Abstract
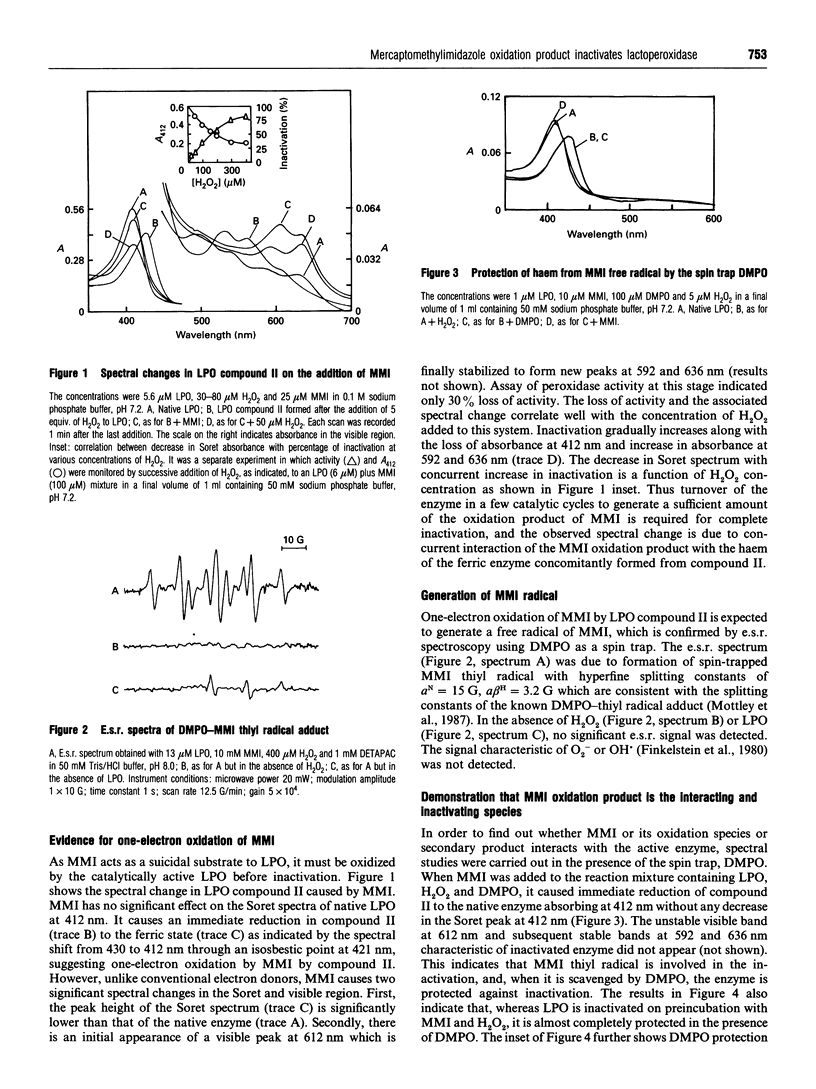
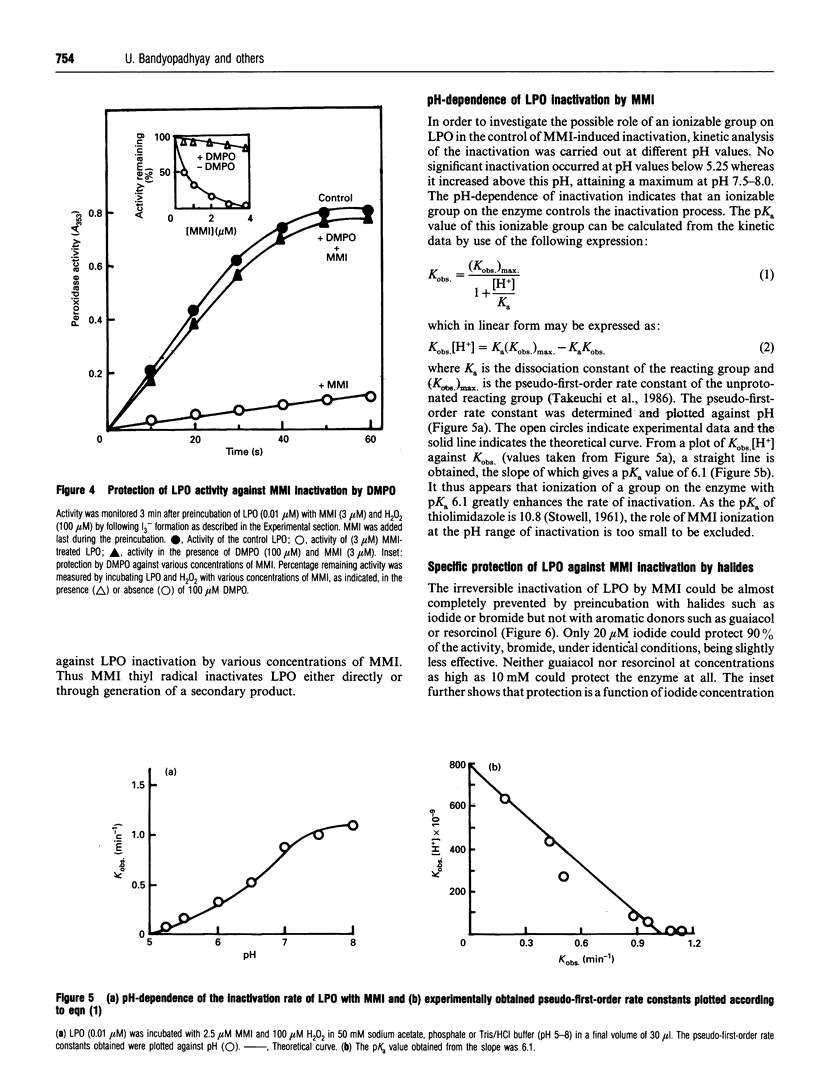
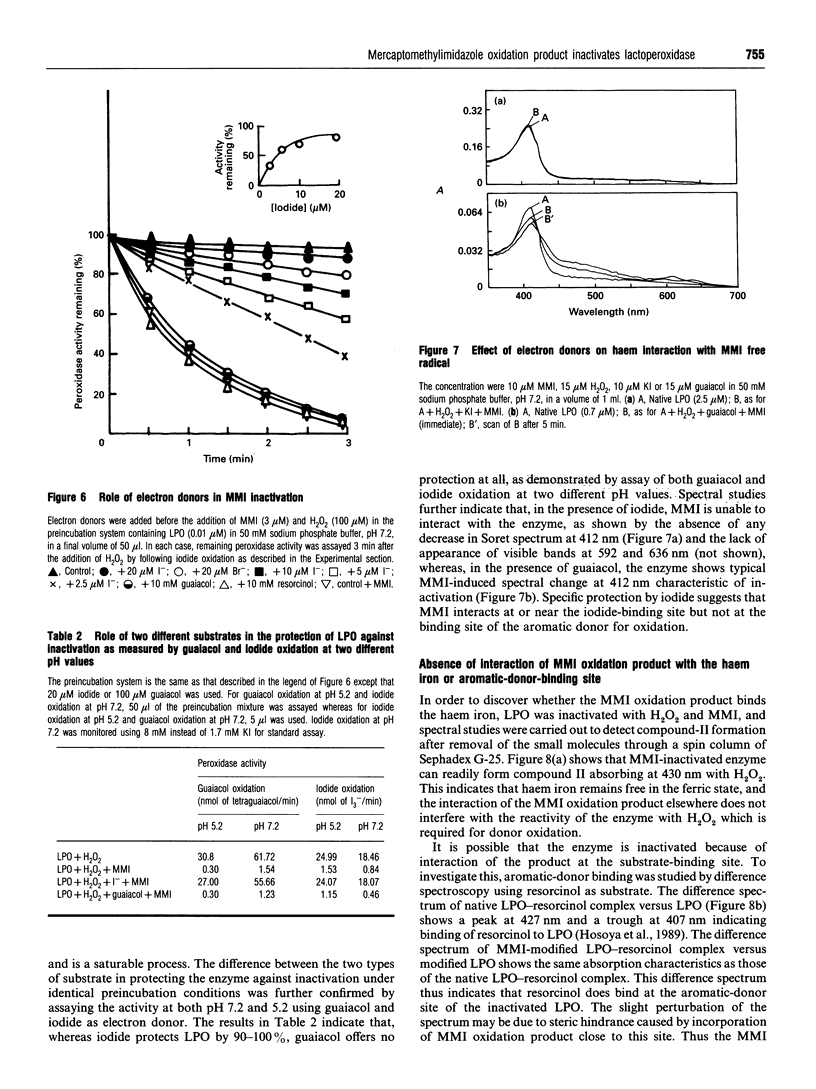
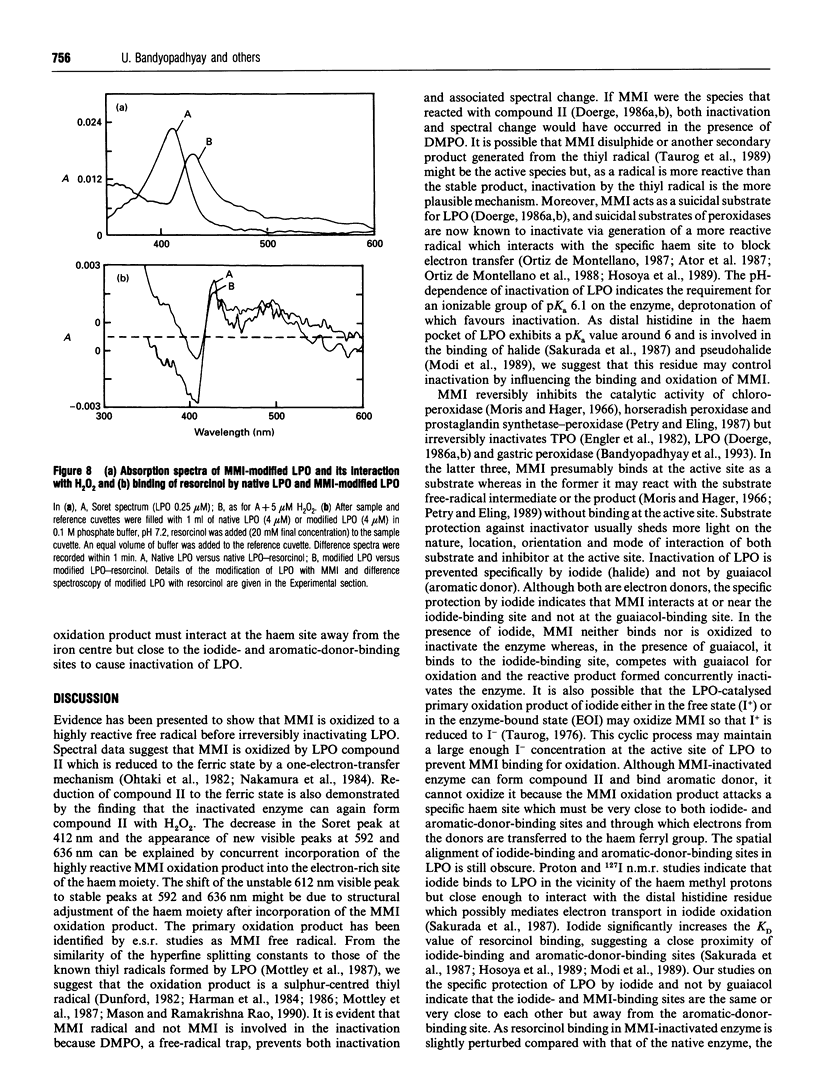
The mechanism of suicidal inactivation of lactoperoxidase (LPO) by mercaptomethylimidazole (MMI) has been studied. Analogue studies indicate a specific requirement for the thiol group of MMI for inactivation of LPO in the presence of H2O2. MMI is oxidized via one-electron transfer by LPO compound II as demonstrated by a spectral shift from 430 to 412 nm through an isosbestic point at 421 nm. A decrease in Soret absorbance at 412 nm and the appearance of visible peaks at 592 and 636 nm are the characteristics of the inactivated enzyme. The one-electron oxidation product of MMI was identified by e.s.r. spectroscopy as the 5,5'-dimethyl-l-pyrroline N-oxide (DMPO) adduct of the sulphur-centred thiyl radical. Both inactivation and spectral change are prevented by the radical trap DMPO, suggesting involvement of the thiyl radical in inactivation. pH-dependent inactivation kinetics indicate the involvement of an ionizable group on LPO (pKa 6.1), deprotonation of which favours inactivation. The enzyme is protected by iodide and not by guaiacol, suggesting that MMI interacts at or near the iodide-binding site which is away from the aromatic-donor-binding site. The inactive enzyme can form compound II and bind aromatic donor, indicating that the MMI oxidation product does not attack haem iron or aromatic-donor-binding site. We suggest that MMI interacts at the iodide-binding site for oxidation and the reactive product, probably the thiyl radical, is incorporated into the adjacent electron-rich site of haem porphyrin to cause inactivation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER N. M. A spectrophotometric assay for iodide oxidation by thyroid peroxidase. Anal Biochem. 