Abstract
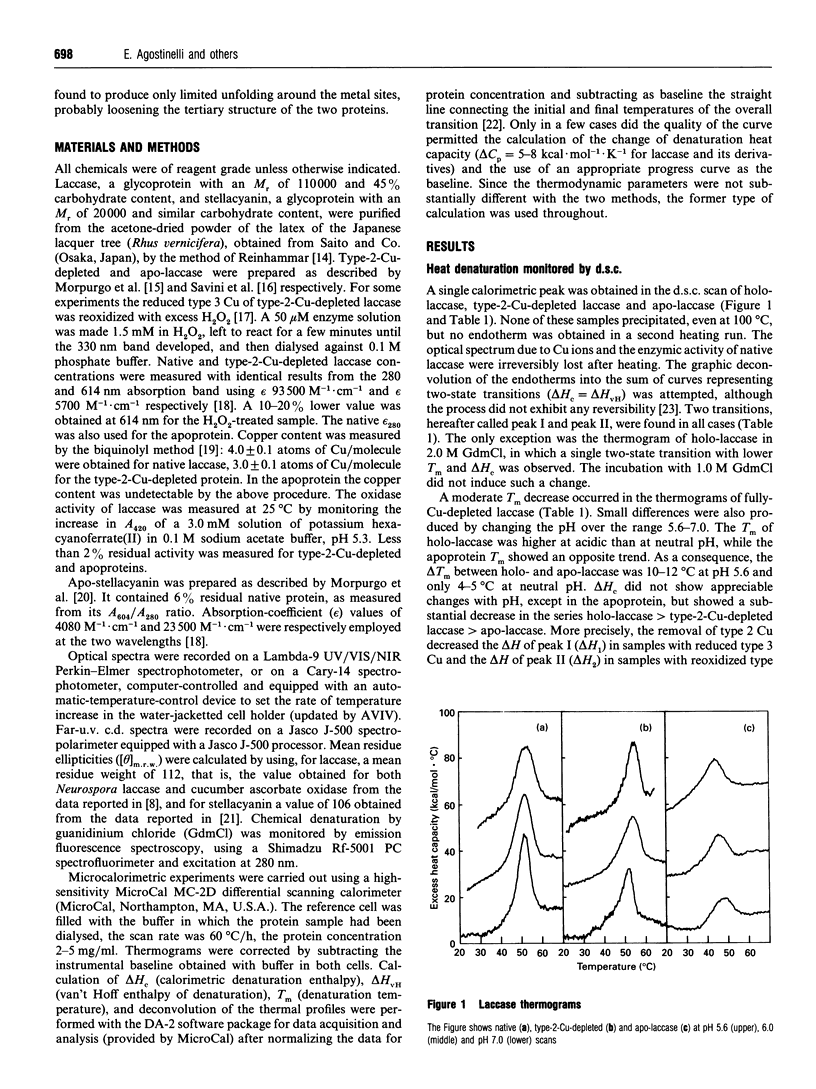
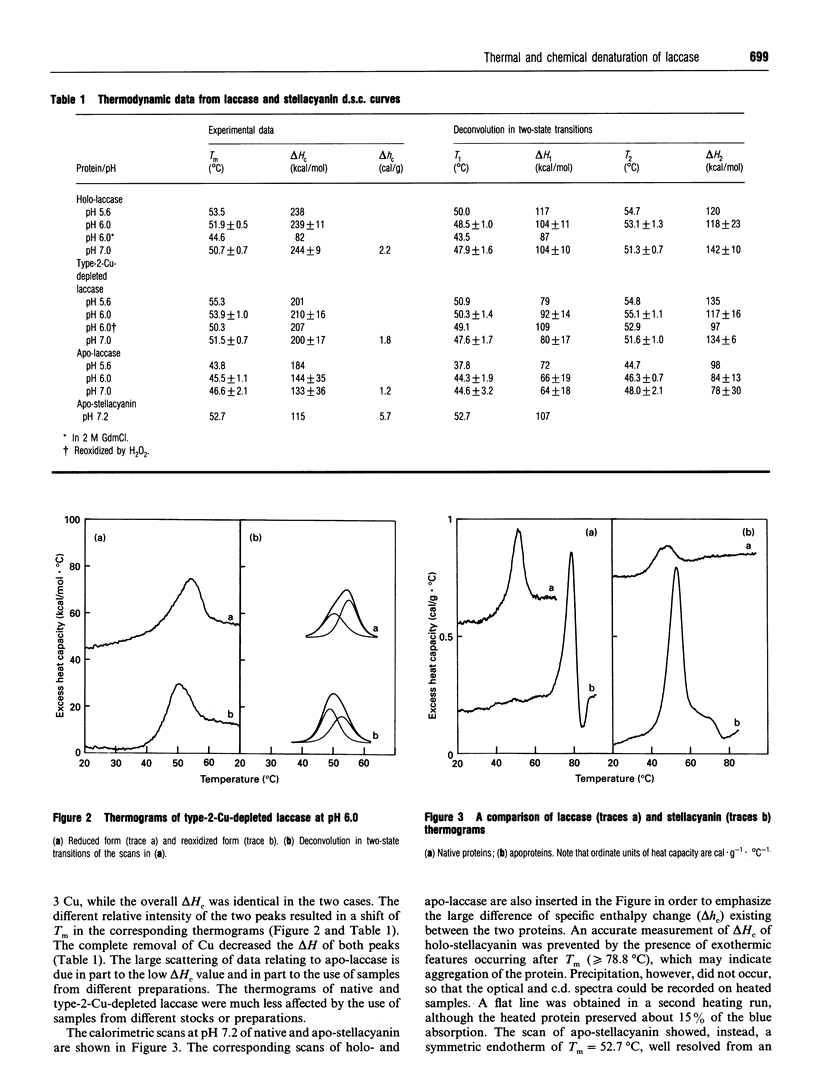
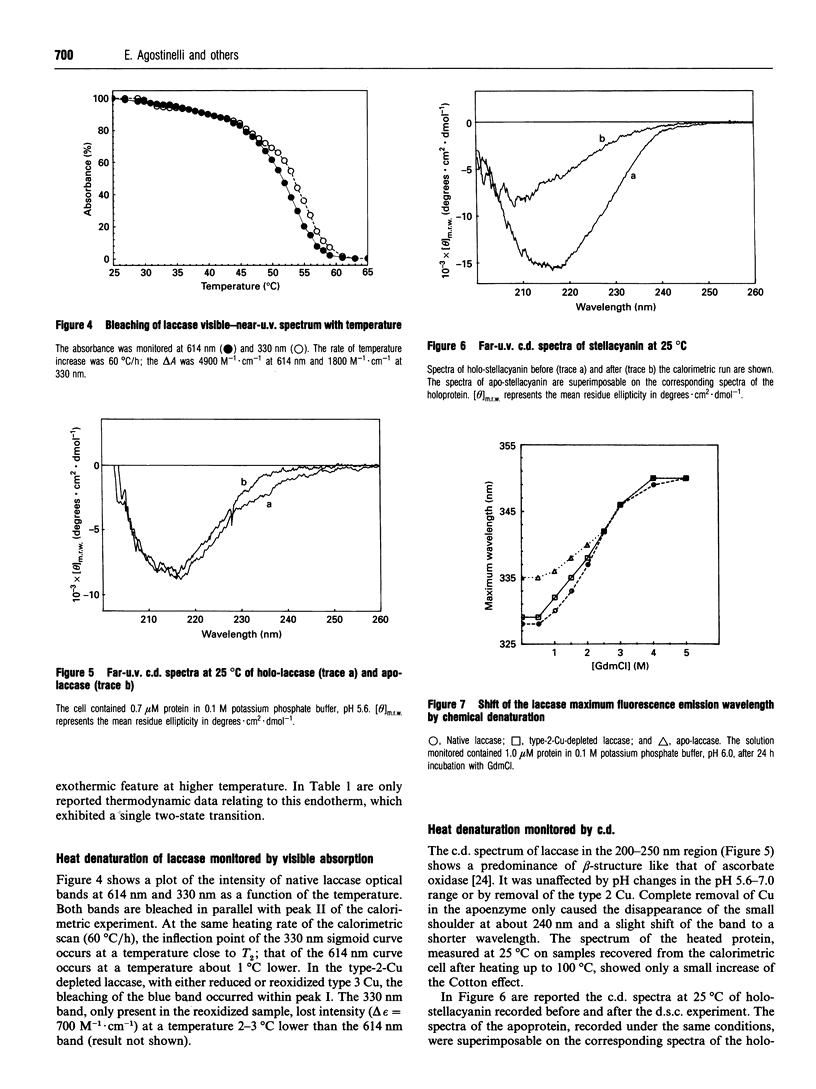
The thermal denaturation of laccase from the Japanese lacquer tree (Rhus vernicifera) was studied by differential scanning calorimetry. The endotherms of holo-laccase, type 2-Cu-depleted laccase and apo-laccase were deconvoluted into two independent two-state transitions, providing evidence for a domain structure of the protein. The correlation of the two transitions with the bleaching of copper optical bands and the decrease of the transitions' enthalpy on Cu removal show that the process involves the denaturation of Cu sites. No detectable unfolding of secondary structure was observed, since the thermal transitions, characterized by low overall specific enthalpy, did not modify either the laccase c.d. spectra in the beta-fold region or the maximum wavelength of the fluorescence emission. On chemical denaturation, however, the emission was red-shifted by about 20 nm. The laccase behaviour is substantially different from that of stellacyanin, a protein containing a single blue Cu ion, in which the thermal transition had higher specific enthalpy and induced a large change of the c.d. spectrum in the beta-fold region. The laccase denaturation behaviour is similar to that of ascorbate oxidase from zucchini (courgette; Cucurbita pepo) [Savini, D'Alessio, Giartosio, Morpurgo and Avigliano (1990) Eur. J. Biochem. 190, 491-495], suggesting a structural analogy. In both proteins heating may cause a change of tertiary structure through modifications of Cu co-ordination with loosening of the bonds between the structural domains at the interface of which the trinuclear Cu cluster is located.