Abstract
Purpose:
Mpox is a rare infectious disease. Lack of knowledge among eye care professionals regarding mpox keratitis greatly reduces the likelihood of diagnosis and effective management. This report and review seek to increase the knowledge of mpox keratitis among eye care professionals.
Methods:
We report a patient with mpox keratitis who underwent successful penetrating keratoplasty, with 20 years of follow-up. A systematic literature search and review of cases of mpox keratitis from 1970 to 2024 was performed.
Results:
A total of 24 articles and 2 abstracts reporting 35 cases of mpox keratitis were identified. A frequency of 0.5% to 1.0% may be the lower range of mpox keratitis among symptomatic patients with a confirmed mpox diagnosis. Mpox keratitis occurred with and without systemic mpox. Initial misdiagnoses were common (40%). Polymerase chain reaction results aided clinical diagnosis. Corneal disease ranged from mild epitheliopathy to fulminant ulcerative keratitis. Outcomes ranged from 20/20 acuity to no light perception. In the absence of fulminant systemic disease, tecovirimat was associated with clinical improvement of mpox keratitis in almost all cases. Our case is the only known report of successful penetrating keratoplasty for mpox keratitis and the only case whereby monkeypox virus was cultured from the corneal surface.
Conclusions:
Mpox keratitis is rare but can result in severe vision loss and blindness. Systemic tecovirimat seems to be effective in treating mpox keratitis, although the low frequency of keratitis precludes clinical trials. Topical steroids may extend virus survival in the cornea. Polymerase chain reaction may help confirm mpox corneal involvement.
Key Words: mpox, mpox ocular disease, mpox keratitis, mpox related ocular disease
Mpox is a systemic disease caused by monkeypox virus (MPXV), an orthopoxvirus related to variola virus, the causative agent of smallpox.1 Ocular involvement can result in vision loss and blindness, typically from keratitis.2 The infrequency of severe ocular involvement and the absence of randomized clinical trials require that treatment strategies draw from past experiences with smallpox and vaccinia, case reports from the recent mpox outbreak, and knowledge from other viral ocular conditions.3–5
To collate and organize the literature on mpox keratitis, we reviewed all English-language publications on human mpox from 1970 through 2023. We report the only known successful penetrating keratoplasty for mpox keratitis, which occurred during the 2003 US outbreak and reinterpret the clinical findings and course considering reports from the 2022–2023 outbreak. Features of mpox keratitis are summarized, and diagnostic and treatment considerations are discussed.
METHODS
Report of a Case
The first mpox outbreak outside of Africa occurred in 2003 in the United States.6 The patient described herein was a confirmed case.6,7 An immunocompetent 32-year-old veterinarian developed tender cervical lymphadenopathy and right eye (OD) conjunctivitis 12 days after an mpox-infected prairie dog was treated at her workplace. She was not involved in the animal's care but handled the leather gloves contaminated by the animal and potentially inoculated her eye during surgical mask donning.
On day 2 after symptom onset, she presented to an urgent care center for eyelid swelling and was treated for conjunctivitis with oral amoxicillin. On day 3, topical ofloxacin was added and she was referred to an ophthalmologist. On day 4, ophthalmic examination noted 20/30 visual acuity (VA) OD, with severe lid swelling and conjunctival injection. The cornea was clear, and the anterior chamber was without inflammation. The left eye was normal with 20/20 VA and remained unaffected. Swab specimens from the OD conjunctiva and a skin lesion (Fig. 1A) were sent on day 5 for culture and polymerase chain reaction (PCR) to the Poxvirus Section of the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. PCR and viral culture were MPXV-positive, and she was quarantined per public health protocols.
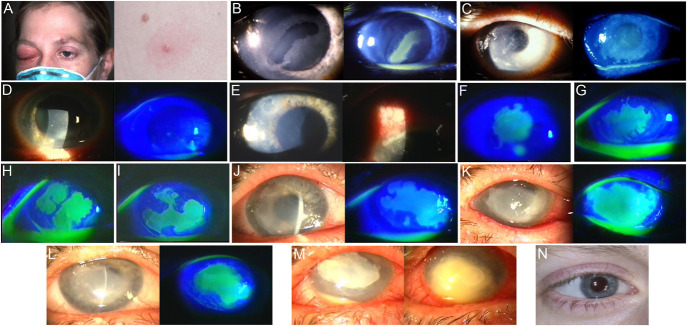
FIGURE 1.
Clinical progression of case patient ocular findings. A, Day 2 after symptom onset with right eyelid swelling and left eyebrow and back mpox lesions. B, Day 27, on referral to cornea service with a new corneal epithelial defect. C, Day 41, showing an enlarged irregular epithelial defect. D, Day 48, a week after increasing prednisolone acetate; the epithelial defect was almost resolved. E, Day 52, new limbitis with an associated corneal epithelial defect. F, Day 55, enlargement of the corneal epithelial defect. G–I, Over the following 2 weeks (days 62–76) with changing geographic epithelial ulcers. J, Day 88, pan-cultures, PCR, and lateral tarsorrhaphy performed. K, Day 100, initiation of trifluridine after positive MPXV culture, increasing stromal opacification. L, Day 119, trifluridine stopped after 2 consecutive weeks of negative MPXV cultures, with the epithelial defect persistent since day 50. M, Day 132, new-onset hypopyon with stromal infiltrate grows Streptococcus pneumoniae; corneal perforation occurs despite appropriate response to antibiotics. N, 20 years later with a clear corneal graft.
On day 18, fever, malaise, lymphadenopathy, and pox lesions were resolved, but she had worsening eye discomfort and VA of 20/50. Slit-lamp examination revealed a corneal arcuate immune line, punctate epithelial fluorescein staining, and mild iritis. Prednisolone acetate 1% qid and a cycloplegic were initiated. On day 20, keratic precipitates (KP) were noted and PA1% was increased to q2 hours. On day 25, VA was 20/100 with a central corneal epithelial defect, and she was referred to the cornea service.
On day 27, the initial cornea service examination, VA was 20/400, with 3+ lid edema, a peripheral corneal immune ring, and central corneal epithelial defect (Fig. 1B). A bandage contact lens (BCL) was placed, with PA1% qid and atropine 1% daily. A topical antibiotic was used throughout the treatment.
On day 30, the epithelial defect and stromal inflammation were unchanged; PA1% and atropine were stopped, and oral prednisone 60 mg daily was begun to minimize topical medications and attempt better inflammation control. On day 41, the epithelial defect had enlarged (Fig. 1C) and significant stromal inflammation remained, so PA1% q2 hours was resumed. Corneal surface specimens were sent to CDC. MPXV culture was negative, and MPXV PCR remained positive.
On day 48, the epithelium had a small defect, with less stromal inflammation (Fig. 1D). PA1% was reduced to q3 hours, but 4 days later, limbitis developed (Fig. 1E), so PA1% was increased. This reduced pain and inflammation, but the associated epithelial defect enlarged (Fig. 1F). Preservative-free PA1% was obtained to reduce preservative toxicity and used thenceforth. In the following 2 weeks, stromal inflammation remained stable, but geographic epithelial defects developed with changing morphology at each visit (Figs. 1G–I). Another BCL was placed, without improvement. The BCL was removed, and taped lid closure was performed for 5 days, without improvement.