1962 Oct;4:341–345. doi: 10.1016/0003-2697(62)90097-0. [DOI] [PubMed] [Google Scholar]
- Ator M. A., David S. K., Ortiz de Montellano P. R. Structure and catalytic mechanism of horseradish peroxidase. Regiospecific meso alkylation of the prosthetic heme group by alkylhydrazines. J Biol Chem. 1987 Nov 5;262(31):14954–14960. [PubMed] [Google Scholar]
- Ator M. A., Ortiz de Montellano P. R. Protein control of prosthetic heme reactivity. Reaction of substrates with the heme edge of horseradish peroxidase. J Biol Chem. 1987 Feb 5;262(4):1542–1551. [PubMed] [Google Scholar]
- Bandyopadhyay U., Bhattacharyya D. K., Banerjee R. K. Mechanism-based inactivation of gastric peroxidase by mercaptomethylimidazole. Biochem J. 1993 Nov 15;296(Pt 1):79–84. doi: 10.1042/bj2960079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay U., Bhattacharyya D. K., Chatterjee R., Banerjee R. K. Localization of gastric peroxidase and its inhibition by mercaptomethylimidazole, an inducer of gastric acid secretion. Biochem J. 1992 Jun 1;284(Pt 2):305–312. doi: 10.1042/bj2840305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R. K., De S. K., Bose A. K., Datta A. G. Horseradish peroxidase-catalyzed conversion of iodine to iodide in presence of EDTA and H2O2. J Biol Chem. 1986 Aug 15;261(23):10592–10597. [PubMed] [Google Scholar]
- Bhattacharyya D. K., Bandyopadhyay U., Banerjee R. K. Chemical and kinetic evidence for an essential histidine residue in the electron transfer from aromatic donor to horseradish peroxidase compound I. J Biol Chem. 1993 Oct 25;268(30):22292–22298. [PubMed] [Google Scholar]