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergaman C., Gandvik E. K., Nyman P. O., Strid L. The amino acid sequence of Stellacyanin from the lacquer tree. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1052–1059. doi: 10.1016/s0006-291x(77)80084-3. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi di Patti M. C., Musci G., Giartosio A., D'Alessio S., Calabrese L. The multidomain structure of ceruloplasmin from calorimetric and limited proteolysis studies. J Biol Chem. 1990 Dec 5;265(34):21016–21022. [PubMed] [Google Scholar]
- Briving C., Gandvik E. K., Nyman P. O. Structural studies around cysteine and cystine residues in the "blue" oxidase fungal laccase B. Similarity in amino acid sequence with ceruloplasmin. Biochem Biophys Res Commun. 1980 Mar 28;93(2):454–461. doi: 10.1016/0006-291x(80)91099-2. [DOI] [PubMed] [Google Scholar]
- Edge V., Allewell N. M., Sturtevant J. M. Differential scanning calorimetric study of the thermal denaturation of aspartate transcarbamoylase of Escherichia coli. Biochemistry. 1988 Oct 18;27(21):8081–8087. doi: 10.1021/bi00421a017. [DOI] [PubMed] [Google Scholar]
- Engeseth H. R., McMillin D. R. Studies of thermally induced denaturation of azurin and azurin derivatives by differential scanning calorimetry: evidence for copper selectivity. Biochemistry. 1986 May 6;25(9):2448–2455. doi: 10.1021/bi00357a023. [DOI] [PubMed] [Google Scholar]
- Fields B. A., Guss J. M., Freeman H. C. Three-dimensional model for stellacyanin, a "blue" copper-protein. J Mol Biol. 1991 Dec 20;222(4):1053–1065. doi: 10.1016/0022-2836(91)90593-u. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships. Eur J Biochem. 1990 Jan 26;187(2):341–352. doi: 10.1111/j.1432-1033.1990.tb15311.x. