Abstract
Sevoflurane is a volatile anesthetic that can tolerate inhalation induction and is widely used for inducing anesthesia due to its pleasant odor. As a drug that has been on the market for nearly 30 years, the vast majority of adverse reactions have been documented. This study aims to improve the adverse reactions related to Sevoflurane through the mining, organizing and analysis of Food and Drug Administration Adverse Event Reporting System database data. We collected, organized, and analyzed reports from the first quarter of 2004 to the fourth quarter of 2022. We performed disproportionality analysis algorithms, including reporting odds ratio, the proportional reporting ratio values, to quantify the signal values of different adverse events (AEs). A total of 1126 AEs and 27 system organ classes were identified by performing statistics analysis system software. By combining algorithm calculations, we create a forest map of the top 30 AEs of the reporting odds ratio signal. Based on the reviewing relevant literature, we found that the vast majority of AEs have been reported in relevant studies. However, there is currently no study revealing the correlation between atrial fibrillation and Sevoflurane, which means that atrial fibrillation may be an unreported AE of Sevoflurane. In the present study, we found that atrial fibrillation may be a new adverse reaction of Sevoflurane through the Food and Drug Administration Adverse Event Reporting System database, which can function as a novel guideline to guide us in the more standardized use of Sevoflurane in clinical practice.
Keywords: adverse events, atrial fibrillation, FAERS, Sevoflurane
1. Introduction
Sevoflurane was first discovered by Pos Terell and successfully synthesized by Regan in 1968.[1] However, it was not put into clinical use until phase III clinical trial from Japan in 1986, which was related to the potential AEs it may have.[2] Sevoflurane is a colorless, transparent, aromatic, and nonvolatile liquid with stable chemical properties. At commonly used clinical concentrations, it will not burn or explode when in contact with oxygen.[3] Due to its pleasant odor and nonirritating effect on trachea, Sevoflurane is permitted for inhalation induction in children and adults.[4] In inhalation anesthesia, 95% to 98% of Sevoflurane is excreted through the lungs, while the rest is excreted through the liver and kidney pathways.[5] Since its launch, there have been many reports of adverse events related to Sevoflurane, including neurological, respiratory, and circulatory systems. However, overall, Sevoflurane is considered a relatively safe and reliable drug.[6] This study aims to explore the unreported adverse reactions of Sevoflurane through the FDA adverse event reporting system (FAERS) database.
Sevoflurane mainly acts on the human nervous system, which can increase dose-dependent intracranial pressure and reduce cerebrovascular resistance.[7] However, there are also relevant studies reporting that Sevoflurane may damage the human nervous system. For instance, Jiang et al[8] found that elderly patients over 65 years old are at increased risk of developing Alzheimer disease after inhaling inhaled anesthetics such as Sevoflurane. Moreover, Constant et al[9] reported that Sevoflurane may be related to cortical epileptic electroencephalogram signs and usually has no clinical symptoms. Moreover, according to research conducted by Sun et al,[10] we found an increased incidence of emergence delirium in children receiving Sevoflurane induced anesthesia. Malignant hyperthermia is a hereditary disease, which is characterized by hypermetabolic reaction to strong volatile anesthetic gases, including Halothane, Sevoflurane, etc.[11] However, increasing evidence has demonstrated that Sevoflurane may induce hyperthermia malignant, postoperative restlessness, and agitation in children, which may be closely related to early pain.[12,13] Zhao et al[14] conducted a meta-analysis revealing that compared to propofol anesthesia, children who received Sevoflurane anesthesia had a higher risk of restlessness, postoperative nausea, vomiting, and postoperative pain. According to Kraus review,[15] we found a significant increase in the incidence of diabetes insipidus in critically ill patients receiving Sevoflurane. Therefore, the incidence of neurological related adverse events (AEs) significantly increases in patients undergoing inhalation of Sevoflurane induced anesthesia.
In addition, Sevoflurane may also lead to complications in other systems. For example, similar to other halogenated volatile anesthetics, Sevoflurane can lead to dose-dependent cardiovascular suppression, which may affect blood flow in different organ systems.[16] The Gonzalo Pascual team[17] believes that the incidence of severe acute hepatitis significantly increases after receiving Sevoflurane anesthesia. Additionally, Goa et al[18] proposed that similar to Halothane, Sevoflurane may cause emergency events such as cough, laryngospasm, agitation, and excitement, but Sevoflurane has a lower probability of causing arrhythmia than Halothane. Furthermore, Shutes et al[19] found an unusual complication of Sevoflurane in severe childhood cases, which can cause hypercapnia. Besides, Barrons et al[20] published a case report reporting on a 32 year old young woman who experienced rhabdomyolysis after receiving Sevoflurane.