On day 88 (Fig. 1J), a 50% tarsorrhaphy was performed with corneal scraping for bacterial and Acanthamoeba culture and PCR and culture for viruses (MPXV, herpes simplex virus, and varicella zoster virus). On day 96, all results were negative except for MPXV detection, which was culture- and PCR-positive. Confirmatory scrapings on day 97 for MPXV culture and PCR were also positive.
On day 100, topical trifluridine 6x daily was initiated. Tecovirimat did not yet exist. Preservative-free PA1% was decreased to bid but not discontinued because of pain and worsening stromal opacification (Fig. 1K). Trifluridine was used for 16 days, with weekly corneal swabs sent to CDC for MPXV culture and PCR. On day 109, culture was negative (PCR remained positive). On day 116, trifluridine was discontinued after 2 weeks of negative cultures. From day 50, until subsequent keratoplasty, an epithelial defect and stromal inflammation persisted (Fig. 1L).
On day 130, after 4 weeks of negative MPXV cultures (PCR remained positive), the weekly surveillance culture became positive and trifluridine was restarted. Days later, Streptococcus pneumoniae keratitis developed (Fig. 1M). Steroids were stopped, and topical vancomycin was started. Trifluridine was continued for 15 days; vancomycin was tapered as the infiltrate improved, but perforation occurred requiring tectonic penetrating keratoplasty on day 145. Postoperatively, standard (with preservatives) PA1% and antibiotic were administered, but not trifluridine. A week after keratoplasty, the eye showed minimal inflammation and intact epithelium. Subsequent recovery was uneventful. The corneal graft remains clear after 20 years with 20/20 acuity (Fig. 1N).
Literature Reviewed
A systematic literature search was performed on PubMed, Embase, and EBSCO to identify published reports of mpox keratitis from 1970 to January 5, 2024 (Table 1). Articles were excluded if they were not available in English or did not provide either a description or photograph of mpox keratitis with a clinical history. Abstracts and text were reviewed and excluded if there were no reports of keratitis. In total, 24 articles and 2 abstracts reporting 35 cases of mpox keratitis were identified and their results summarized (Table 2).
TABLE 1.
Search Terms
| Database | Search Terms |
| PubMed | (“Monkeypox virus” [Mesh] OR “Monkeypox” [Mesh] OR monkeypox [tw] OR mpox [tw] OR “monkeypox virus*” [tw] OR “mpox virus*” [tw]) AND (“eye diseases” [Mesh] OR “Ophthalmology” [Mesh] OR ophthalmology [tw] OR “eye disease*” [tw] OR “eye lesion*” [tw] OR “ocular infection*” [tw] OR “ocular inflammation*” [tw] OR uveitis[tw] OR scleritis [tw] OR conjunctivitis [tw] OR keratitis [tw] OR “corneal ulcer” [tw] OR eye [tw] OR “monkeypox-related ophthalmic disease” [tw] OR MPXROD [tw]) |
| EBSCO | (“monkeypox”/exp OR “monkeypox” OR “monkeypox virus”/exp OR “monkeypox virus”) AND (“ophthalmology”/exp OR “ophthalmology” OR “eye disease” OR “eye injury” OR “eye infection’ OR “eye inflammation” OR “uveitis” OR “scleritis” OR “conjunctivitis” OR “keratitis” OR “cornea ulcer”) |
| Embase | (Monkeypox OR mpox OR monkeypox virus OR mpox virus) AND (ophthalmology OR eye disease OR eye lesion OR ocular infection OR ocular inflammation OR uveitis OR scleritis OR conjunctivitis OR keratitis OR corneal ulcer OR eye OR monkeypox-related ophthalmic disease OR MPXROD) |
TABLE 2.
Reported Cases of Mpox Keratitis
| Year | Reference | Age, Sex | Key Corneal Findings | Nonocular MPOX Lesions | Ocular PCR | Immune Compromise | Topical Therapy | Systemic Antivirals or Corticosteroid | Final Acuity, Outcome |
| Published manuscripts | |||||||||
| 2022 | Lamas-Francis et al8 | 45 yr Male |
Geographic epithelial ulcer Limbitis AC cell |
Genital Perioral |
+MPXV Cornea, conjunctiva |
HIV+ On ART “poor adherence” CD4 873 cells/mm3 |
Ganciclovir Povidone iodine Moxifloxacin |
PO-tecovirimat | 20/25 Resolution with faint subepithelial haze |
| 2022 | Cash-Goldwasser et al9 | 20–29 yr Male |
Peripheral keratitis | Buttocks Chest Arms Hands Face |
+OPXV Conjunctiva |
HIV+ Off ART CD4 25 cells/mm3 |
Trifluridine | PO-tecovirimat (failed) IV-tecovirimat PO-tecovirimat (failed) IV-tecovirimat |
20/800 “Profound visual impairment" |
| 30–39 yr Male |
Nonspecific epithelial defect/ulcer | Chest Legs Perianal Face Medial canthus |
+OPXV Face/scalp |
HIV+ Off ART CD4 78 cells cells/mm3 |
Trifluridine Antibacterial drops |
PO-tecovirimat (recurred) PO-tecovirimat |
“Regression of the eye lesion” | ||
| 2022 | Bacorn et al10 | 47 yr Male |
Perforation Fulminant disease |
Extremities Trunk Face Eyelids Fulminant systemic disease |
No info | HIV + Off ART CD4 28 cells/mm3 |
Trifluridine Moxifloxacin |
Tecovirimat | No light perception Death |
| 2022 | Alexis et al11 | 38 yr Male |
Superficial punctate epithelial erosions | Anogenital Upper limb(s) Lower limb(s) |
+MPXV Conjunctiva |
No | Chloramphenicol | PO-tecovirimat | Acuity not reported Resolution |
| 2023 | Vasquez-Perez et al12 | 63 yr Male |
Corneal edema Geographic epithelial ulcer Stromal keratitis |
Face Arms Back Groin |
+MPXV Conjunctiva |
HIV+ On ART CD4 495 cells/mm3 |
Trifluridine Moxifloxacin Chloramphenicol Dexamethasone |
PO-tecovirimat | Count fingers “Corneal scarring with a negative effect on vision.” |
| 2023 | Finamor et al13 | 27 yr Male |
Serpiginous/arcuate epithelial keratitis Stromal infiltrates Keratic precipitates AC cell |
Genitals Face Upper back Hands Feet Eyelid |
+MPXV “Eye swab” |
No | Sodium hyaluronate Moxifloxacin Fluorometholone |
PO-valacyclovir (progressed) PO-tecovirimat |
20/30 Resolution with “superficial corneal opacities and residual keratic precipitates” |
| 2023 | Doan et al14 | 34 yr Male |
Serpiginous/arcuate epithelial keratitis | Penis Perineum Eyelid |
+MPXV Cornea |
No | Ofloxacin Dexamethasone Trifluridine |
PO-valacyclovir (failed) Prednisone (self-admin) |