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Ladenstein R., Huber R., Bolognesi M., Avigliano L., Petruzzelli R., Rossi A., Finazzi-Agró A. Refined crystal structure of ascorbate oxidase at 1.9 A resolution. J Mol Biol. 1992 Mar 5;224(1):179–205. doi: 10.1016/0022-2836(92)90583-6. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Luecke H., Huber R. X-ray structures and mechanistic implications of three functional derivatives of ascorbate oxidase from zucchini. Reduced, peroxide and azide forms. J Mol Biol. 1993 Apr 5;230(3):997–1014. doi: 10.1006/jmbi.1993.1215. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Rossi A., Ladenstein R., Huber R., Bolognesi M., Gatti G., Marchesini A., Petruzzelli R., Finazzi-Agró A. X-ray crystal structure of the blue oxidase ascorbate oxidase from zucchini. Analysis of the polypeptide fold and a model of the copper sites and ligands. J Mol Biol. 1989 Apr 5;206(3):513–529. doi: 10.1016/0022-2836(89)90498-1. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Steigemann W., Huber R., Lang G., Kroneck P. M. X-ray crystallographic characterisation of type-2-depleted ascorbate oxidase from zucchini. Eur J Biochem. 1992 Oct 15;209(2):597–602. doi: 10.1111/j.1432-1033.1992.tb17325.x. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Finazzi-Agrò A., Rotilio G., Mondovì B. Studies of the metal sites of copper proteins. IV. Stellacyanin: preparation of apoprotein and involvement of sulfhydryl and tryptophan in the copper chromophore. Biochim Biophys Acta. 1972 Jul 21;271(2):292–299. doi: 10.1016/0005-2795(72)90203-6. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Graziani M. T., Finazzi-Agrò A., Rotilio G., Mondovì B. Optical properties of japanese-lacquer-tree (Rhus vernicifera) laccase depleted of type 2 copper(II). Involvement of type-2 copper(II) in the 330nm chromophore. Biochem J. 1980 May 1;187(2):361–366. doi: 10.1042/bj1870361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpurgo L., Savini I., Gatti G., Bolognesi M., Avigliano L. Reassessment of copper stoichiometry in ascorbate oxidase. Biochem Biophys Res Commun. 