Abstract
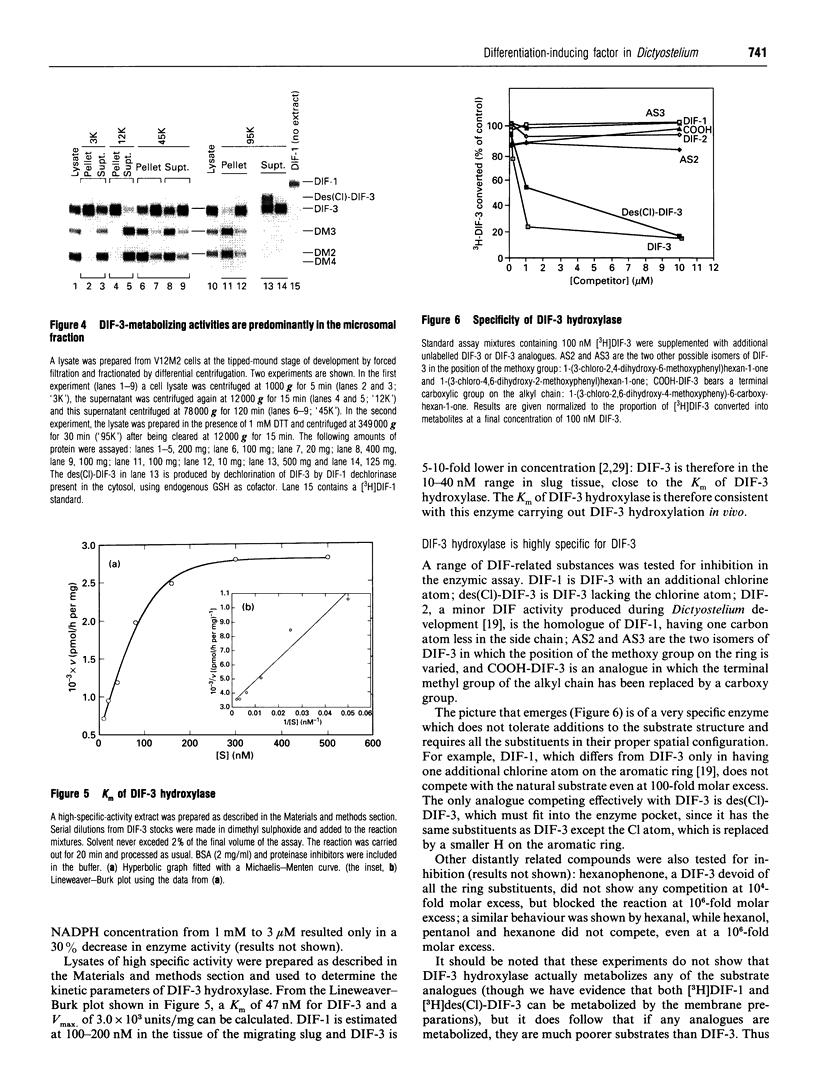
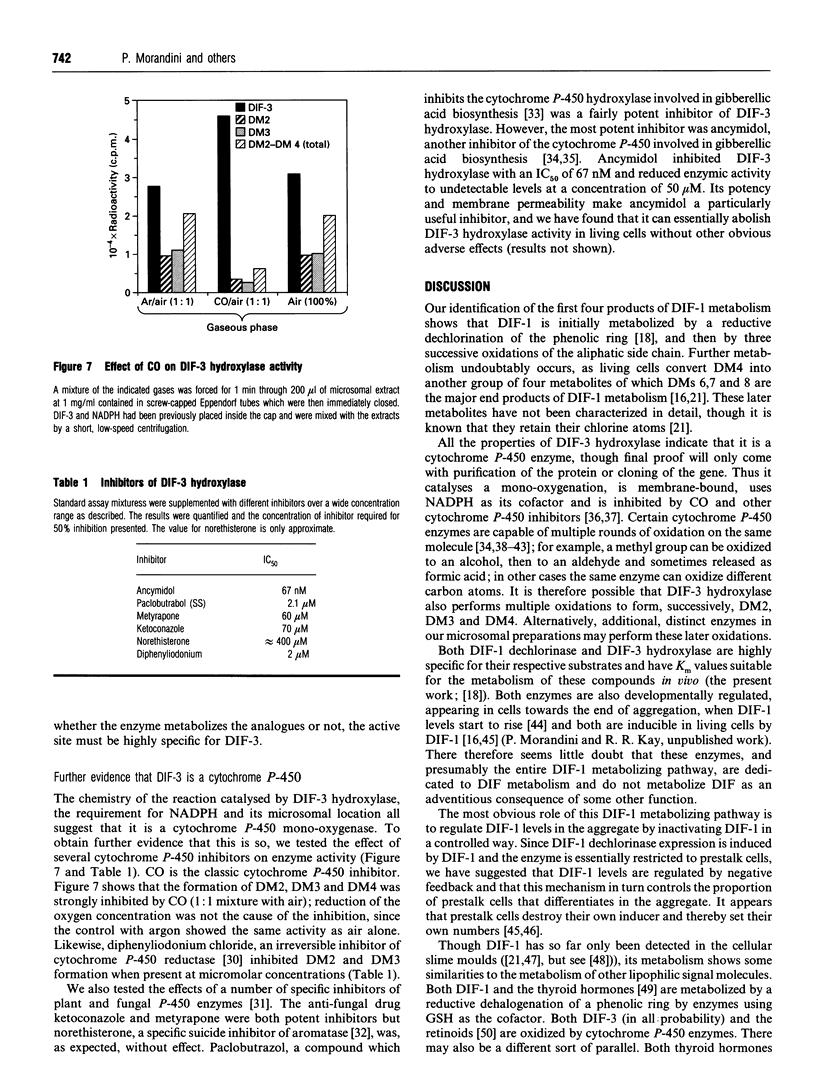
Stalk cell differentiation during development of the slime mould Dictyostelium is induced by a chlorinated alkyl phenone called differentiation-inducing factor-1 (DIF-1). Inactivation of DIF-1 is likely to be a key element in the DIF-1 signalling system, and we have shown previously that this is accomplished by a dedicated metabolic pathway involving up to 12 unidentified metabolites. We report here the structure of the first four metabolites produced from DIF-1, as deduced by m.s., n.m.r. and chemical synthesis. The structures of these compounds show that the first step in metabolism is a dechlorination of the phenolic ring, producing DIF metabolite 1 (DM1). DM1 is identical with the previously known minor DIF activity, DIF-3. DIF-3 is then metabolized by three successive oxidations of its aliphatic side chain: a hydroxylation at omega-2 to produce DM2, oxidation of the hydroxy group to a ketone group to produce DM3 and a further hydroxylation at omega-1 to produce DM4, a hydroxyketone of DIF-3. We have investigated the enzymology of DIF-1 metabolism. It is already known that the first step, to produce DIF-3, is catalysed by a novel dechlorinase. The enzyme activity responsible for the first side-chain oxidation (DIF-3 hydroxylase) was detected by incubating [3H]DIF-3 with cell-free extracts and resolving the reaction products by t.l.c. DIF-3 hydroxylase has many of the properties of a cytochrome P-450. It is membrane-bound and uses NADPH as co-substrate. It is also inhibited by CO, the classic cytochrome P-450 inhibitor, and by several other cytochrome P-450 inhibitors, as well as by diphenyliodonium chloride, an inhibitor of cytochrome P-450 reductase. DIF-3 hydroxylase is highly specific for DIF-3: other closely related compounds do not compete for the activity at 100-fold molar excess, with the exception of the DIF-3 analogue lacking the chlorine atom. The Km for DIF-3 of 47 nM is consistent with this enzyme being responsible for DIF-3 metabolism in vivo. The two further oxidations necessary to produce DM4 are also performed in vitro by similar enzyme activities. One of the inhibitors of DIF-3 hydroxylase, ancymidol (IC50 67 nM) is likely to be particularly suitable for probing the function of DIF metabolism during development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman P. J., Mackenzie A., Bramley P. M. Characterization of ent-kaurene oxidase activity from Gibberella fujikuroi. Biochim Biophys Acta. 1990 Nov 9;1036(2):151–157. doi: 10.1016/0304-4165(90)90027-t. