Abstract
Background
Although Janus kinase (JAK) inhibitors have expanding indications, deep vein thrombosis (DVT) and pulmonary embolism (PE) are serious adverse events associated with their use. Moreover, their analysis using the Japanese database of spontaneous adverse drug reaction reports has not yet been conducted.
Objective
The objective of this study was to analyze the Japanese Adverse Drug Event Report database (JADER) to evaluate the association between JAK inhibitors and DVT and PE.
Methods
JADER reports from April 2004 to October 2023 were analyzed. A classification of reports for the period covered was performed by drug, and an imbalance analysis was performed with oral JAK inhibitors as the target drug and DVT, PE, and “embolic and thrombotic events, venous” (Standardised MedDRA Query; SMQ) as the target adverse events. Reported odds ratios (ROR) and information components (IC) were calculated for signal detection.
Results
Overall, 6631 JAK inhibitor-related adverse events were reported, including 60 and 41 cases of DVT and PE, respectively. The ROR and IC of the JAK inhibitors for DVT were 2.52 (1.95–3.25) and 1.27 (0.41–2.13), while those of baricitinib for DVT were 4.37 (2.83–6.73) and 1.90 (0.47–3.33), respectively. ROR signals were detected for JAK inhibitors for PE and “embolic and thrombotic events, venous (SMQ),” overall and for several JAK inhibitors but none for IC.
Conclusions
Several JAK inhibitors are under postmarketing phase vigilance, and the number of reported adverse events is low. However, when administering these drugs, care should be taken to avoid the development of thromboembolism, considering the patient’s background.
Key Points
| Deep vein thrombosis and pulmonary embolism are serious side effects of Janus kinase inhibitors. |
| We analyzed spontaneous reports of adverse events from people of Japanese ethnicity, who are known to have a lower incidence of thrombosis. |
| Some JAK inhibitors may increase the risk of DVT in Japanese patients, warranting cautious use. |
Introduction
Janus kinase (JAK) inhibitors have been increasingly used to treat rheumatoid arthritis and other immune-mediated inflammatory diseases in Japan since 2013. However, thromboembolism is a serious side effect of JAK inhibitors. Therefore, they should be used with caution, especially in at-risk patients, such as those with smoking habits or a history of coronary artery disease because their mechanism is unknown [1]. Several pharmacovigilance analyses have been reported for JAK inhibitors and thromboembolism using the Food and Drug Administration Adverse Event Reporting System (FAERS) or the World Health Organization (WHO) Vigibase [2–4]. These reports showed an association between JAK inhibitors and venous thromboembolisms such as deep vein thrombosis (DVT) and pulmonary embolism (PE). FAERS and Vigibase include reports from Japan but are skewed with a higher percentage from North America and Europe. Japanese people are known to have a lower incidence of venous thromboembolism than Westerners [5, 6]. Additionally, and factor V Leiden and prothrombin mutations, one of the causes of activated protein C resistance seen in Westerners, have not been identified in the Japanese population, among other characteristics. Moreover, the proportion of protein C or S deficiency is higher in Japanese patients with DVT than in Westerners [7]; therefore, it is useful to analyze Japanese patients with different backgrounds compared to their Western counterparts.
Consequently, this study aims to evaluate the association between JAK inhibitors use and DVT/PE in Japanese patients using the latest Japanese Adverse Drug Event Report database (JADER) data.
Methods
Source of Data
We used JADER data from April 2004 to October 2023, downloaded from the Pharmaceuticals and Medical Devices Agency (PMDA) website. The JADER database contains “adverse drug reaction case report information” and consists of four datasets: patient demographic information (DEMO), drug information (DRUG), adverse events (REAC), and primary disease (HIST) tables. The DEMO table contains patient age and sex data. The DRUG table contains drug name, dose, reason for use, and duration of use. The REAC table contains the name of the adverse event, the outcome, and the date of occurrence of the adverse event. Finally, the HIST table provides information on the underlying disease. The JAK inhibitors included in this study were tofacitinib, ruxolitinib, baricitinib, peficitinib, upadacitinib, filgotinib, and abrocitinib, which are oral drugs marketed in Japan as of October 2023. Trends in the number of reports for each JAK inhibitor and the background of the cases were analyzed. “Deep vein thrombosis” and “pulmonary embolism” were the investigated adverse events which are the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms (PT) according to MedDRA version 26.1. Additionally, the Standardised MedDRA Query (SMQ), which encompasses various types of venous thromboembolisms, was included as a target adverse event. “Embolic and thrombotic events, venous” (SMQ code: 20000084) contains 97 PTs.