“Normal” vision Resolution with limbal neovascularization |
| 30 yo Male |
Limbitis Serpiginous/arcuate epithelial keratitis |
Penis Eyelid |
+MPXV Cornea |
No | Ganciclovir Dexamethasone Trifluridine |
IV-cidofovir (progressed) IV-cidofovir PO-tecovirimat |
20/32 Linear corneal scar, limbal stem cell deficiency, corneal neovascularization, hazy epithelium |
||
| 2023 | Alsarhani et al15 | 36 yr Male |
Serpiginous/arcuate epithelial keratitis disciform edema Keratic precipitates AC cell |
Face Scalp Torso Genitals Arms Legs |
+MPXV Conjunctiva |
No | Prednisolone | PO-tecovirimat | 20/40 “Small subepithelial corneal opacity” |
| 2023 | Androudi et al16 | 50 yr Male |
Serpiginous/arcuate epithelial keratitis geographic epithelial ulcer AC cell |
Eyelid Body |
+MPXV Tears |
HIV + “High…viral load” |
Artificial tears Moxifloxacin Cyclopentolate Dexamethasone PF-dexamethasone Levofloxacin |
PO-valacyclovir (worsened) PO- tecovirimat |
20/200 “Hazy cornea” |
| 31 yr Male |
Keratic precipitates Geographic epithelial ulcer AC cell |
Face Eyelid Neck |
+MPXV Tears |
No | Dexamethasone “antiglaucoma drops” Vitamin A ointment Trifluridine Autologous serum |
PO-valacyclovir (worsened) PO- tecovirimat |
20/20 Resolution |
||
| 2023 | Bhamray-Sanchez et al17 | 28 yr Male |
Stromal keratitis Peripheral ulcerative keratitis Nonspecific opacification |
Penis Back Shoulder |
+OPXV Cornea |
HIV + On ART CD4 951 cells/mm3 |
Cyclopentolate Erythromycin Trifluridine |
PO-valacyclovir (worsened) PO-tecovirimat |
Hand motions “Keratitis and ulceration healed” |
| 36 yr Male |
Stromal keratitis | Face | +OPXV Cornea |
HIV status not reported Initiated ART during treatment course |
Moxifloxacin Vancomycin Tobramycin Trifluridine Tobramycin Prednisolone Erythromycin |
PO-valacyclovir PO- tecovirimat |
“Vision did not improve” “Photophobia, tearing, and eye pain resolved after 6 wk of treatment” |
||
| 2023 | Carrubba et al18 | 33 yr Male |
Perforation Fulminant disease |
Face Torso Extremities Fulminant systemic disease |
+OPXV Crystalline lens (possible eyelid contamination) |
HIV+ Off ART CD4 55 cells/mm3 |
Not reported | PO-tecovirimat IV-tecovirimat Vaccinia IVIG |
Bilateral globe rupture Death |
| 2023 | Quites et al19 | 32 yr Male |
Serpiginous/arcuate epithelial keratitis Geographic epithelial ulcer Immune ring |
Genital Body |
+MPXV Cornea, conjunctiva |
No | Ciprofloxacin Gatifloxacin Diclofenac |
PO-Acyclovir (worsened) IV-Acyclovir (worsened) PO-tecovirimat |
20/100 |
| 2023 | Nguyen et al20 | 53 yr Male |
Keratic precipitates Geographic epithelial ulcer Limbitis Perforation Fulminant disease |
None (semen deposition into affected eye) | +MPXV (RNA sequence) Aqueous +MPXV Cornea Sclera Conjunctiva |
Lymphopenia Chronic lymphocytic leukemia |
Erythromycin ointment Moxifloxacin Prednisolone Voriconazole (route not specified) Trifluridine Betadine |
PO-valacyclovir (worsened) PO-prednisone (worsened) PO-tecovirimat (worsened) IV-tecovirimat IV-cidofovir |
Light perception Opacified vascularized cornea |
| 2023 | Pazos et al21 | 24 yr Male |
Geographic epithelial ulcer Peripheral ulcerative keratitis |
Genital Proctitis |
+MPXV Conjunctiva |
No | 0.6% Povidone iodine Erythromycin ointment Lubrication |
PO-tecovirimat | “No significant vision loss” Peripheral corneal scarring |
| 36 yr Male |
Serpiginous/arcuate epithelial keratitis Stromal keratitis AC cell |
Genital | +MPXV Conjunctiva |
HIV On ART |
0.6% Povidone iodine Erythromycin ointment Lubrication |
PO-tecovirimat | No vision loss | ||
| 46 yr Male |
Nonspecific epithelial defect/ulcer | Chest Peri-rectal |
+MPXV Conjunctiva |
No | 0.6% Povidone iodine Erythromycin ointment Dexamethasone Lubrication |
PO-tecovirimat | No vision loss | ||
| 35 yr Male |
Nonspecific epithelial defect Stromal keratitis Endotheliitis |
Skin | +MPXV Conjunctiva |
No | 0.6% Povidone iodine Erythromycin ointment Dexamethasone Lubrication |
PO-tecovirimat | No vision loss | ||
| 2023 | Uner et al22 | 47 yr Male |
Geographic epithelial ulcer | Groin Back Feet Face Eyelid |
+OPXV Conjunctiva |
No | Artificial tears Erythromycin Moxifloxacin Prednisolone |
PO-tecovirimat | 20/25 Resolution with symblepharon |
| 2023 | Raccagni et al23 | 35 yr Male |
Geographic epithelial ulcer Stromal infiltrate |
Hand | +MPXV Conjunctiva +MPXV Conjunctiva (after relapse) +MPXV Conjunctiva (after s relapse) |
No | Moxifloxacin Tobramycin Polymyxin “Topical steroids” Dexamethasone |
PO-Acyclovir PO-tecovirimat (relapsed) PO-tecovirimat (relapsed) PO-tecovirimat IV-cidofovir |
20/30 Resolution with stromal opacity |
| 2023 | Ditta et al24 | 8 yr Female |
Limbitis Stromal infiltrate |
Eyelid | Eye not tested (skin lesions +MPXV) | No | Erythromycin Trifluridine |
IV-Acyclovir Clindamycin PO-tecovirimat |
Resolution with “subepithelial and stromal infiltrates” |
| 2023 | Arteaga-Rivera et al25 | 24 yr Female |
Serpiginous/arcuate epithelial keratitis AC cell |
Hands Face Armpits Abdomen Right foot |
+MPXV Cornea, conjunctiva |
No | Prednisolone acetate Moxifloxacin Interferon alpha 2b Prednisolone acetate Tropicamide/phenylephrine |
PO-prednisone PO-Acyclovir |
20/30 Resolution |
| 2023 | Rodríguez-Badillo et al26 | 19 yr Male |
Corneal edema Stromal infiltrate Peripheral ulcerative keratitis |
Face Chest Abdomen |
+MPXV Cornea, conjunctiva |
Not reported | “Topical analgesics” “Antibiotics” “Topical corticosteroids” Interferon alfa-2b |
PO-corticosteroids | “Decreased visual acuity” |
| 2023 | Bloom et al27 | 38 yr Male |
Superficial punctate epithelial erosions | Eyelid Face |
Eye sample error | HIV+ ART/immune status not reported |
Moxifloxacin | Acyclovir Azithromycin Tecovirimat |
Lost to follow-up |
| 2023 | Oprea et al28 | 42 yr Male |
Nonspecific epithelial defect/ulcer | Face Limbs Inguinal area Scrotum |
+MPXV Conjunctiva |
HIV+ on ART | “Ocular drops prescribed by the ophthalmologist” | Tecovirimat | Not reported |