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berks M., Kay R. R. Combinatorial control of cell differentiation by cAMP and DIF-1 during development of Dictyostelium discoideum. Development. 1990 Nov;110(3):977–984. doi: 10.1242/dev.110.3.977. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Brookman J. J., Jermyn K. A., Kay R. R. Nature and distribution of the morphogen DIF in the Dictyostelium slug. Development. 1987 May;100(1):119–124. doi: 10.1242/dev.100.1.119. [DOI] [PubMed] [Google Scholar]
- Brookman J. J., Town C. D., Jermyn K. A., Kay R. R. Developmental regulation of a stalk cell differentiation-inducing factor in Dictyostelium discoideum. Dev Biol. 1982 May;91(1):191–196. doi: 10.1016/0012-1606(82)90022-7. [DOI] [PubMed] [Google Scholar]
- Coolbaugh R. C., Hell D. R., West C. A. Comparative Effects of Substituted Pyrimidines on Growth and Gibberellin Biosynthesis in Gibberella fujikuroi. Plant Physiol. 1982 Mar;69(3):712–716. doi: 10.1104/pp.69.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolbaugh R. C., Hirano S. S., West C. A. Studies on the Specificity and Site of Action of alpha-Cyclopropyl-alpha-[p-methoxyphenyl]-5-pyrimidine Methyl Alcohol (Ancymidol), a Plant Growth Regulator. Plant Physiol. 1978 Oct;62(4):571–576. doi: 10.1104/pp.62.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early A. E., Williams J. G. A Dictyostelium prespore-specific gene is transcriptionally repressed by DIF in vitro. Development. 1988 Jul;103(3):519–524. doi: 10.1242/dev.103.3.519. [DOI] [PubMed] [Google Scholar]
- Esch R. K., Firtel R. A. cAMP and cell sorting control the spatial expression of a developmentally essential cell-type-specific ras gene in Dictyostelium. Genes Dev. 1991 Jan;5(1):9–21. doi: 10.1101/gad.5.1.9. [DOI] [PubMed] [Google Scholar]
- Fosnaugh K. L., Loomis W. F. Coordinate regulation of the spore coat genes in Dictyostelium discoideum. Dev Genet. 1991;12(1-2):123–132. doi: 10.1002/dvg.1020120120. [DOI] [PubMed] [Google Scholar]
- Gambino M., Kay R. R., Bozzaro S. Morphogenesis and differentiation of Dictyostelium cells interacting with immobilized glucosides: dependence on DIF production. Differentiation. 1992 Apr;49(3):133–141. doi: 10.1111/j.1432-0436.1992.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Reactions and significance of cytochrome P-450 enzymes. J Biol Chem. 1991 Jun 5;266(16):10019–10022. [PubMed] [Google Scholar]