In the present study, we obtained the AEs and clinical data of patients from the FAERS database, and then conducted disproportionality analysis to calculate the reporting odds ratios (RORs) values and corresponding 95% CIs of different system organ classes (SOCs) and PTs. Subsequently, we ranked the RORs values to obtain the AEs of Sevoflurane. Our research findings can help doctors better understand the risks associated with the use of Sevoflurane, thereby enabling more thorough communication with patients before surgery.
2. Materials and methods
2.1. Data acquisition
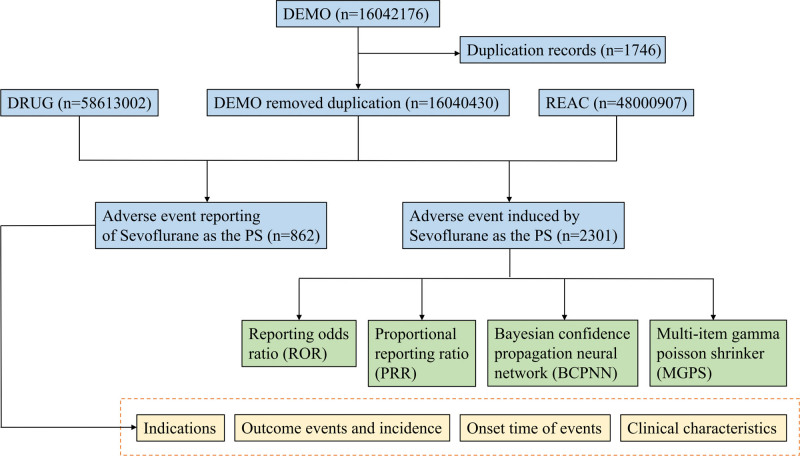
This study is a retrospective study aimed at exploring the adverse reactions of Sevoflurane through the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). The AEs and clinical information of patients from over 100 countries were acquired from FAERS database. The FAERS database is a free and publicly available database that is updated quarterly and records adverse reactions from the first quarter of 2004 to the fourth quarter of 2022. Sevoflurane for inhalation was FDA certified and approved for sale in the United States in June 1995. Therefore, the AEs of Sevoflurane from the first quarter of 2004 to the fourth quarter of 2022 were included in our subsequent research (Fig. 1).
Figure 1.
Flowchart for the safety analysis of Sevoflurane.
2.2. Data processing
The duplicate data were removed, and the obtained data were extracted and merged subsequently. To collect all the names of Sevoflurane (“Sevorane,” “Ultane,” and “BAX 3084”), we retrieved the Cochrane Library (https://www.cochranelibrary.com)[21] and The Medical Subject Headings (MeSH, https://www.ncbi.nlm.nih.gov/).[22] The Medical Dictionary for Regulatory Activities (MedDRA, version 26.0)[23] were utilized for further supplementing the name of Sevoflurane.
2.3. Detection of drug signals
The disproportionality analysis was conducted to evaluate the relationship between the AE signals and Sevoflurane by performing the ROR and proportional reporting ratio (PRR) algorithm.[24] The formula of ROR and PRR were listed as follows:
Equation: a, number of reports containing both the target drug and target adverse drug reaction; b, number of reports containing other adverse drug reaction of the target drug; c, number of reports containing the target adverse drug reaction of other drugs; d, number of reports containing other drugs and other adverse drug reactions.
2.4. Signal analysis
Based on the AE signals, we explored the potential relationship among the AE signals, SOC and preferred term (PT). We recognized the clinical information of patients (e.g., survival outcomes, reporter, age, and reporting country), which was shown in Table 1. The forest map was generated to exhibit the RORs and 95% CI of AEs related to Sevoflurane.
Table 1.
The survival outcomes, reporter, age, and reporting country of AE patients.