| 2023 | Trawally Flores et al29 | 37 yr Male |
Peripheral ulcerative keratitis | Hands Feet Chest |
+MPXV Conjunctiva |
No | Tobramycin Dexamethasone Artificial tears Povidone iodine Ozonized oil Ofloxacin Ganciclovir Serum tears Fluorometholone |
PO-tecovirimat | 20/200 Corneal leukoma, symblepharon |
| 2023 | Malciolu-Nica et al30 | 41 yr Male |
Peripheral ulcerative keratitis | Skin Oral |
+MPXV “Eye lesions” |
HIV+ ART/immune status not reported |
Chloramphenicol Dexamethasone Lubricants Acyclovir Antibiotics Mydriatic |
“Systemic” tecovirimat | 20/25 Corneal scarring, peripheral pannus |
| 2023 | Avila and Gallego-Suarez31 | 24 yr Male |
Limbitis AC cell Corneal edema |
Eyelid | +MPXV Conjunctiva |
No | Ganciclovir Lubrication Prednisolone Ice |
IV-Acyclovir IV-ceftriaxone |
20/50 Resolution |
| 2024 | This report | 32 yr Female |
AC cell with keratic precipitates Geographic epithelial ulcer Limbitis Stromal keratitis |
Eyebrow Torso Limbs |
+MPXV Cornea |
No | Ofloxacin Mydriatic Moxifloxacin Prednisolone acetate Trifluridine |
PO-prednisone | 20/20 Stromal opacification, secondary bacterial keratitis, perforation Penetrating keratoplasty |
| Abstracts | |||||||||
| 2023 | Cuppari et al32 | 28 yr Male |
Peripheral ulcerative keratitis Stromal keratitis |
Not reported | +MPXV Cornea |
HIV+, syphilis | Trifluridine | “Systemic” tecovirimat | Count fingers at 2 feet “Healed ulcer” |
| 36 yr Male |
Nonspecific epithelial defect/ulcer Stromal infiltrate |
Not reported | +MPXV Cornea |
HIV+, latent TB, syphilis | Trifluridine | PO-tecovirimat | 20/70 Subepithelial and stromal opacities |
||
| 2023 | Carrubba et al33 | NR | “Limited keratouveitis” | Not reported | Not reported | No | Trifluridine Steroids |
Tecovirimat | Not reported |
| NR | Fulminant disease | Not reported | Not reported | HIV+ CD4 < 200 cells/mm3 |
Not reported | Not reported | Not reported | ||
AC cell, iritis, anterior uveitis; IV, intravenous; PO, oral; TB, tuberculosis.
RESULTS
The Changing Epidemiology of Mpox
Until the 2022–2023 global outbreak, mpox was a rare zoonotic disease that occurred sporadically in rural Africa.34 The mode of infection often involved direct interaction with infected rodents and nonhuman primates.35 In 2014, the number of cases in Africa surpassed the number of cumulative cases from the previous 40 years.36 A 2017 outbreak in Nigeria raised concern about the role of men having sex with men (MSM) as a new mode of transmission.36,37 Sexual contact was the primary mode of transmission in the 2022–2023 global outbreak involving more than 115 countries.38
Frequency of Mpox Keratitis
Determining mpox keratitis frequency is hampered by its low occurrence rate and probable underdiagnosis and underreporting. Potential explanatory factors include poor health care access and possible cultural and legal risks related to MSM.39 To accurately determine frequency, each patient within an mpox cohort would need to undergo an eye examination to confirm lack of corneal involvement, which has not been reported. However, an estimate of minimum mpox keratitis frequency can be made from published series (Table 3).
TABLE 3.
Number and Percentage of Patients With Mpox Keratitis and Vision Loss in Published Case Series*
| Reference Number | Country | Years | Total Number of Mpox Cases | Mpox Diagnostic Laboratory methods | No. (%) of Men | Age Range | No. (%) of Patients With Keratitis | No. (%) of Patients With Vision Loss | |
| 1 | Zaire (Democratic Republic of Congo) | 1981–1986 | 338 | Electron microscopy (EM), chorioallantoic membrane inoculation, serology | 182 (54%) | 3 month–69 yrs | 12 (3.6%) | 12 (3.6%) | |
| 40 | United States | 2003 | 47 | Polymerase chain reaction (PCR), culture, immunohistochemical staining, EM | 22 (47%) | 1–51 yrs | 1 (2.1%) | 1 (2.1%) | |
| 41 | France | 2022 | 264 | PCR | 262 (99%) | 30–41 yrs | 1 (0.38%) | Not reported | |
| 14 | France | 2022 | 588 | PCR | Not available (NA) | NA | 2 (0.34%) | 0 | |
| 21 | Spain | 2022 | 880 | PCR | 880 (100%) | 38 years (mean) | 4 (0.45%) | 0 | |
| 26 | Mexico | 2022 | 100 | PCR | NA | NA | 1 (1.0%) | Reported but not quantified | |
| 42 | Nigeria | 2022–2023 | 160 | PCR | 114 (71%) | 4 month–75 yrs | 10 (6%)† | Not reported | |
Patients could have had unilateral or bilateral involvement.
No case descriptions or photographs. 30% of patients in cohort had concomitant varicella zoster infection, raising concern for possible overdiagnosis of mpox keratitis.
Two series with keratitis cases are reported before 2022: a 1980s World Health Organization Democratic Republic of Congo mpox surveillance report, where 12 (4%) of 338 patients had keratitis,1 and the 2003 US outbreak, where 1 (2%) of 47 patients had keratitis.40 In both, presumably, only symptomatic patients received eye examinations. Two series without reported keratitis include a 2011-2015 Democratic Republic of Congo surveillance report of 1057 mpox cases in which conjunctivitis occurred in 20% and photophobia in 30%.43 Given that keratitis is a common cause of photophobia, a 30% photophobia prevalence without any occurrence of keratitis underscores potential underdiagnosis without ocular examination of all patients. Similarly, a 2017-2018 outbreak of 122 cases in Nigeria recorded facial rash in 96%, with conjunctivitis in over 20%, but no keratitis.37
Among 5 series from the 2022–2023 outbreak, keratitis was reported in 1 (0.4%) of 264 patients41 and 2 (0.3%) of 588 in France,14 10 (6%) of 160 patients in Nigeria,42 in 4 (0.5%) of 880 patients in Spain,21 and 1 (1.0%) of 100 patients in Mexico.26 In the Spanish and Mexican reports, patients with ocular symptoms underwent examination by an ophthalmologist.21,26 Based on these latter 2 series, an estimated frequency of 0.5% to 1.0% may be the lower range of mpox keratitis among patients with a confirmed mpox diagnosis and ocular symptoms (Table 3).