Signal Detection
Two types of signal detection methods, the reporting odds ratio (ROR) [8] and information component (IC) [9], were used for each combination of the target drug and target adverse event. The signal detection criterion for ROR was a lower limit of 95% confidence interval (CI) exceeding 1, whereas that for IC was a lower limit of 95% CI exceeding 0. Although the ROR is a widely used indicator, it can lead to false positives when the number of reports is low; therefore, we used it in conjunction with IC. Analyses were performed using JMP Pro 17.0.0 (SAS Institute Inc., Cary, NC).
Results
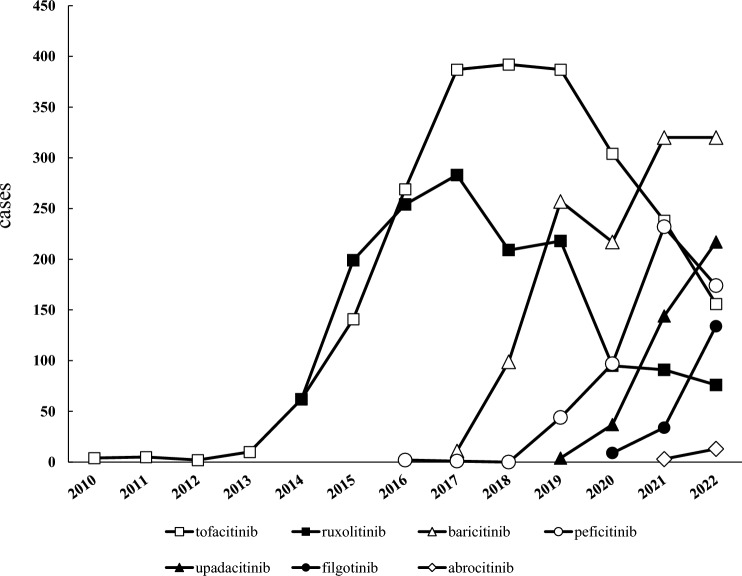
During the study period, 880,999 cases were reported in the JADER, with 6631 patients reporting adverse events caused by the use of JAK inhibitors. The most commonly reported adverse event associated with JAK inhibitors was pneumonia (596 cases), followed by herpes zoster (535 cases) and anemia (442 cases). Adverse event reports for the oral JAK inhibitors included in this study have accumulated since 2010, peaking in 2017 at 283 cases for ruxolitinib and in 2018 at 392 cases for tofacitinib, declining thereafter (Fig. 1). The backgrounds of the reported cases for each JAK inhibitor are shown in Table 1. Analysis of report type showed a high percentage of “report from study” for tofacitinib, ruxolitinib, baricitinib, and peficitinib, particularly for peficitinib (81.3%). In contrast, upadacitinib, filgotinib, and abrocitinib showed high spontaneous reporting rates. Analysis of the ages of the reported cases showed that tofacitinib, ruxolitinib, baricitinib, peficitinib, upadacitinib, and filgotinib had the highest number of patients in their 70s. Overall, 33.0% of the reported patients were aged > 70 years. Adverse events with tofacitinib, baricitinib, peficitinib, upadacitinib, and filgotinib were reported more frequently by women, whereas those with ruxolitinib and abrocitinib were reported more frequently by men. In terms of the primary disease and reason for JAK inhibitor use in the reported cases, rheumatoid arthritis was the most common reason for the use of all five drugs except ruxolitinib and abrocitinib: tofacitinib in 78.7%, baricitinib in 80.4%, peficitinib in 96.8%, upadacitinib in 75.4%, and filgotinib in 85.0%. Abrocitinib was indicated in Japan only for atopic dermatitis with an inadequate response to existing therapy at the time of analysis; 86.7% of the JADER reports included patients with atopic dermatitis. There were no reports on the use of upadacitinib for the treatment of spondyloarthritis axialis.
Fig. 1.
Number of reported cases for each JAK inhibitor in JADER. There were no reports of Peficitinib in 2018
Table 1.