| Female | % | Male | % | |
|---|---|---|---|---|
| Outcome | ||||
| Death | 71 | 6.81 | 108 | 9.77 |
| Hospitalization | 253 | 24.28 | 251 | 22.71 |
| Life-threatening | 146 | 14.01 | 173 | 15.66 |
| Disability | 19 | 1.82 | 31 | 2.81 |
| Other serious | 520 | 49.90 | 504 | 45.61 |
| Required intervention | 26 | 2.50 | 32 | 2.90 |
| Not specified | 7 | 0.67 | 6 | 0.54 |
| Reporter | ||||
| Healthcare professional | 694 | 84.94 | 705 | 84.74 |
| Consumer | 29 | 3.55 | 25 | 3.00 |
| Not specified | 94 | 11.51 | 102 | 12.26 |
| Age | ||||
| <18 | 177 | 21.66 | 244 | 29.33 |
| 18 to 64 | 327 | 40.02 | 309 | 37.14 |
| ≥65 | 125 | 15.30 | 111 | 13.34 |
| Not specified | 188 | 23.01 | 168 | 20.19 |
| Reporting countries | ||||
| United States | 69 | 8.45 | 244 | 22.26 |
| Other country | 44 | 5.39 | 836 | 76.28 |
| Not specified | 704 | 86.17 | 16 | 1.46 |
3. Results
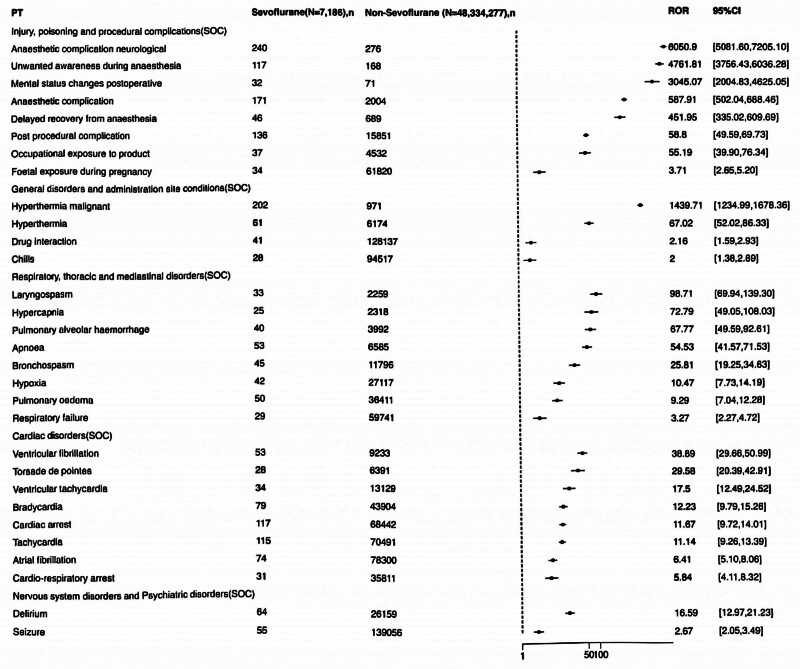
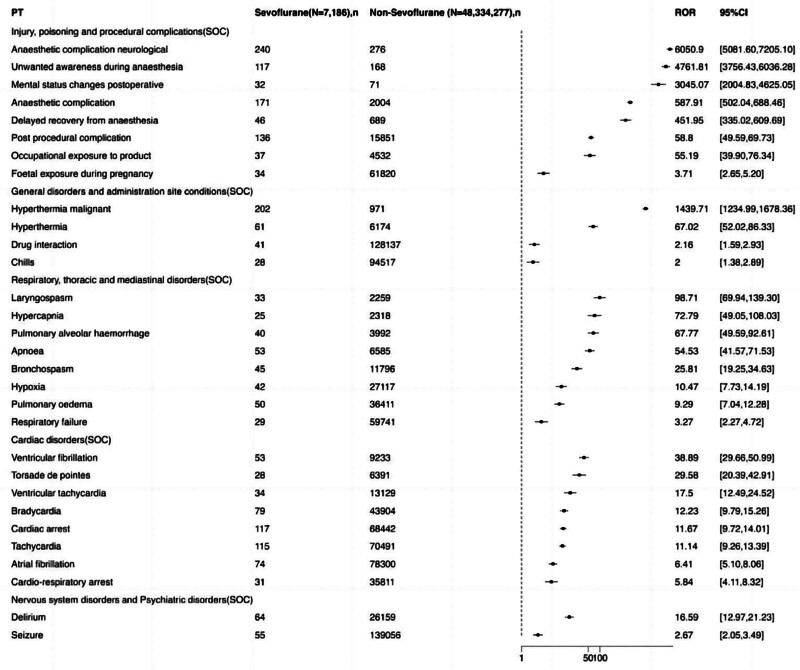
Data extraction obtained 1649 Sevoflurane AE reports, 817 women and 832 men. The survival outcomes, reporter, age, and reporting country of AE patients are shown in (Table 1). Men reported more ADEs, and the reported clinical outcomes of ADEs were more serious. We filtered 1126 AE signals related to Sevoflurane, 558 of which were identified as positive signals (Fig. 2). Regarding AE signals, we obtained 5 SOCs (injury, poisoning and procedural complications, general disorders and administration site conditions, respiratory, thoracic and mediastinal disorders, cardiac disorders, and nervous system disorders and psychiatric disorders), in which injury, poisoning and procedural complications exhibited the strongest signals (Table 2). Additionally, it is worth mentioning that according to time to onset of Sevoflurane-associated AEs in Figure 3, we found that the vast majority of AEs occurred within 30 days after receiving Sevoflurane anesthesia.