Diagnostic Considerations
Diagnosis of mpox keratitis by the ophthalmologist may be delayed because of lack of awareness of disease manifestations. Clinical manifestations of mpox typically include a prodromal fever, lymphadenopathy, and vesiculopapular rash. Dermatologic manifestations are the most common sign, reported in 90% of cases in the 2022–2023 outbreak.44 Mpox-related ocular disease (MPXROD) may rarely be the only or first presenting sign of mpox,20 but it more often presents in a patient with known mpox who subsequently develops ocular complaints. In reported cases, mpox keratitis presented at a median of 17.5 days after systemic manifestations (range, 0–60 days) and was not associated with systemic manifestations in 2 cases.20,29
Among the 35 patients with mpox keratitis from the 2022–2023 outbreak, 31 (89%) were male, 2 (6%) were female, and sex was unreported in 2 (6%). Fifteen patients (43%) had HIV infection (Table 2). MSM was the primary mode of transmission (83%) during the 2022-2023 outbreak45; if MPXROD is suspected, a sexual history should be obtained.
Misdiagnosis of mpox keratitis occurred initially in 14 patients (40%) because of lack of known mpox exposure, lack of nonocular signs or symptoms, or findings suggestive of other conditions.12,16,17,19,20,22–25,27,29–31 The 16 patients (46%) initially diagnosed with mpox keratitis had a diagnosis of systemic mpox before presentation to the eye clinic.8–11,13–15,17,18,21,30 No diagnostic history was provided for 5 patients.26,32,33 Misdiagnoses included bacterial preseptal cellulitis, syphilis, adenoviral conjunctivitis, and, frequently, herpetic keratitis.12,16,17,19,20,22–25,27,29–31 PCR of skin, corneal, or conjunctival samples was instrumental in revising the diagnosis for patients with mpox keratitis who were initially misdiagnosed.12,16,17,19,20,22–25,27,29–31
Current CDC and World Health Organization guidelines recommend use of real time (RT)-PCR for diagnosis of acute mpox infection, either by 2-stage testing with nonvariola orthopoxvirus (OPXV), followed by MPXV, or if available, with MPXV initially. PCR detects viral DNA, which may not reflect the presence of infectious virus. Thus, its role in guiding treatment is undetermined. Serial quantitative PCR may help clarify clinical observations, but availability is limited. MPXV culture should not be performed routinely because of the risk of accidental occupational exposure to infectious virus.46
Mpox Corneal Manifestations
Among reported cases of mpox keratitis, corneal disease ranges from mild epitheliopathy to fulminant ulcerative keratitis.2 It is unclear whether mpox keratitis occurs as part of the primary eruption, as secondary to autoinoculation from a periocular or nonocular lesion, as contiguous spread from the involved conjunctiva, or as the primary ocular manifestation.
Thirty-five cases with sufficient clinical details were identified in the systematic search, and our added case yields 36. Based on these cases, mpox keratitis can affect the corneal epithelium, stroma, and endothelium. In most cases, mpox keratitis occurred in concert with conjunctival, eyelid, or facial lesions, but it has occurred in systemic disease without ocular or facial findings and as isolated keratitis (Table 2).
Mpox keratitis shares similarities with variola and vaccinia keratitis, which can manifest with punctate and geographic epithelial defects, dendritiform lesions, stromal keratitis, and disciform keratitis with KP (resembling herpes simplex keratitis).4,5 Review of the published cases and the case reported here provides insight into common features of epithelial, stromal, and inflammatory/endothelial manifestations of mpox keratitis. The manifestations of mpox keratitis are presented in Table 4 to show their relative frequency. Most patients had multiple manifestations.
TABLE 4.
Reported Mpox Keratitis Manifestations and Recommended Nomenclature†
| Type of Lesion | Reported Descriptions | Number | Reference* | ||||||||||||
| Epithelial | |||||||||||||||
| Mpox infectious epithelial keratitis (mpox IEK)† | |||||||||||||||
| Mpox arcuate or serpiginous peripheral epithelial keratitis† | |||||||||||||||
| Elevated leading edge that stains with fluorescein Usually peripheral, though location likely dependent on time point of observation |
“Serpiginous epitheliopathy" “Arcuate staining epithelial lines" “Arcuate corneal infiltrates which stained" “Corneal epithelial ridge which stained" |
10 | Doan et al14 (2 cases) Pazos et al21 (1 case) Androudi et al16 (1 case) Quites et al19 (1 case) Arteaga-Rivera et al25 (1 case) Cash-Goldwasser et al9 (1 case) Finamor et al13 (1 case) Alsarhani et al15 (1 case) Case report herein (1 case) |
||||||||||||
| Mpox geographic epithelial ulcer† | |||||||||||||||
| Geographic epithelial defect which stains with fluorescein | “Geographic corneal ulcers" | 12 | Pazos et al21 (1 case) Nguyen et al20 (1 case) Vasquez-Perez et al12 (1 case) Androudi et al16 (2 cases) Quites et al19 (1 case) Uner et al22 (1 case) Raccagni et al23 (1 case) Malciolu-Nica et al30 (1 case) Lamas-Francis et al8 (1 case) Cuppari et al32 (1 case) Case report herein (1 case) |
||||||||||||
| Other epithelial manifestations | |||||||||||||||
| Nonspecific defect | 5 | Pazos et al21 (2 cases) Cash-Goldwasser et al9 (1 case) Cuppari et al32 (1 case) Oprea et al28 (1 case) |
|||||||||||||
| Punctate epithelial keratitis | 3 | Bloom et al27 (1 case) Alexis et al11 (1 case) Case report herein (1 case) |
|||||||||||||
| Stromal | |||||||||||||||
| Mpox stromal keratitis† | |||||||||||||||
| Description or image of stromal infiltrate and/or opacity | 10 | Vasquez-Perez et al12 (1 case) Finamor et al13 (1 case) Bhamray-Sanchez et al17 (2 cases) Raccagni et al23 (1 case) Ditta et al24 (1 case) Rodríguez-Badillo et al26 (1 case) Cuppari32 (2 cases) Case report herein (1 case) |
|||||||||||||
| Peripheral ulcerative keratitis Description or image of peripheral stromal infiltrate and thinning |
7 | Pazos et al21 (2 cases) Rodríguez-Badillo et al26 (1 case) Trawally Flores et al29 (1 case) Bhamray-Sanchez et al17 (1 case) Malciolu-Nica et al30 (1 case) Cuppari et al32 (1 case) |
|||||||||||||
| Inflammatory/Endothelial | |||||||||||||||
| Limbitis | |||||||||||||||
| Peripheral or limbal epithelial ulcer Limbal infiltrate which stains with fluorescein Uncertain if infectious |
6 | Doan et al14 (1 case) Nguyen et al20 (1 case) Ditta et al24 (1 case) Avila et al31 (1 case) Lamas-Francis et al8 (1 case) Case report herein (1 case) |
|||||||||||||
| Other Inflammatory/Endothelial Manifestations | |||||||||||||||
| Anterior chamber reaction | “Iritis” “Anterior uveitis” |
10 | Pazos et al21 (2 cases) Androudi et al16 (2 cases) Atreaga-Rivera et al25 (1 case) Lamas-Francis et al8 (1 case) Finamor et al13 (1 case) Alsarhani et al15 (1 case) Carrubba et al33 (1 case) Case report herein (1 case) |
||||||||||||
| Keratic precipitates | 5 | Finamor13 (1 case) Androudi et al16 (1 case) Nguyen et al20 (1 case) Alsarhani15 (1 case) Case report herein (1 case) |
|||||||||||||
| Stromal edema | 5 | Pazos et al21 (2 cases) Rodríguez-Badillo et al26 (1 case) Vasquez-Perez et al12 (1 case) Avila et al31 (1 case) |
|||||||||||||
| Immune ring | 2 | Quites et al19 (1 case) Case report herein (1 case) |
|||||||||||||
| Disciform edema | 1 | Alsarhani et al15 (1 case) | |||||||||||||
| Other | |||||||||||||||
| Fulminant ocular disease with perforation | 2 | Bacorn et al10 (1 case) Carrubba et al18 (1 case) |
|||||||||||||
| Progressive treatment-resistant keratouveitis with stromal melt, intraocular seeding | 2 | Nguyen et al20 (1 case) Carrubba et al33 (1 case) |
|||||||||||||
One or more manifestations usually occurred in each patient.
