 14;207(4):367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Prelli F., Pras M., Frangione B. Degradation and deposition of amyloid AA fibrils are tissue specific. Biochemistry. 1987 Dec 15;26(25):8251–8256. doi: 10.1021/bi00399a035. [DOI] [PubMed] [Google Scholar]
- Rebeck G. W., Reiter J. S., Strickland D. K., Hyman B. T. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993 Oct;11(4):575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E., Saunders A. M., Strittmatter W. J., Crain B. J., Hulette C. M., Joo S. H., Pericak-Vance M. A., Goldgaber D., Roses A. D. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzman A. L., Gregori L., Vitek M. P., Lyubski S., Strittmatter W. J., Enghilde J. J., Bhasin R., Silverman J., Weisgraber K. H., Coyle P. K. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Smyrk T. C. Colon cancer connections. Cancer syndrome meets molecular biology meets histopathology. Am J Pathol. 1994 Jul;145(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Weisgraber K. H., Goedert M., Saunders A. M., Huang D., Corder E. H., Dong L. M., Jakes R., Alberts M. J., Gilbert J. R. Hypothesis: microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp Neurol. 1994 Feb;125(2):163–174. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigushin D. M., Gough J., Allan D., Alguacil A., Penner B., Pettigrew N. M., Quinonez G., Bernstein K., Booth S. E., Booth D. R. Familial nephropathic systemic amyloidosis caused by apolipoprotein AI variant Arg26. Q J Med. 1994 Mar;87(3):149–154. [PubMed] [Google Scholar]
- Whitson J. S., Mims M. P., Strittmatter W. J., Yamaki T., Morrisett J. D., Appel S. H. Attenuation of the neurotoxic effect of A beta amyloid peptide by apolipoprotein E. Biochem Biophys Res Commun. 1994 Feb 28;199(1):163–170. doi: 10.1006/bbrc.1994.1209. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Castano E., Ghiso J., Frangione B. Cerebrospinal fluid inhibits Alzheimer beta-amyloid fibril formation in vitro. Ann Neurol. 1993 Oct;34(4):631–633. doi: 10.1002/ana.410340422. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Ghiso J., Frangione B. Alzheimer's disease and soluble A beta. Neurobiol Aging. 1994 Mar-Apr;15(2):143–152. doi: 10.1016/0197-4580(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Ghiso J., Frangione B. Peptides homologous to the amyloid protein of Alzheimer's disease containing a glutamine for glutamic acid substitution have accelerated amyloid fibril formation. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1247–1254. doi: 10.1016/0006-291x(91)91706-i. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Golabek A., Matsubara E., Ghiso J., Frangione B. Apolipoprotein E: binding to soluble Alzheimer's beta-amyloid. Biochem Biophys Res Commun. 1993 Apr 30;192(2):359–365. doi: 10.1006/bbrc.1993.1423. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Lalowski M., Levy E., Marques M. R., Frangione B. The amino acid sequence of neuritic plaque amyloid from a familial Alzheimer's disease patient. Ann Neurol. 1994 Feb;35(2):245–246. doi: 10.1002/ana.410350223. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]