- Hamerski D., Matern U. Elicitor-induced biosynthesis of psoralens in Ammi majus L. suspension cultures. Microsomal conversion of demethylsuberosin into (+)marmesin and psoralen. Eur J Biochem. 1988 Jan 15;171(1-2):369–375. doi: 10.1111/j.1432-1033.1988.tb13800.x. [DOI] [PubMed] [Google Scholar]
- Insall R., Kay R. R. A specific DIF binding protein in Dictyostelium. EMBO J. 1990 Oct;9(10):3323–3328. doi: 10.1002/j.1460-2075.1990.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insall R., Nayler O., Kay R. R. DIF-1 induces its own breakdown in Dictyostelium. EMBO J. 1992 Aug;11(8):2849–2854. doi: 10.1002/j.1460-2075.1992.tb05352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juchau M. R. Substrate specificities and functions of the P450 cytochromes. Life Sci. 1990;47(26):2385–2394. doi: 10.1016/0024-3205(90)90482-7. [DOI] [PubMed] [Google Scholar]
- Kay R. R., Dhokia B., Jermyn K. A. Purification of stalk-cell-inducing morphogens from Dictyostelium discoideum. Eur J Biochem. 1983 Oct 17;136(1):51–56. doi: 10.1111/j.1432-1033.1983.tb07703.x. [DOI] [PubMed] [Google Scholar]
- Kay R. R., Jermyn K. A. A possible morphogen controlling differentiation in Dictyostelium. Nature. 1983 May 19;303(5914):242–244. doi: 10.1038/303242a0. [DOI] [PubMed] [Google Scholar]
- Kay R. R., Large S., Traynor D., Nayler O. A localized differentiation-inducing-factor sink in the front of the Dictyostelium slug. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):487–491. doi: 10.1073/pnas.90.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R. R., Taylor G. W., Jermyn K. A., Traynor D. Chlorine-containing compounds produced during Dictyostelium development. Detection by labelling with 36Cl. Biochem J. 1992 Jan 1;281(Pt 1):155–161. doi: 10.1042/bj2810155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Judd D., Stolee A. Aromatization of delta4-androstene-3,17-dione, 19-hydroxy-delta4-androstene-3,17-dione, and 19-oxo-delta4-androstene-3,17-dione at a common catalytic site in human placental microsomes. Biochemistry. 1977 Jan 11;16(1):140–145. doi: 10.1021/bi00620a024. [DOI] [PubMed] [Google Scholar]
- Kubohara Y., Okamoto K., Tanaka Y., Asahi K., Sakurai A., Takahashi N. Differanisole A, an inducer of the differentiation of Friend leukemic cells, induces stalk cell differentiation in Dictyostelium discoideum. FEBS Lett. 1993 May 3;322(1):73–75. doi: 10.1016/0014-5793(93)81114-f. [DOI] [PubMed] [Google Scholar]
- Kuspa A., Loomis W. F. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J. D., Kitchen S. E., Farooqui A. A., Tuckey R., Kamin H. Cytochrome P-450scc-substrate interactions. Studies of binding and catalytic activity using hydroxycholesterols. J Biol Chem. 1982 Feb 25;257(4):1876–1884. [PubMed] [Google Scholar]