Cited reports may include several patients.
Recommended nomenclature.
Epithelium
Epithelial involvement was the most common mpox keratitis finding, occurring in 31 (86%) of the 36 patients as a distinct clinical manifestation and as a component of peripheral keratitis, perforation, or nonspecific “ulcer” (Table 2). As a distinct clinical manifestation, the 2 primary epithelial abnormalities were arcuate or serpiginous epithelial keratitis and geographic epithelial defects, occurring in 19 patients (Table 4).8,9,12–16,19–23,25,30,32 Descriptions and photos of the arcuate and serpiginous keratitis show an elevated epithelial ridge stained with fluorescein,9,13–16,19,21 located at and potentially emanating from the peripheral cornea. Progressive central migration of the ridge and coalescence was observed in 4 cases,13,14,19,25 and in 2 cases, the peripheral epitheliopathy preceded a central geographic epithelial defect.16,19 An arcuate epithelial defect was noted in our patient early in the disease course and preceded the central geographic epithelial defects. A geographic epithelial defect, which stained with fluorescein, was a major epithelial manifestation in 12 of the 19 patients.8,12,16,19,21,23,30 Three of the 19 patients, including our patient, had both serpiginous and geographic epithelial keratitis.16,19
With one exception, all patients with these epithelial manifestations were OPXV or MPXV PCR positive from the ocular surface9,13–16,19,21; the exception was positive from a skin lesion.27 PCR positivity usually persisted beyond resolution of the keratitis and up to 46 days.13–15,21 Our patient was still PCR-positive when last tested on day 130, with active keratitis and before penetrating keratoplasty. In contrast to current guidelines, the CDC performed MPXV cultures for our patient during the 2003 US outbreak. This is the only known report of MPXV recovery by culture from the corneal surface of human mpox keratitis. Quantitative MPXV RT-PCR values supported the clinical observations in our patient, with high values when culture-positive and decreasing values after initiation of trifluridine and when cultures were negative. The recovery of infectious virus by culture from the geographic epithelial defects of our patient is direct evidence to support that these epithelial manifestations represent mpox infectious epithelial keratitis. We suggest mpox arcuate and serpiginous epithelial keratitis and mpox geographic epithelial ulcer as the 2 primary types of mpox infectious epithelial keratitis (Table 4).
Stroma
Stromal involvement varied significantly in clinical descriptions, case photographs, and used terminology. Stromal findings were reported in 11 cases and ranged from advanced imaging findings suggestive of stromal involvement31 and anterior stromal infiltration without stromal thinning or significant scarring, to severe inflammation, thinning (including perforation), opacification, and visually significant scarring (Tables 2 and 4).12,13,17,21,23,24,26,29,30,32 Our patient had prolonged stromal inflammation for 5 months, which included periods of infectious epithelial keratitis. Including the case herein, 10 cases had stromal infiltrates or opacity.12,13,17,21,23,24,32 Seven cases showed peripheral stromal infiltrates with varying degrees of associated stromal thinning, with several reports using the terminology “peripheral ulcerative keratitis.”17,21,26,29,30,32 Four cases reported “stromal keratitis” or “stromal interstitial keratitis” without additional detail.12,17,32 Fulminant stromal disease is also discussed below.10,18,20
The combination of few cases, inconsistent terminology, and cooccurrence with epithelial disease limits conclusions regarding the pathologic mechanisms of stromal inflammation. Based on published reports, we are unable to distinguish stromal inflammation from MPXV particles in the stroma from stromal inflammation secondary to epithelial disease. Until such understanding may occur, mpox stromal keratitis may encompass the various manifestations of stromal inflammation (Table 4).
Endothelium and Other Inflammatory Manifestations
Endothelial manifestations included endotheliitis with KP, with or without anterior uveitis (Tables 2 and 4).8,13,15,16,20,21,25,33,47 These findings may accompany the epithelial manifestations,8,15,16 appear later in the disease course,13 or occur as an independent cornea-sparing anterior uveitis with conjunctival disease.47 Stromal edema occurred in several cases of epithelial keratitis or inflammatory disease and thus is unlikely to be a primary manifestation of mpox. By contrast, disciform edema was reported in one case and resolved after receiving systemic tecovirimat and topical steroids.15 Three patients had ocular hypertension with concomitant anterior uveitis.16,17 Limbal inflammation (limbitis) was described in 6 patients, including ours.8,14,20,24 As with stromal inflammation, it is unclear whether these inflammatory manifestations occur because of infection or as a subsequent inflammatory response.
Fulminant Disease
Severe keratitis with perforation, loss of vision, and death was reported in 2 patients with fulminant systemic mpox and immune compromise from HIV.10,18 A prolonged case of severe uveitis, scleritis, and keratitis occurred in a patient exposed to direct inoculation of the eye by semen. He did not have fulminant systemic mpox but was immune compromised because of chronic lymphocytic leukemia.20 Another case of severe keratitis was reported in a patient with HIV and a CD4 count <200 cells/μL but did not include significant detail.18,33 Patients with immune compromise and patients with severe systemic mpox seem at greater risk of severe keratitis, even with appropriate antiviral treatment.
Treatment Observations
Treatment varied significantly across cases. Different combinations of topical and systemic antibiotics, steroids, and antivirals (anti-herpes and anti-OPX) were administered, initiated at different stages of disease, and for variable durations. This fact, together with the small number of cases and absence of clinical trials, limits conclusions regarding treatment efficacy for mpox keratitis. CDC guidance recommends systemic antiviral therapy for all patients with severe mpox disease, which includes ocular manifestations.48
Anti-OPX treatments used include systemic tecovirimat, systemic cidofovir, vaccinia IVIG, topical trifluridine, and topical interferon alpha. Thirty (83%) of 36 patients received oral or intravenous tecovirimat. Tecovirimat administration was associated with clinical improvement and resolution of mpox epithelial keratitis in almost all cases. Seven of 10 patients with arcuate or serpiginous epithelial keratitis and 11 of 12 with geographic epithelial defects received tecovirimat with disease resolution.8,9,12–16,19,21–23,25,30 One case progressed despite intravenous cidofovir before tecovirimat administration.14 One case of epithelial disease, 2 cases of nonspecific “keratitis,” and a fulminant keratitis case received more than 1 course of tecovirimat because of incomplete keratitis resolution or recurrence.9,20,23 Among cases that did not receive tecovirimat, 1 serpiginous epitheliopathy case resolved with corticosteroids and trifluridine,14 2 cases of serpiginous epithelial keratitis resolved with topical interferon alpha,25,26 1 case of geographic epithelial defect resolved after prolonged disease and treatment that included trifluridine,12 1 case with predominantly inflammatory disease resolved with topical steroids,31 and our case did not demonstrate viral clearance despite topical trifluridine until cessation of topical steroids and penetrating keratoplasty.