Characteristics of JAK inhibitor use cases in JADER reports
| Totala | Tofacitinib | Ruxolitinib | Baricitinib | Peficitinib | Upadacitinib | Filgotinib | Abrocitinib | |
|---|---|---|---|---|---|---|---|---|
| Approval year | – | 2013 | 2014 | 2017 | 2019 | 2020 | 2020 | 2021 |
| Cases (n) | 6631 | 2431 | 1513 | 1338 | 651 | 580 | 233 | 30 |
| Report type | ||||||||
| Report from study | 3828 (57.7) | 1357 (55.8) | 939 (62.1) | 772 (57.7) | 529 (81.3) | 258 (44.5) | 68 (29.2) | 5 (16.7) |
| Spontaneous | 2795 (42.2) | 1070 (44.0) | 574 (37.9) | 565 (42.2) | 121 (18.6) | 320 (55.2) | 165 (70.8) | 25 (83.3) |
| Other or unknown | 8 (0.1) | 4 (0.2) | 0 (0.0) | 1 (0.07) | 1 (0.2) | 2 (0.3) | 0 (0.0) | 0 (0.0) |
| Age | ||||||||
| < 10 | 16 (0.2) | 4 (0.2) | 12 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥ 10 to < 20 | 63 (1.0) | 18 (0.7) | 11 (0.7) | 0 (0.0) | 2 (0.3) | 22 (3.8) | 2 (0.9) | 11 (36.7) |
| ≥ 20 to < 30 | 98 (1.5) | 68 (2.8) | 3 (0.2) | 11 (0.8) | 2 (0.3) | 10 (1.7) | 1 (0.4) | 3 (10.0) |
| ≥ 30 to < 40 | 132 (2.0) | 65 (2.7) | 12 (0.8) | 24 (1.8) | 5 (0.8) | 21 (3.6) | 1 (0.4) | 5 (16.7) |
| ≥ 40 to < 50 | 339 (5.1) | 153 (6.3) | 44 (2.9) | 77 (5.8) | 24 (3.7) | 30 (5.2) | 11 (4.7) | 2 (6.7) |
| ≥ 50 to < 60 | 722 (10.9) | 294 (12.1) | 123 (8.1) | 171 (12.8) | 56 (8.6) | 63 (10.9) | 29 (12.4) | 3 (10.0) |
| ≥ 60 to < 70 | 1460 (22.0) | 614 (25.3) | 325 (21.5) | 287 (21.5) | 112 (17.2) | 105 (18.1) | 39 (16.7) | 0 (0.0) |
| ≥ 70 to < 80 | 2189 (33.0) | 724 (29.8) | 575 (38.0) | 437 (32.7) | 231 (35.5) | 202 (34.8) | 80 (34.3) | 1 (3.3) |
| ≥ 80 to < 90 | 1024 (15.4) | 303 (12.5) | 193 (0.13) | 219 (16.4) | 189 (29.0) | 97 (16.7) | 52 (22.3) | 2 (6.7) |
| ≥ 90 to < 100 | 62 (0.9) | 16 (0.7) | 4 (0.3) | 20 (1.5) | 13 (2.0) | 10 (1.7) | 1 (0.4) | 0 (0.0) |
| Youth | 1 (0.02) | 1 (0.04) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adult | 63 (1.0) | 8 (0.3) | 34 (2.2) | 16 (1.2) | 0 (0.0) | 2 (0.3) | 4 (1.7) | 3 (10.0) |
| Elderly | 100 (1.5) | 11 (0.5) | 74 (4.9) | 2 (0.1) | 5 (0.8) | 3 (0.5) | 5 (2.1) | 0 (0.0) |
| Unknown | 362 (5.5) | 152 (6.3) | 103 (6.8) | 74 (5.5) | 12 (1.8) | 15 (2.6) | 8 (3.4) | 0 (0.0) |
| Sex | ||||||||
| Female | 4032 (60.8) | 1578 (67.8) | 644 (42.6) | 877 (70.0) | 482 (74.0) | 381 (66.8) | 168 (72.1) | 10 (33.3) |
| Male | 2300 (34.7) | 749 (32.2) | 795 (52.5) | 366 (29.2) | 164 (25.2) | 188 (33.0) | 53 (22.7) | 18 (60.0) |
| Unknown or missing | 299 (4.5) | 0 (0.0) | 74 (4.9) | 9 (0.7) | 5 (0.8) | 1 (0.2) | 12 (5.2) | 2 (6.7) |
| Disease or reason for use | ||||||||
| Rheumatoid arthritis | 1914b (78.7) | – | 1076 (80.4) | 630 (96.8) | 438 (75.4) | 198 (85.0) | – | |
| Ulcerative colitis | 287 (11.8) | – | – | – | 46 (7.9) | 28 (12.0) | – | |
| Myelofibrosis | – | 1115 (73.7) | – | – | – | – | – | |
| Polycythemia vera | – | 281 (18.6) | – | – | – | – | – | |
| Graft-versus-host disease | – | 34 (2.2) | – | – | – | – | – | |