- Carlström A. Lactoperoxidase. Some spectral properties of a haemoprotein with a prosthetic group of unknown structure. Acta Chem Scand. 1969;23(1):203–213. doi: 10.3891/acta.chem.scand.23-0203. [DOI] [PubMed] [Google Scholar]
- Davidson B., Soodak M., Neary J. T., Strout H. V., Kieffer J. D., Mover H., Maloof F. The irreversible inactivation of thyroid peroxidase by methylmercaptoimidazole, thiouracil, and propylthiouracil in vitro and its relationship to in vivo findings. Endocrinology. 1978 Sep;103(3):871–882. doi: 10.1210/endo-103-3-871. [DOI] [PubMed] [Google Scholar]
- De S. K., Banerjee R. K. Purification, characterization and origin of rat gastric peroxidase. Eur J Biochem. 1986 Oct 15;160(2):319–325. doi: 10.1111/j.1432-1033.1986.tb09974.x. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Decker C. J., Takazawa R. S. Chemical and enzymatic oxidation of benzimidazoline-2-thiones: a dichotomy in the mechanism of peroxidase inhibition. Biochemistry. 1993 Jan 12;32(1):58–65. doi: 10.1021/bi00052a009. [DOI] [PubMed] [Google Scholar]
- Doerge D. R. Mechanism-based inhibition of lactoperoxidase by thiocarbamide goitrogens. Identification of turnover and inactivation pathways. Biochemistry. 1988 May 17;27(10):3697–3700. doi: 10.1021/bi00410a026. [DOI] [PubMed] [Google Scholar]
- Doerge D. R. Mechanism-based inhibition of lactoperoxidase by thiocarbamide goitrogens. Biochemistry. 1986 Aug 12;25(16):4724–4728. doi: 10.1021/bi00364a041. [DOI] [PubMed] [Google Scholar]