Use of additional systemic anti-OPX medications was also reported, but the efficacy of these agents is less clear. Cidofovir was used in 3 cases, either before or concurrent with tecovirimat, and was not given as a monotherapy.14,20,23 Vaccinia IVIG was part of a complex treatment regimen in 1 case of severe mpox keratitis with bilateral globe rupture.18 Acyclovir, valacyclovir, and ganciclovir antiherpes medications are not effective against orthopoxviruses; mpox keratitis worsened in patients receiving systemic antiherpetic therapy before receiving tecovirimat.13,14,16,19,25,27
Including our case, topical trifluridine was used in 15 cases, 13 of which also received tecovirimat; thus, it is uncertain whether trifluridine monotherapy could effectively treat mpox keratitis.9,10,12,14,16,17,20,24,32,33 Our patient demonstrated culture-positive mpox keratitis for over 4 months despite 2 courses of topical trifluridine but also received chronic steroids.
Most cases received topical steroids for stromal or endothelial inflammation or iritis at various time points (Table 2). Viral persistence in the setting of topical steroids has occurred in vaccinia keratitis in a rabbit model,49 as well as in our patient, which is notable because orthopoxviruses do not establish latency in humans. Tecovirimat appeared to resolve infectious mpox keratitis in most patients who did not have fulimnant systemic disease, despite systemic or topical steroid use. One report described persistent keratitis for 8 months in an immunocompetent patient; topical steroids were used for 4 months, and disease resolution occurred after IV cidofovir was administered consecutively to a third course of tecovirimat.23
Surgical intervention was rare. Two patients had amniotic membrane placement for corneal epithelial defects.12,22 One patient with fulminant disease and perforation underwent penetrating keratoplasty with poor outcome (no light perception),10 and corneal glue application complicated by expulsion of intraocular contents was attempted on 1 patient with fulminant disease and corneal perforation.18 Our patient underwent successful penetrating keratoplasty.
Study Limitations
We emphasize the challenge of categorizing the various findings across highly heterogenous reports. Descriptions and nomenclature varied widely, even when referring to potentially related processes. For example, limbitis, peripheral keratitis, peripheral ulcerative keratitis, and semilunar corneal ulceration all describe peripheral corneal manifestations whereby both epithelial and stromal abnormalities occur. These could represent various stages of the same mpox corneal disease process, or they could represent distinct subtypes of peripheral mpox keratitis, but published reports make it difficult to draw such distinctions. Regardless, the broad categories of common and shared clinical manifestations should be noted as manifestations of mpox keratitis (Table 4).
CONCLUSIONS
Mpox keratitis is a rare but potentially devastating complication of MPXROD. Underdiagnosis of mpox keratitis will likely remain a major challenge because of worldwide unfamiliarity with mpox, the sporadic nature of cases, and the rarity of mpox keratitis as a complication.
Mpox keratitis usually occurs within the context of systemic mpox at a median of 2 and a half weeks after systemic symptoms but rarely occurs in the absence of systemic disease. PCR may help confirm mpox keratitis, especially when the clinical diagnosis is ambiguous. Common features of mpox keratitis include infectious epithelial keratitis (including serpiginous/arcuate epithelial keratitis and geographic epithelial ulcer), stromal keratitis, and endotheliitis with or without anterior uveitis.
Tecovirimat tended to result in improvement or resolution of mpox keratitis. The efficacy of other anti-OPX treatments for mpox keratitis is less certain. Topical steroids should be used with caution in mpox keratitis and should generally not be used in the absence of effective systemic anti-mpox therapy.
The prognosis of mpox keratitis depends on early recognition and treatment before the development of vision-limiting sequelae. Clinicians should maintain a high index of suspicion for mpox keratitis in patients with confirmed or suspected mpox, patients at high risk of mpox, and cases of atypical or persistent keratitis, particularly in the setting of a local mpox outbreak.
ACKNOWLEDGMENTS
The authors extend their gratitude to Kari Severson, DVM, Medical Director, Wisconsin Veterinary Referral Center/Ethos Veterinary Health, for her assistance with this report.
Footnotes
The authors have no funding or conflicts of interest to disclose.
C. R. Croasdale and E. Weinlander had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, and interpretation of data: C. R. Croasdale and E. Weinlander. Drafting of the manuscript: C. R. Croasdale and E. Weinlander. Critical revision of the manuscript for important intellectual content: All authors.
Contributor Information
Eric Weinlander, Email: eweinlander@wisc.edu.
Thomas G. Boyce, Email: boyce.thomas@marshfieldclinic.org.
REFERENCES
- 1.Jezek Z, Fenner F. Human monkeypox.Monographs in Virology. Vol 17. Basel, Switzerland: Karger; 1988. [Google Scholar]
- 2.Kaufman AR, Chodosh J, Pineda R. Monkeypox virus and ophthalmology—a primer on the 2022 monkeypox outbreak and monkeypox-related ophthalmic disease. JAMA Ophthalmol. 2023;141:78–83. [DOI] [PubMed] [Google Scholar]
- 3.Gessain A, Nakoune E, Yazdanpanah Y. Monkeypox. N Engl J Med. 2022;387:1783–1793. [DOI] [PubMed] [Google Scholar]
- 4.Semba RD. The ocular complications of smallpox and smallpox immunization. Arch Ophthalmol. 2003;121:715–719. [DOI] [PubMed] [Google Scholar]
- 5.Pepose JS, Margolis TP, LaRussa P, et al. Ocular complications of smallpox vaccination. Am J Ophthalmol. 2003;136:343–352. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:642–646. [PubMed] [Google Scholar]
- 7.Croasdale C, Wise J, Holland E. Human monkeypox ocular infection: first Western Hemisphere case report. Paper presented at: 2003 Federated Scientific Session of the Cornea Society and Eye Bank Association of America; November 15, 2003; Anaheim, CA.