Figure 2.
Identification of the AE signals related to Sevoflurane. We filtered 1126 AE signals related to Sevoflurane, 558 of which were identified as positive signals (as shown in this figure). Regarding AE signals, we obtained 5 SOCs (injury, poisoning, and procedural complications, general disorders and administration site conditions, respiratory, thoracic and mediastinal disorders, cardiac disorders, and nervous system disorders and psychiatric disorders), in which injury, poisoning, and procedural complications exhibited the strongest signals. AE = adverse event, SOCs = system organ classes.
Table 2.
Signal strength of reports of Sevoflurane at the Top 30 PTs in FAERS database.
| PT | Sevoflurane (N = 7186), n | Non-Sevoflurane (N = 48,334,277), n | ROR | RORL | RORU | PRR | χ2 | EBGM | EBGM05 | IC2 | ICO25 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Injury, poisoning, and procedural complications (SOC) | |||||||||||
| Anaesthetic complication neurological | 240 | 276 | 6050.902114 | 5081.599149 | 7205.097318 | 5848.845823 | 750579.8696 | 3128.917533 | 2703.666523 | 11.61144792 | 9.940394323 |
| Unwanted awareness during anesthesia | 117 | 168 | 4761.812772 | 3756.431582 | 6036.276817 | 4684.298842 | 322932.6767 | 2761.681423 | 2264.605489 | 11.43133119 | 9.75639037 |
| Mental status changes postoperative | 32 | 71 | 3045.070013 | 2004.830766 | 4625.054415 | 3031.514455 | 66825.94424 | 2089.995401 | 1473.199672 | 11.02928405 | 9.339204364 |
| Anaesthetic complication | 171 | 2004 | 587.9060612 | 502.0372255 | 688.4619691 | 573.9398858 | 90116.81085 | 528.8949569 | 463.4392795 | 9.046837409 | 7.379461418 |
| Delayed recovery from anesthesia | 46 | 689 | 451.9490042 | 335.0206032 | 609.6875846 | 449.0623281 | 19278.20332 | 421.0203321 | 327.72726 | 8.717746096 | 7.048126837 |
| Post-procedural complication | 136 | 15,851 | 58.80386933 | 49.59117096 | 69.7280379 | 57.70989129 | 7516.914698 | 57.22746524 | 49.62320993 | 5.838635802 | 4.17225131 |
| Occupational exposure to product | 37 | 4532 | 55.19262241 | 39.90354231 | 76.33972805 | 54.91358998 | 1942.800393 | 54.47699492 | 41.52815901 | 5.767575218 | 4.100800374 |
| Foetal exposure during pregnancy | 34 | 61,820 | 3.712118358 | 2.650041008 | 5.199852629 | 3.699286181 | 67.01563016 | 3.697802433 | 2.78914761 | 1.886668146 | 0.220399532 |
| General disorders and administration site conditions (SOC) | |||||||||||
| Hyperthermia malignant | 202 | 971 | 1439.707976 | 1234.991379 | 1678.35913 | 1399.265587 | 233648.1306 | 1158.473048 | 1018.944737 | 10.17800876 | 8.509653821 |
| Hyperthermia | 61 | 6174 | 67.01593628 | 52.02241074 | 86.33078806 | 66.45554495 | 3894.732931 | 65.81516191 | 53.24704363 | 6.040348072 | 4.373719241 |
| Drug interaction | 41 | 128,137 | 2.158785187 | 1.588069505 | 2.93460297 | 2.152173694 | 25.34875163 | 2.15180515 | 1.664304559 | 1.105547445 | -0.560700267 |
| Chills | 28 | 94,517 | 1.996464293 | 1.377394907 | 2.893774075 | 1.992581605 | 13.86742849 | 1.992287646 | 1.460386592 | 0.994425959 | -0.671828688 |
| Respiratory, thoracic, and mediastinal disorders (SOC) | |||||||||||