- Doerge D. R. Oxygenation of organosulfur compounds by peroxidases: evidence of an electron transfer mechanism for lactoperoxidase. Arch Biochem Biophys. 1986 Feb 1;244(2):678–685. doi: 10.1016/0003-9861(86)90636-3. [DOI] [PubMed] [Google Scholar]
- Edelhoch H., Irace G., Johnson M. L., Michot J. L., Nunez J. The effects of thioureylene compounds (goitrogens) on lactoperoxidase activity. J Biol Chem. 1979 Dec 10;254(23):11822–11830. [PubMed] [Google Scholar]
- Engler H., Taurog A., Nakashima T. Mechanism of inactivation of thyroid peroxidase by thioureylene drugs. Biochem Pharmacol. 1982 Dec 1;31(23):3801–3806. doi: 10.1016/0006-2952(82)90296-9. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Harman L. S., Carver D. K., Schreiber J., Mason R. P. One- and two-electron oxidation of reduced glutathione by peroxidases. J Biol Chem. 1986 Feb 5;261(4):1642–1648. [PubMed] [Google Scholar]
- Harman L. S., Mottley C., Mason R. P. Free radical metabolites of L-cysteine oxidation. J Biol Chem. 1984 May 10;259(9):5606–5611. [PubMed] [Google Scholar]
- Hosoya T., Sakurada J., Kurokawa C., Toyoda R., Nakamura S. Interaction of aromatic donor molecules with lactoperoxidase probed by optical difference spectra. Biochemistry. 1989 Mar 21;28(6):2639–2644. doi: 10.1021/bi00432a042. [DOI] [PubMed] [Google Scholar]
- Huwiler M., Kohler H. Pseudo-catalytic degradation of hydrogen peroxide in the lactoperoxidase/H2O2/iodide system. Eur J Biochem. 1984 May 15;141(1):69–74. doi: 10.1111/j.1432-1033.1984.tb08158.x. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Rao D. N. Thiyl free radical metabolites of thiol drugs, glutathione, and proteins. Methods Enzymol. 1990;186:318–329. doi: 10.1016/0076-6879(90)86125-f. [DOI] [PubMed] [Google Scholar]