1988 Apr 29;152(2):623–628. doi: 10.1016/s0006-291x(88)80084-6. [DOI] [PubMed] [Google Scholar]
- Musci G., Bonaccorsi di Patti M. C., Fagiolo U., Calabrese L. Age-related changes in human ceruloplasmin. Evidence for oxidative modifications. J Biol Chem. 1993 Jun 25;268(18):13388–13395. [PubMed] [Google Scholar]
- Ortel T. L., Takahashi N., Putnam F. W. Structural model of human ceruloplasmin based on internal triplication, hydrophilic/hydrophobic character, and secondary structure of domains. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4761–4765. doi: 10.1073/pnas.81.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L., Potekhin S. A. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986;131:4–51. doi: 10.1016/0076-6879(86)31033-4. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Savini I., D'Alessio S., Giartosio A., Morpurgo L., Avigliano L. The role of copper in the stability of ascorbate oxidase towards denaturing agents. Eur J Biochem. 1990 Jul 5;190(3):491–495. doi: 10.1111/j.1432-1033.1990.tb15600.x. [DOI] [PubMed] [Google Scholar]
- Savini I., Morpurgo L., Avigliano L. Full, reversible copper removal from ascorbate oxidase. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1251–1255. doi: 10.1016/0006-291x(85)90225-6. [DOI] [PubMed] [Google Scholar]
- Wherland S., Farver O., Pecht I. Three-dimensional model of stellacyanin and its implications for electron transfer reactivity. J Mol Biol. 1988 Nov 20;204(2):407–415. doi: 10.1016/0022-2836(88)90585-2. [DOI] [PubMed] [Google Scholar]