| Laryngospasm | 33 | 2259 | 98.7061954 | 69.94378565 | 139.2963352 | 98.25750288 | 3131.242159 | 96.85719852 | 72.60396592 | 6.59778737 | 4.930469923 |
| Hypercapnia | 25 | 2318 | 72.79261173 | 49.04931031 | 108.0293339 | 72.54284617 | 1745.179239 | 71.7794782 | 51.58678409 | 6.16549953 | 4.498205774 |
| Pulmonary alveolar hemorrhage | 40 | 3992 | 67.76817699 | 49.58948363 | 92.61088191 | 67.39652001 | 2590.713498 | 66.73782437 | 51.39022466 | 6.060432751 | 4.39358514 |
| Apnoea | 53 | 6585 | 54.53105766 | 41.57370577 | 71.52685081 | 54.1362419 | 2742.506128 | 53.71198447 | 42.80384471 | 5.74717212 | 4.080567603 |
| Bronchospasm | 45 | 11,796 | 25.81475103 | 19.24578725 | 34.62583068 | 25.65935668 | 1062.632291 | 25.56564237 | 19.9961342 | 4.676134371 | 3.009695172 |
| Hypoxia | 42 | 27,117 | 10.4731593 | 7.73116746 | 14.18764582 | 10.41779154 | 357.2265277 | 10.40322741 | 8.069732853 | 3.378959263 | 1.712639049 |
| Pulmonary edema | 50 | 36,411 | 9.294167574 | 7.036015871 | 12.27705458 | 9.236456973 | 367.0093911 | 9.225162087 | 7.308469345 | 3.20557426 | 1.539277514 |
| Respiratory failure | 29 | 59,741 | 3.274255765 | 2.273459852 | 4.715610354 | 3.265077722 | 45.60340001 | 3.263978722 | 2.405400319 | 1.706631652 | 0.04036163 |
| Cardiac disorders (SOC) | |||||||||||
| Ventricular fibrillation | 53 | 9233 | 38.88956346 | 29.65800111 | 50.99460818 | 38.61011079 | 1930.997164 | 38.39545046 | 30.60578086 | 5.262863469 | 3.596366233 |
| Torsade de pointes | 28 | 6391 | 29.57980577 | 20.39222929 | 42.90678068 | 29.46844555 | 766.8093954 | 29.34426476 | 21.4963619 | 4.875006656 | 3.208389305 |
| Ventricular tachycardia | 34 | 13,129 | 17.49673469 | 12.48646126 | 24.51741277 | 17.41868167 | 524.9709095 | 17.37627226 | 13.10268277 | 4.119046708 | 2.452628694 |
| Bradycardia | 79 | 43,904 | 12.22636188 | 9.792899488 | 15.26452151 | 12.10294376 | 803.9460687 | 12.08300123 | 10.03518428 | 3.594906937 | 1.92862191 |
| Cardiac arrest | 117 | 68,442 | 11.67199283 | 9.721529568 | 14.01378411 | 11.49823508 | 1121.14516 | 11.48031922 | 9.851639664 | 3.521090853 | 1.85482619 |
| Tachycardia | 115 | 70,491 | 11.13537169 | 9.260216501 | 13.39023799 | 10.9731719 | 1042.219298 | 10.95692803 | 9.390313702 | 3.453771464 | 1.787507857 |
| Atrial fibrillation | 74 | 78,300 | 6.41252871 | 5.099378983 | 8.063829851 | 6.356791565 | 334.270206 | 6.351733733 | 5.243570526 | 2.667150435 | 1.000890336 |
| Cardio-respiratory arrest | 31 | 35,811 | 5.843444783 | 4.105716195 | 8.316660311 | 5.822550434 | 123.8078906 | 5.818379376 | 4.330592561 | 2.540617368 | 0.874319439 |
| Nervous system disorders and psychiatric disorders (SOC) | |||||||||||
| Delirium | 64 | 26,159 | 16.59498963 | 12.97084464 | 21.23174614 | 16.45609743 | 927.3148803 | 16.41837519 | 13.35956326 | 4.037239456 | 2.370915959 |
| Seizure | 55 | 139,056 | 2.673169495 | 2.050141986 | 3.485531832 | 2.660363439 | 57.1357407 | 2.659706985 | 2.130131175 | 1.411267315 | -0.254978865 |
Figure 3.
Time to onset of sevoflurane-associated adverse events. The vast majority of AEs occurred within 30 days after receiving Sevoflurane anesthesia. AEs = adverse events.