| Atopic dermatitis | – | – | 43 (3.2) | – | 55 (9.5) | – | 26 (86.7) | |
| COVID-19 | – | – | 153 (11.4) | – | – | – | – | |
| Alopecia areata | – | – | 7c (0.5) | – | – | – | – | |
| Psoriatic arthritis | – | – | – | – | 17 (2.9) | – | – | |
| Spondyloarthritis axialis | – | – | – | – | 0 | – | – | |
| Ankylosing spondylitis | – | – | – | – | 5 (0.9) | – | – | |
| Crohn’s disease | – | – | – | – | 4 (0.7) | – | – | |
| Other | 69 (2.8) | 54 (3.6) | 7 (0.5) | 2 (0.3) | 4 (0.7) | 0 | 0 | |
| Unknown | 161 (6.6) | 29 (1.9) | 52 (3.9) | 19 (2.9) | 12 (2.1) | 7 (3.0) | 4 (13.3) | |
Results as number and percentage, unless otherwise specified. In the Disease or reason for use section, a hyphenated column indicates that the drug is not indicated for use in Japan
JAK Janus kinase, JADER Japanese Adverse Drug Event Report database, COVID-19 coronavirus disease 2019
aValues that include multiple JAK inhibitor use cases
bIncluding two cases of rheumatoid arthritis and ulcerative colitis
cIncluding one case of alopecia areata combined with atopic dermatitis
DVT
The signal detection results for DVT are presented in Table 2. Overall, 60 cases of JAK inhibitors were reported, and both ROR and IC signals were detected, with a ROR of 2.52 (1.95–3.25) and an IC of 1.27 (0.41–2.13). In the per-drug results, both ROR and IC signals were detected for baricitinib. Baricitinib was reported in 21 cases, with ROR and IC of 4.37 (2.83–6.73) and 1.90 (0.47–3.33), respectively. Tofacitinib was reported in 24 cases, with an ROR (95% CI) of 2.73 (1.82–4.09) and IC (95% CI) of 1.34 (− 0.004 to 2.68), respectively; the signal was detected only in the ROR. Similarly, upadacitinib was positive with an ROR of 3.33 (1.58–7.03) but negative with an IC of 1.36 (− 1.01 to 3.72).
Table 2.
Signal scores for JAK inhibitors-associated DVT
| JAK inhibitor | Total (n) | Cases (n) | ROR (95% CI) | IC (95% CI) |
|---|---|---|---|---|
| JAK inhibitors | 6631 | 60 | 2.52* (1.95–3.25) | 1.27* (0.41–2.13) |
| Tofacitinib | 2431 | 24 | 2.73* (1.82–4.09) | 1.34 (− 0.004 to 2.68) |
| Ruxolitinib | 1513 | 2 | 0.36 (0.09–1.44) | − 1.12 (− 4.97 to 2.72) |
| Baricitinib | 1338 | 21 | 4.37* (2.83–6.73) | 1.90* (0.47–3.33) |
| Peficitinib | 651 | 5 | 2.11 (0.87–5.09) | 0.83 (− 1.90 to 3.55) |
| Upadacitinib | 580 | 7 | 3.33* (1.58–7.03) | 1.36 (− 1.01 to 3.72) |
| Filgotinib | 233 | 2 | 2.36 (0.59–9.50) | 0.69 (− 3.17 to 4.55) |
| Abrocitinib | 30 | 0 | – | – |
JAK Janus kinase, DVT deep vein thrombosis, ROR reported odds ratio, IC information component
*The adverse events are detected as signals
PE
The signal detection results for the PE are listed in Table 3. Overall, 41 cases of PE due to JAK inhibitors were, with signals detected only in the ROR: 1.71 (1.26–2.34). The highest number of reported cases was 14 for baricitinib, with signals detected only in ROR: 2.91 (1.71–4.93).
