Abstract
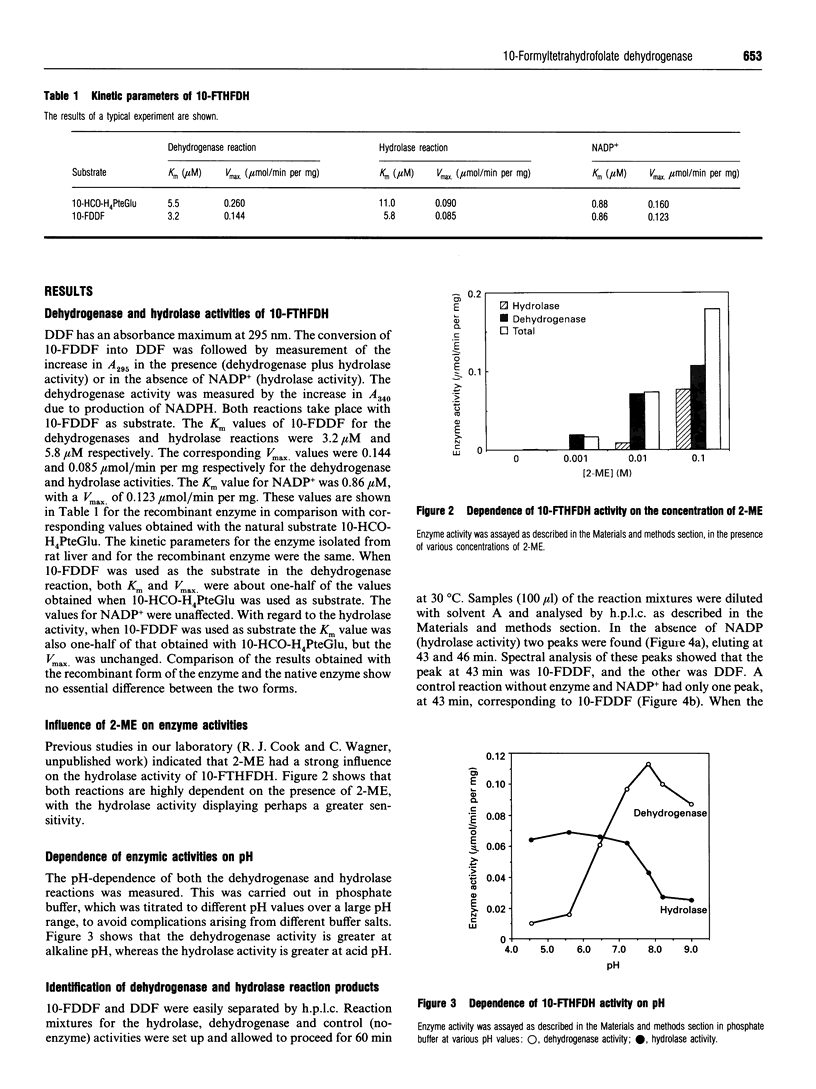
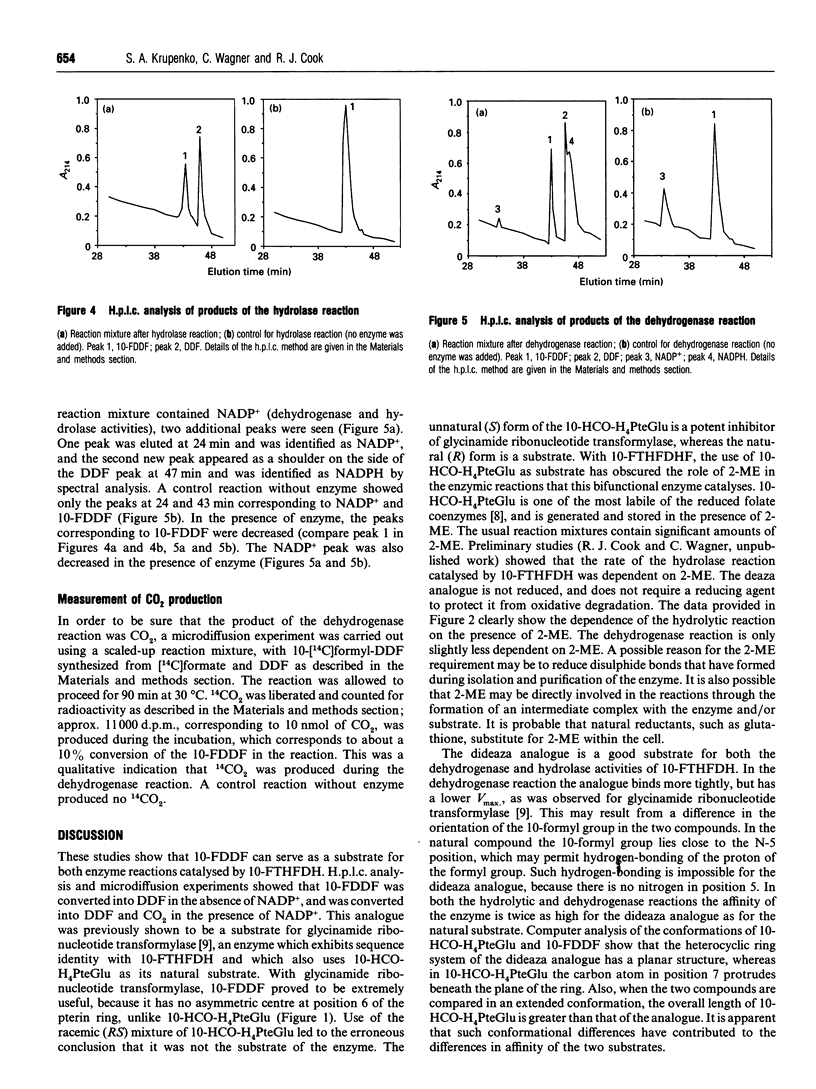
10-Formyltetrahydrofolate dehydrogenase (EC 1.5.1.6) is a bifunctional enzyme, displaying both NADP(+)-dependent dehydrogenase activity for the formation of tetrahydrofolate and CO2, and NADP(+)-independent hydrolase activity for the formation of tetrahydrofolate and formate. A previous report [Case, Kaisaki and Steele (1988) J. Biol. Chem. 263, 1024-1027] claimed that dehydrogenase and hydrolase activities were products of separate cytosolic and mitochondrial forms of this enzyme. Here we report that recombinant 10-formyltetrahydrofolate dehydrogenase carries out both enzymic reactions, proving that a product of a single gene, i.e. one protein, not two, has both activities. The stable synthetic analogue 10-formyl-5,8-dideazafolate can substitute for the labile natural substrate, 10-formyltetrahydrofolate, in both reactions. This was shown with both native and recombinant rat liver enzyme. The Km values for 10-formyl-5,8-dideazafolate were half of those for 10-formyltetrahydrofolate in both the dehydrogenase and hydrolytic reactions. The Vmax, values were similar for both substrates. Both dehydrogenase and hydrolase reactions were dependent on the presence of 2-mercaptoethanol. The pH optima were 7.8 and 5.6 for the dehydrogenase and hydrolase reactions respectively, consistent with the presence of two active sites in the enzyme.