The injury, poisoning, and procedural complications included anesthetic complication neurological, unwanted awareness during anesthesia, mental status changes postoperative, anesthetic complication, delayed recovery from anesthesia, post-procedural complication, occupational exposure to product, and fetal exposure during pregnancy. General disorders and administration site conditions exhibited the second strongest signal strength, including hyperthermia malignant, hyperthermia, drug interaction, and chills. Respiratory, thoracic and mediastinal disorders showed the third strongest signal strength, including laryngospasm, hypercapnia, pulmonary alveolar hemorrhage, apnoea, bronchospasm, hypoxia, pulmonary edema, and respiratory failure. Cardiac disorders emerged as the fourth strongest signal strength, including ventricular fibrillation, torsade de pointes ventricular tachycardia, bradycardia, cardiac arrest, tachycardia, atrial fibrillation, and cardio-respiratory arrest. Nervous system disorders and psychiatric disorders revealed the fifth strongest signal strength, including delirium and seizure.
4. Discussion
According to the calculation of adverse reaction signals of Sevoflurane in the FAERS database, we found that most AEs have been reported. For instance, as stated in the introduction, the main AE of Sevoflurane is its neurotoxicity, which can also cause epilepsy, delirium, postoperative restlessness, and agitation. This means that it undeniably causes anesthetic complication neurological, unwanted awareness during anesthesia, and mental status changes postoperative.[25–27] Furthermore, as an inhaled anesthetic, Sevoflurane inevitably causes anesthesia complications (postoperative nausea, vomiting, postoperative pain), Delayed recovery from anesthesia, post-procedural complication, and occupational exposure to product. As for fetal exposure during pregnancy, increasing evidence have demonstrated that the fetal toxicity caused by exposure to Sevoflurane during pregnancy involves oxidative stress, neuroinflammation, and cognitive impairment.[10]
As reported, Sevoflurane can cause hyperthermia malignant, hyperthermia, and drug interaction. According to relevant research, children receive Sevoflurane induced anesthesia are often more prone to hyperthermia malignant and hyperthermia, which is related to the damage of thermoregulation center.[28] Additionally, Sevoflurane can generate additive interactions with N(2)O and synergistic interactions with opioid drugs.[29]
As for the complications of the respiratory, thermal, and medical disorders system, they are consistent with the reported findings as well. For instance, after receiving Sevoflurane inhalation anesthesia, the child can cause laryngospasm and can be treated with intramuscular injection of succinylcholine.[30] Furthermore, Brittany team has reported that Sevoflurane may cause hypercapnia in children.[19] Ahmed-Khan et al[31] reported that Sevoflurane could cause diffuse pulmonary alveolar hemorrhage. Therefore, if hemoptysis and hypoxemia occur after Sevoflurane induction, we should be alert to the possibility of alveolar hemorrhage. In addition, the recent literature have also reported that Sevoflurane can cause hypoxia, apnea, hypoxemia, bronchial obstruction, and pneumothorax.[32] According to a case report from Japan, we found that a 9-year-old Japanese boy developed bronchospasm and hypercapnia after Sevoflurane anesthesia.[33] Moreover, related literature have reported that Sevoflurane can cause pulmonary edema and airway bleeding in goats, and can also cause pulmonary edema in humans.[34,35] In addition, according to a retrospective study of 5864 cases conducted in Spain, we found that although Sevoflurane could have good anesthesia effects, it had also led to severe respiratory failure in several cases.[36]
Regarding cardiac disorders, we found that the vast majority of AEs were the same as reported. For example, under Sevoflurane anesthesia, it is more likely to cause ventricular fibrillation and torsade de pointe.[37,38] Besides, Sevoflurane can cause multiform ventricular tachycardia, which is believed to be caused by prolonging the Q-T interval.[39] Furthermore, related studies have also found that children experience significant rapid onset of bradycardia during Sevoflurane induced anesthesia.[40] Furthermore, related cases have also been reported to experience sudden cardiac arrest after receiving Sevoflurane anesthesia.[41]
However, there are also several AEs have not been reported yet, which are also the AE need to be focused on. At present, there are no relevant research reports on the correlation between atrial fibrillation and Sevoflurane. However, based on our ROR values, we found that there is a certain possibility of inducing atrial fibrillation in patients receiving Sevoflurane anesthesia. Therefore, we do not recommend using Sevoflurane anesthesia for patients with arrhythmia.
We were the first to conduct a systematic analysis of adverse reactions of Sevoflurane drugs through the FAERS database. In addition, based on our results, we found that atrial fibrillation is also a common AE of Sevoflurane, but there are currently no relevant reports on the correlation between the 2. However, our research is still remain an analysis of public database, and the correlation between atrial fibrillation and Sevoflurane needs further clinical research to confirm.
In Summary, we downloaded and analyzed the results of the FAERS database, and obtained the most common adverse reactions of Sevoflurane, most of which are closely related to clinically discovered adverse reactions. In addition, we also found that Sevoflurane can cause a novel AE, namely atrial fibrillation, which provides a theoretical basis for guiding our clinical use of Sevoflurane.