- 8.Lamas-Francis D, Treviño M, Pérez-Freixo H, et al. Corneal ulcer due to monkeypox infection. Ocul Immunol Inflamm. 2024;32:259–261. [DOI] [PubMed] [Google Scholar]
- 9.Cash-Goldwasser S, Labuda SM, McCormick DW, et al. Ocular monkeypox—United States, July–September 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1343–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacorn C, Majidi S, Schultz H, et al. Severe mpox infection of the eye and periocular region. Ophthalmic Plast Reconstr Surg. 2023;39:e176–e179. [DOI] [PubMed] [Google Scholar]
- 11.Alexis J, Hohnen H, Kenworthy M, et al. Ocular manifestation of monkeypox virus in a 38-year old Australian male. IDCases. 2022;30:e01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasquez-Perez A, Magan T, Volpe G, et al. Necrotizing blepharoconjunctivitis and keratitis in human monkeypox. JAMA Ophthalmol. 2023;141:285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finamor LPS, De Freitas D, Andrade G, et al. Tecovirimat treatment for monkeypox virus keratouveitis. JAMA Ophthalmol. 2023;141:210–212. [DOI] [PubMed] [Google Scholar]
- 14.Doan S, Houry R, Cristea I, et al. Severe corneal involvement associated with mpox infection. JAMA Ophthalmol. 2023;141:402–403. [DOI] [PubMed] [Google Scholar]
- 15.Alsarhani WK, Chan CC, Boyd SR, et al. Monkeypox-associated disciform keratitis. Cornea. 2023;42:641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Androudi S, Kaufman AR, Kouvalakis A, et al. Non-healing corneal ulcer and uveitis following monkeypox disease: diagnostic and therapeutic challenges. Ocul Immunol Inflamm. 2023;32:253–258. [DOI] [PubMed] [Google Scholar]
- 17.Bhamray-Sanchez D, Subramanian S, Dever LL, et al. Ocular MPox: a report of two cases. IDCases. 2023;31:e01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrubba S, Geevarghese A, Solli E, et al. Novel severe oculocutaneous manifestations of human monkeypox virus infection and their historical analogues. Lancet Infect Dis. 2023;23:e190–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quites TO, Landeira LFL, Santana TGV, et al. Monkeypox keratoconjunctivitis with associated Wessley immune ring in an immunocompetent patient. J Med Virol. 2023;95:e28545. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen MT, Mentreddy A, Schallhorn J, et al. Isolated ocular mpox without skin lesions, United States. Emerg Infect Dis. 2023;29:1285–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pazos M, Riera J, Moll-Udina A, et al. Characteristics and management of ocular involvement in individuals with monkeypox disease. Ophthalmology. 2023;130:655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uner OE, Hubbard DC, Torres-Quinones C, et al. Human MPox (monkeypox) virus membranous keratoconjunctivitis with transient corneal hypoesthesia and late symblepharon formation: a novel case and clinical implications. Cornea. 2023;42:751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raccagni AR, Clemente T, Ranzenigo M, et al. Persistent ocular mpox infection in an immunocompetent individual. Lancet Infect Dis. 2023;23:652–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ditta LC, Wojcik P, Minniear TD. Ocular presentation of Mpox in a healthy child without known exposure. J AAPOS. 2023;27:97–100. [DOI] [PubMed] [Google Scholar]
- 25.Arteaga-Rivera JY, Vigderovich-Cielak I, Ramirez-Miranda A, et al. Adjuvant topical interferon alpha 2b for the treatment of monkeypox ocular manifestations. Cornea. 2023;42:1578–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Badillo P, Rodríguez-Aldama JC, Gabián-Fortes LDC, et al. Mpox-related ophthalmic disease: a retrospective observational study in a single center in Mexico. J Infect Dis. 2024;229(suppl 2):S255–S259. [DOI] [PubMed] [Google Scholar]
- 27.Bloom J, Parise M, Saeed O, et al. Monkeypox presenting with blepharoconjunctivitis. Case Rep Ophthalmol. 2023;14:647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oprea C, Costescu C, Ianache I, et al. A severe case of mpox complicated with penile necrosis and keratitis in a patient living with HIV. Travel Med Infect Dis. 2023;55:102646. [DOI] [PubMed] [Google Scholar]
- 29.Trawally Flores A, Guedes Guedes II, Espinoza González JP, et al. Ocular involvement secondary to Monkeypox virus infection. Arch Soc Esp Oftalmol (Engl Ed). 2024;99:33–37. [DOI] [PubMed] [Google Scholar]
- 30.Malciolu-Nica MA, Costescu C, Popescu CP, et al. Mpox-related ophthalmic disease: a rare case report. AIDS Res Hum Retroviruses. 2023;39:616–620. [DOI] [PubMed] [Google Scholar]
- 31.Avila MY, Gallego-Suarez LJ. Confocal microscopy, anterior segment optical coherence tomography and clinical findings in a non-granulomatous uveitis case for mpox infection. Ocul Immunol Inflamm. 2023. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32.Cuppari N, Shah MK, Dastjerdi M. Report of two cases of monkeypox keratitis. Invest Ophthalmol Vis Sci. 2023;64:2336. [Google Scholar]
- 33.Carrubba S, Jennings E, Guttha S, et al. Monkeypox keratouveitis: novel reports and analysis of host immune responses. Invest Ophthalmol Vis Sci. 2023;64:1714. [Google Scholar]
- 34.Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damon IK. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29(suppl 4):D54–D59. [DOI] [PubMed] [Google Scholar]
- 36.McCollum AM, Shelus V, Hill A, et al. Epidemiology of human mpox—worldwide, 2018–2021. MMWR Morb Mortal Wkly Rep. 2023;72:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–2018: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Multi-Country Outbreak of mpox, External Situation Report #21–27. April 2023. Vol. Emergency Situational Updates. 2023. Multi-Country Outbreak of mpox, External Situation Report#21–27 April 2023 (who.int).
- 39.Happi C, Adetifa I, Mbala P, et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol. 2022;20:e3001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–780. [DOI] [PubMed] [Google Scholar]
- 41.Mailhe M, Beaumont AL, Thy M, et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: an observational cohort study. Clin Microbiol Infect. 2023;29:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogoina D, Dalhat MM, Denue BA, et al. Clinical characteristics and predictors of human mpox outcome during the 2022 outbreak in Nigeria: a cohort study. Lancet Infect Dis. 2023;23:1418–1428. [DOI] [PubMed] [Google Scholar]
- 43.Whitehouse ER, Bonwitt J, Hughes CM, et al. Clinical and epidemiological findings from enhanced monkeypox surveillance in Tshuapa Province, Democratic Republic of the Congo during 2011–2015. J Infect Dis. 2021;223:1870–1878. [DOI] [PubMed] [Google Scholar]
- 44.2022–2023 Mpox (Monkeypox) Outbreak. Global Trends. Basel, Switzerland: World Health Organization (WHO): 2024. Available at: https://worldhealthorg.shinyapps.io/mpx_global/. Accessed January 5, 2024. [Google Scholar]
- 45.World Health Organization . Multi-Country Outbreak of Mpox External Situation Report 29. Emergency Situational Updates. Geneva, Switzerland: World Health Organization; 2023. [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC) . Biosafety laboratory guidance for handling and processing mpox specimens. 2023. Available at: https://www.cdc.gov/poxvirus/mpox/lab-personnel/lab-procedures.html. Accessed January 5, 2024.
- 47.Carvalho EM, Medeiros M, Veloso VG, et al. Monkeypox infection causing conjunctival vesicles and anterior uveitis. Ocul Immunol Inflamm. 2024;32:266–267. [DOI] [PubMed] [Google Scholar]
- 48.Treatment Information for Healthcare Professionals . Centers for Disease Control and Prevention (CDC). 2023. Available at: https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html. Accessed January 5, 2024. [Google Scholar]
- 49.Altmann S, Brandt CR, Murphy CJ, et al. Evaluation of therapeutic interventions for vaccinia virus keratitis. J Infect Dis. 2011;203:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]