- Masento M. S., Morris H. R., Taylor G. W., Johnson S. J., Skapski A. C., Kay R. R. Differentiation-inducing factor from the slime mould Dictyostelium discoideum and its analogues. Synthesis, structure and biological activity. Biochem J. 1988 Nov 15;256(1):23–28. doi: 10.1042/bj2560023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. R., Masento M. S., Taylor G. W., Jermyn K. A., Kay R. R. Structure elucidation of two differentiation inducing factors (DIF-2 and DIF-3) from the cellular slime mould Dictyostelium discoideum. Biochem J. 1988 Feb 1;249(3):903–906. doi: 10.1042/bj2490903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Masento M. S., Jermyn K. A., Kay R. R. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. 1987 Aug 27-Sep 2Nature. 328(6133):811–814. doi: 10.1038/328811a0. [DOI] [PubMed] [Google Scholar]
- Murphy P. J., West C. A. The role of mixed function oxidases in kaurene metabolism in Echinocystis macrocarpa Greene endosperm. Arch Biochem Biophys. 1969 Sep;133(2):395–407. doi: 10.1016/0003-9861(69)90468-8. [DOI] [PubMed] [Google Scholar]
- Nayler O., Insall R., Kay R. R. Differentiation-inducing-factor dechlorinase, a novel cytosolic dechlorinating enzyme from Dictyostelium discoideum. Eur J Biochem. 1992 Sep 1;208(2):531–536. doi: 10.1111/j.1432-1033.1992.tb17217.x. [DOI] [PubMed] [Google Scholar]
- Newell P. C., Telser A., Sussman M. Alternative developmental pathways determined by environmental conditions in the cellular slime mold Dictyostelium discoideum. J Bacteriol. 1969 Nov;100(2):763–768. doi: 10.1128/jb.100.2.763-768.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa Y., Yarborough C., Osawa Y. Norethisterone, a major ingredient of contraceptive pills, is a suicide inhibitor of estrogen biosynthesis. Science. 1982 Mar 5;215(4537):1249–1251. doi: 10.1126/science.7058343. [DOI] [PubMed] [Google Scholar]
- Pijnappel W. W., Hendriks H. F., Folkers G. E., van den Brink C. E., Dekker E. J., Edelenbosch C., van der Saag P. T., Durston A. J. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature. 1993 Nov 25;366(6453):340–344. doi: 10.1038/366340a0. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Coon M. J. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991 Jul 25;266(21):13469–13472. [PubMed] [Google Scholar]
- Reinhard K., Matern U. Different types of microsomal enzymes catalyze ortho- or para-hydroxylation in the biosynthesis of carnation phytoalexins. FEBS Lett. 1991 Dec 2;294(1-2):67–72. doi: 10.1016/0014-5793(91)81345-9. [DOI] [PubMed] [Google Scholar]