Acknowledgments
We thank the FAERS database for generously sharing a large amount of data.
Author contributions
Writing – original draft: Xinxia Yang.
Writing – review & editing: Xinxia Yang, Dongdong Chen.
Formal analysis: Yiming Shen.
Data curation: Hang Chen.
Investigation: Hang Chen.
Software: Dongdong Chen.
Supervision: Dongdong Chen.
Abbreviations:
- AEs
- adverse events
- FAERS
- FDA adverse event reporting system
- MeSH
- Medical Subject Headings
- PRR
- proportional reporting ratio
- PT
- preferred term
- ROR
- reporting odds ratio
- SAS
- statistics analysis system
- SOCs
- system organ classes
The authors have no funding and conflicts to disclose.
Consent for publication is not applicable for this study.
Ethics approval and consent to participate is not applicable for this study.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Yang X, Shen Y, Chen H, Chen D. An analysis of the safety of Sevoflurane drugs: A disproportionality analysis based on Food and Drug Administration Adverse Event Reporting System. Medicine 2024;103:35(e38873).
References
- [1].Wallin R, Regan B, Napoli M, et al. Sevoflurane: a new inhalational anesthetic agent. Anesth Analg. 1975;54:758–66. [DOI] [PubMed] [Google Scholar]
- [2].Eger EJ. New inhaled anesthetics. Anesthesiology. 1994;80:906–22. [DOI] [PubMed] [Google Scholar]
- [3].Murat I, Dubois M, Piat V. Sevoflurane. Ann Fr Anesth Reanim. 1995;14:489–501. [DOI] [PubMed] [Google Scholar]
- [4].Doi M, Ikeda KJ. Airway irritation produced by volatile anaesthetics during brief inhalation: comparison of halothane, enflurane, isoflurane and sevoflurane. Can J Anaesth. 1993;40:122–6. [DOI] [PubMed] [Google Scholar]
- [5].Kharasch EJ. Biotransformation of sevoflurane. Anesth Analg. 1995;81:S27–38. [DOI] [PubMed] [Google Scholar]
- [6].De Hert S, Moerman A. Sevoflurane. F1000Res. 2015;4:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schwender D, End H, Daunderer M, et al. Sevoflurane and the nervous system. Anaesthesist. 1998;47:S37–42. [DOI] [PubMed] [Google Scholar]
- [8].Jiang J, Jiang H. Effect of the inhaled anesthetics isoflurane, sevoflurane and desflurane on the neuropathogenesis of Alzheimer’s disease (review). Mol Med Rep. 2015;12:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth. 2005;15:266–74. [DOI] [PubMed] [Google Scholar]
- [10].Sun M, Xie Z, Zhang J, et al. Mechanistic insight into sevoflurane-associated developmental neurotoxicity. Cell Biol Toxicol. 2022;38:927–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Scholz J. Sevoflurane in pediatric anesthesia. Malignant hyperthermia. Anaesthesist. 1998;47:S43–48. [DOI] [PubMed] [Google Scholar]
- [13].Kuratani N, Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trials. Anesthesiology. 2008;109:225–32. [DOI] [PubMed] [Google Scholar]
- [14].Zhao Y, Qin F, Liu Y, et al. The safety of propofol versus sevoflurane for general anesthesia in children: a meta-analysis of randomized controlled trials. Front Surg. 2022;9:924647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kraus M, Leuzinger K, Reynolds E, et al. Diabetes insipidus related to sedation in the intensive care unit: a review of the literature. J Crit Care. 2023;75:154233. [DOI] [PubMed] [Google Scholar]
- [16].Schindler E, Hempelmann G. Perfusion and metabolism of liver and splanchnic nerve area under sevoflurane anesthesia. Anaesthesist. 1998;47:S19–23. [DOI] [PubMed] [Google Scholar]
- [17].Gonzalo Pascual V, Forner González A, Salvador E, et al. Severe acute hepatitis after anesthesia with sevoflurane. Gastroenterol Hepatol. 2005;28:361–2. [DOI] [PubMed] [Google Scholar]
- [18].Goa K, Noble S, Spencer C. Sevoflurane in paediatric anaesthesia: a review. Paediatr Drugs. 1999;1:127–53. [DOI] [PubMed] [Google Scholar]
- [19].Shutes B, Frazier W, Tobias J. An unusual complication with the administration of a volatile anesthetic agent for status asthmaticus in the pediatric intensive care unit: case report and review of the literature. J Intensive Care Med. 2017;32:400–4. [DOI] [PubMed] [Google Scholar]