- Michot J. L., Nunez J., Johnson M. L., Irace G., Edelhoch H. Iodide binding and regulation of lactoperoxidase activity toward thyroid goitrogens. J Biol Chem. 1979 Apr 10;254(7):2205–2209. [PubMed] [Google Scholar]
- Modi S., Behere D. V., Mitra S. Binding of thiocyanate to lactoperoxidase: 1H and 15N nuclear magnetic resonance studies. Biochemistry. 1989 May 30;28(11):4689–4694. doi: 10.1021/bi00437a027. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Hager L. P. Mechanism of the inhibition of enzymatic halogenation by antithyroid agents. J Biol Chem. 1966 Aug 10;241(15):3582–3589. [PubMed] [Google Scholar]
- Morrison M., Bayse G. S. Catalysis of iodination by lactoperoxidase. Biochemistry. 1970 Jul 21;9(15):2995–3000. doi: 10.1021/bi00817a010. [DOI] [PubMed] [Google Scholar]
- Mottley C., Toy K., Mason R. P. Oxidation of thiol drugs and biochemicals by the lactoperoxidase/hydrogen peroxide system. Mol Pharmacol. 1987 Apr;31(4):417–421. [PubMed] [Google Scholar]
- Nakamura S., Nakamura M., Yamazaki I., Morrison M. Reactions of ferryl lactoperoxidase (compound II) with sulfide and sulfhydryl compounds. J Biol Chem. 1984 Jun 10;259(11):7080–7085. [PubMed] [Google Scholar]
- Ohtaki S., Nakagawa H., Nakamura M., Yamazaki I. Reactions of purified hog thyroid peroxidase with H2O2, tyrosine, and methylmercaptoimidazole (goitrogen) in comparison with bovine lactoperoxidase. J Biol Chem. 1982 Jan 25;257(2):761–766. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., David S. K., Ator M. A., Tew D. Mechanism-based inactivation of horseradish peroxidase by sodium azide. Formation of meso-azidoprotoporphyrin IX. Biochemistry. 1988 Jul 26;27(15):5470–5476. doi: 10.1021/bi00415a013. [DOI] [PubMed] [Google Scholar]