- Song W. C., Brash A. R. Purification of an allene oxide synthase and identification of the enzyme as a cytochrome P-450. Science. 1991 Aug 16;253(5021):781–784. doi: 10.1126/science.1876834. [DOI] [PubMed] [Google Scholar]
- Tew D. G. Inhibition of cytochrome P450 reductase by the diphenyliodonium cation. Kinetic analysis and covalent modifications. Biochemistry. 1993 Sep 28;32(38):10209–10215. doi: 10.1021/bi00089a042. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Isolation of 3,4-didehydroretinoic acid, a novel morphogenetic signal in the chick wing bud. Nature. 1990 Jun 28;345(6278):815–819. doi: 10.1038/345815a0. [DOI] [PubMed] [Google Scholar]
- Thompson E. A., Jr, Siiteri P. K. The involvement of human placental microsomal cytochrome P-450 in aromatization. J Biol Chem. 1974 Sep 10;249(17):5373–5378. [PubMed] [Google Scholar]
- Town C. D., Gross J. D., Kay R. R. Cell differentiation without morphogenesis in Dictyostelium discoideum. Nature. 1976 Aug 19;262(5570):717–719. doi: 10.1038/262717a0. [DOI] [PubMed] [Google Scholar]
- Traynor D., Kay R. R. The DIF-1 signaling system in Dictyostelium. Metabolism of the signal. J Biol Chem. 1991 Mar 15;266(8):5291–5297. [PubMed] [Google Scholar]
- Wada A., Okamoto M., Nonaka Y., Yamano T. Aldosterone biosynthesis by a reconstituted cytochrome P-45011 beta system. Biochem Biophys Res Commun. 1984 Feb 29;119(1):365–371. doi: 10.1016/0006-291x(84)91660-7. [DOI] [PubMed] [Google Scholar]
- Williams J. B., Napoli J. L. Metabolism of retinoic acid and retinol during differentiation of F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4658–4662. doi: 10.1073/pnas.82.14.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Ceccarelli A., McRobbie S., Mahbubani H., Kay R. R., Early A., Berks M., Jermyn K. A. Direct induction of Dictyostelium prestalk gene expression by DIF provides evidence that DIF is a morphogen. Cell. 1987 Apr 24;49(2):185–192. doi: 10.1016/0092-8674(87)90559-9. [DOI] [PubMed] [Google Scholar]
- Wurster B., Kay R. R. New roles for DIF? Effects on early development in Dictyostelium. Dev Biol. 1990 Jul;140(1):189–195. doi: 10.1016/0012-1606(90)90066-r. [DOI] [PubMed] [Google Scholar]
- van Es S., Nieuwenhuijsen B. W., Lenouvel F., van Deursen E. M., Schaap P. Universal signals control slime mold stalk formation. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8219–8223. doi: 10.1073/pnas.91.17.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]