- [20].Barrons R, Nguyen L. Succinylcholine-induced rhabdomyolysis in adults: case report and review of the literature. J Pharm Pract. 2020;33:102–7. [DOI] [PubMed] [Google Scholar]
- [21].Timmer A, Motschall E. The Cochrane Library – a short introduction for gastroenterologists. Z Gastroenterol. 2007;45:259–64. [DOI] [PubMed] [Google Scholar]
- [22].Mao Y, Lu Z. MeSH now: automatic MeSH indexing at PubMed scale via learning to rank. J Biomed Semantics. 2017;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brown E, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20:109–17. [DOI] [PubMed] [Google Scholar]
- [24].Moreland-Head L, Coons J, Seybert A, et al. Use of disproportionality analysis to identify previously unknown drug-associated causes of cardiac arrhythmias using the Food and Drug Administration Adverse Event Reporting System (FAERS) database. J Cardiovasc Pharmacol Ther. 2021;26:341–8. [DOI] [PubMed] [Google Scholar]
- [25].Chai D, Cheng Y, Jiang H. Fundamentals of fetal toxicity relevant to sevoflurane exposures during pregnancy. Int J Dev Neurosci. 2019;72:31–5. [DOI] [PubMed] [Google Scholar]
- [26].Messieha Z. Prevention of sevoflurane delirium and agitation with propofol. Anesth Prog. 2013;60:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miao M, Han Y, Zhang Y, et al. Epileptiform EEG discharges during sevoflurane anesthesia in children: a meta-analysis. Clin Neurophysiol. 2022;143:48–55. [DOI] [PubMed] [Google Scholar]
- [28].Rosenberg H, Davis M, James D, et al. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kreuer S, Bruhn J, Wilhelm W, et al. Pharmacokinetic-pharmacodynamic models for inhaled anaesthetics. Anaesthesist. 2007;56:538–56. [DOI] [PubMed] [Google Scholar]
- [30].Al-alami A, Zestos M, Baraka A. Pediatric laryngospasm: prevention and treatment. Curr Opin Anaesthesiol. 2009;22:388–95. [DOI] [PubMed] [Google Scholar]
- [31].Ahmed-Khan M, Moin K, Funk C, et al. Sevoflurane-induced diffuse alveolar hemorrhage. Arch Clin Cases. 2023;10:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Machotta A. Anaesthetic management for endoscopy of the pediatric airway. Anaesthesist. 2002;51:668–78. [DOI] [PubMed] [Google Scholar]
- [33].Kawahito S, Kitahata H, Kimura H, et al. Bronchospasm during anesthesia in a patient with Prader-Willi syndrome. Masui. 1995;44:1675–9. [PubMed] [Google Scholar]
- [34].Adami C, Levionnois O, Spadavecchia C. Acute pulmonary edema and airway hemorrhage in a goat during sevoflurane anesthesia. Schweiz Arch Tierheilkd. 2011;153:86–8. [DOI] [PubMed] [Google Scholar]
- [35].González-Moya J, Calderón Seoane E, Pastor Ferrer Y. Acute pulmonary edema after anesthesia with sevoflurane. Arch Bronconeumol. 1999;35:194. [DOI] [PubMed] [Google Scholar]
- [36].De Sanctis Briggs V. Sedation with sevoflurane for magnetic resonance imaging in pediatrics: retrospective study of 5864 cases. Rev Esp Anestesiol Reanim. 2009;56:212–6. [DOI] [PubMed] [Google Scholar]
- [37].Abe K, Takada K, Yoshiya I. Intraoperative torsade de pointes ventricular tachycardia and ventricular fibrillation during sevoflurane anesthesia. Anesth Analg. 1998;86:701–2. [DOI] [PubMed] [Google Scholar]
- [38].Tanaka K, Nakamura M, Umeda T, et al. Effects of sevoflurane and halothane on reperfusion-induced arrhythmia in the isolated rat heart. Clin Ther. 1993;15:1085–93. [PubMed] [Google Scholar]
- [39].Kleinsasser A, Kuenszberg E, Loeckinger A, et al. Sevoflurane, but not propofol, significantly prolongs the Q-T interval. Anesth Analg. 2000;90:25–7. [DOI] [PubMed] [Google Scholar]
- [40].Walia H, Ruda J, Tobias J. Sevoflurane and bradycardia in infants with trisomy 21: a case report and review of the literature. Int J Pediatr Otorhinolaryngol. 2016;80:5–7. [DOI] [PubMed] [Google Scholar]
- [41].Nogami K, Taniguchi S, Togami K. Transient cardiac arrest in a child with Down syndrome during anesthesia induction with sevoflurane: a case report. JA Clin Rep. 2016;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]