- Petry T. W., Eling T. E. The mechanism for the inhibition of prostaglandin H synthase-catalyzed xenobiotic oxidation by methimazole. Reaction with free radical oxidation products. J Biol Chem. 1987 Oct 15;262(29):14112–14118. [PubMed] [Google Scholar]
- Sakurada J., Takahashi S., Shimizu T., Hatano M., Nakamura S., Hosoya T. Proton and iodine-127 nuclear magnetic resonance studies on the binding of iodide by lactoperoxidase. Biochemistry. 1987 Oct 6;26(20):6478–6483. doi: 10.1021/bi00394a028. [DOI] [PubMed] [Google Scholar]
- Sinha B. K. Enzymatic activation of hydrazine derivatives. A spin-trapping study. J Biol Chem. 1983 Jan 25;258(2):796–801. [PubMed] [Google Scholar]
- Takeuchi M., Asano N., Kameda Y., Matsui K. Chemical modification by diethylpyrocarbonate of an essential histidine residue in 3-ketovalidoxylamine A C-N lyase. J Biochem. 1986 Jun;99(6):1571–1577. doi: 10.1093/oxfordjournals.jbchem.a135630. [DOI] [PubMed] [Google Scholar]
- Taurog A., Dorris M. L., Guziec F. S., Jr Metabolism of 35S- and 14C-labeled 1-methyl-2-mercaptoimidazole in vitro and in vivo. Endocrinology. 1989 Jan;124(1):30–39. doi: 10.1210/endo-124-1-30. [DOI] [PubMed] [Google Scholar]
- Taurog A., Dorris M. L., Lamas L. Comparison of lactoperoxidase- and thyroid peroxidase-catalyzed iodination and coupling. Endocrinology. 1974 May;94(5):1286–1294. doi: 10.1210/endo-94-5-1286. [DOI] [PubMed] [Google Scholar]
- Taurog A. The mechanism of action of the thioureylene antithyroid drugs. Endocrinology. 1976 Apr;98(4):1031–1046. doi: 10.1210/endo-98-4-1031. [DOI] [PubMed] [Google Scholar]