Full text
PDF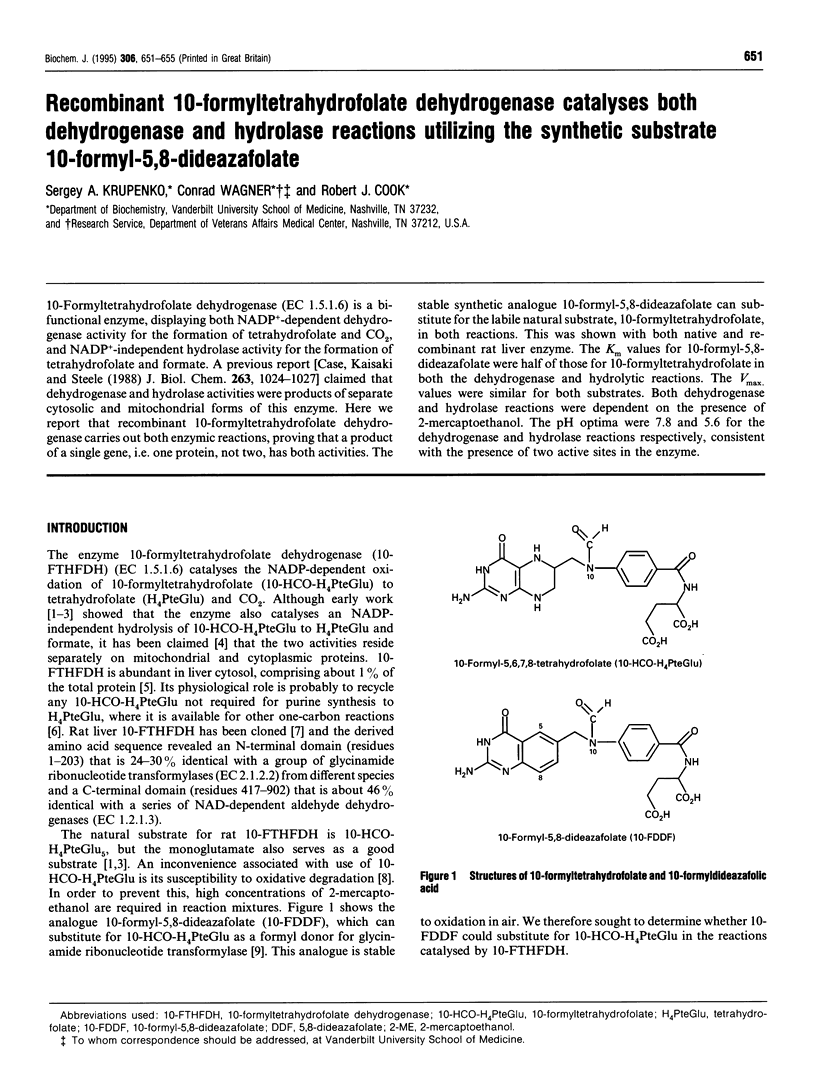


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook R. J., Lloyd R. S., Wagner C. Isolation and characterization of cDNA clones for rat liver 10-formyltetrahydrofolate dehydrogenase. J Biol Chem. 1991 Mar 15;266(8):4965–4973. [PubMed] [Google Scholar]
- Cook R. J., Wagner C. Purification and partial characterization of rat liver folate binding protein: cytosol I. Biochemistry. 1982 Aug 31;21(18):4427–4434. doi: 10.1021/bi00261a036. [DOI] [PubMed] [Google Scholar]
- Cook R. J., Wagner C. Purification of rat liver folate-binding protein: cytosol I. Methods Enzymol. 1986;122:251–255. doi: 10.1016/0076-6879(86)22178-3. [DOI] [PubMed] [Google Scholar]
- Horne D. W., Patterson D., Cook R. J. Effect of nitrous oxide inactivation of vitamin B12-dependent methionine synthetase on the subcellular distribution of folate coenzymes in rat liver. Arch Biochem Biophys. 1989 May 1;270(2):729–733. doi: 10.1016/0003-9861(89)90556-0. [DOI] [PubMed] [Google Scholar]
- Rios-Orlandi E. M., Zarkadas C. G., MacKenzie R. E. Formyltetrahydrofolate dehydrogenase-hydrolase from pig liver: simultaneous assay of the activities. Biochim Biophys Acta. 1986 May 12;871(1):24–35. doi: 10.1016/0167-4838(86)90129-9. [DOI] [PubMed] [Google Scholar]
- Schirch D., Villar E., Maras B., Barra D., Schirch V. Domain structure and function of 10-formyltetrahydrofolate dehydrogenase. J Biol Chem. 1994 Oct 7;269(40):24728–24735. [PubMed] [Google Scholar]
- Scrutton M. C., Beis I. Inhibitory effects of histidine and their reversal. The roles of pyruvate carboxylase and N10-formyltetrahydrofolate dehydrogenase. Biochem J. 1979 Mar 1;177(3):833–846. doi: 10.1042/bj1770833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. K., Mueller W. T., Benkovic P. A., Benkovic S. J. On the cofactor specificity of glycinamide ribonucleotide and 5-aminoimidazole-4-carboxamide ribonucleotide transformylase from chicken liver. Biochemistry. 1981 Mar 3;20(5):1241–1245. doi: 10.1021/bi00508a029. [DOI] [PubMed] [Google Scholar]