Table 3.
Signal scores for JAK inhibitors-associated PE
| JAK inhibitor | Total (n) | Cases (n) | ROR (95% CI) | IC (95% CI) |
|---|---|---|---|---|
| JAK inhibitors | 6631 | 41 | 1.71* (1.26–2.34) | 0.74 (− 0.29 to 1.78) |
| Tofacitinib | 2431 | 13 | 1.48 (0.85–2.55) | 0.50 (− 1.28 to 2.29) |
| Ruxolitinib | 1513 | 8 | 1.46 (0.73–2.92) | 0.47 (− 1.76 to 2.69) |
| Baricitinib | 1338 | 14 | 2.91* (1.71–4.93) | 1.35 (− 0.38 to 3.08) |
| Peficitinib | 651 | 2 | 0.84 (0.21–3.39) | − 0.17 (− 4.01 to 3.68) |
| Upadacitinib | 580 | 5 | 2.39 (0.99–5.76) | 0.95 (− 1.78 to 3.68) |
| Filgotinib | 233 | 0 | – | – |
| Abrocitinib | 30 | 0 | – | – |
JA janus kinase, PE pulmonary embolism, ROR reported odds ratio, IC information component
*The adverse events are detected as signals
Embolic and Thrombotic Events, Venous (SMQ)
The signal detection results for “embolic and thrombotic events, venous” (SMQ) are presented in Table 4. Overall, 115 SMQ due to JAK inhibitors have been reported, wherein only the ROR signal was detected at 1.57 (1.30–1.89). Signals were also detected for tofacitinib, baricitinib, and upadacitinib only for RORs of 1.82 (1.37–2.42), 2.17 (1.53–3.08), and 1.87 (1.05–3.31), respectively. IC was not detected for any of the JAK inhibitors. There have been no reports on the effects of abrocitinib.
Table 4.
Signal scores for JAK inhibitors-associated embolic and thrombotic events, venous (SMQ)
| JAK inhibitor | Total (n) | Cases (n) | ROR (95% CI) | IC (95% CI) |
|---|---|---|---|---|
| JAK inhibitors | 6631 | 115 | 1.57* (1.30–1.89) | 0.63 (− 0.0001 to 1.25) |
| Tofacitinib | 2431 | 49 | 1.82* (1.37–2.42) | 0.83 (− 0.13 to 1.78) |
| Ruxolitinib | 1513 | 15 | 0.89 (0.53–1.47) | − 0.16 (− 1.84 to 1.51) |
| Baricitinib | 1338 | 32 | 2.17* (1.53–3.08) | 1.05 (− 0.13 to 2.22) |
| Peficitinib | 651 | 5 | 0.68 (0.28–1.65) | − 0.47 (− 3.19 to 2.26) |
| Upadacitinib | 580 | 12 | 1.87* (1.05–3.31) | 0.79 (− 1.07 to 2.66) |
| Filgotinib | 233 | 3 | 1.15 (0.37–3.60) | 0.14 (− 3.21 to 3.50) |
| Abrocitinib | 30 | 0 | – | − |
JAK janus kinase, SMQ Standardised Medical Dictionary for Regulatory Activities Query, ROR reported odds ratio, IC information component
*The adverse events are detected as signals
Discussion
In this study, we analyze the reporting status of JAK inhibitors in the JADER and used imbalance analysis to detect the signals of JAK inhibitors as a whole and baricitinib alone on DVT.
The number of adverse drug reaction reports for JAK inhibitors has increased since each drug was marketed, whereas the number of adverse drug reaction reports for tofacitinib, ruxolitinib, and peficitinib has decreased, possibly due to the Weber effect [10]. The number of reports on other JAK inhibitors is increasing and is expected to continue to increase. The JADER reports were categorized into reports from trials and spontaneous reports. The 57.9% spontaneous reports of adverse events for JAK inhibitors in this study were from clinical trials, and this percentage of spontaneous reports is expected to increase in the future. However, some JAK inhibitors are under post-marketing surveillance in Japan, which affects reporting [11].
Regarding the background of the reported cases, approximately 70% of the patients were women in the adverse event reports of cases with JAK inhibitors because rheumatoid arthritis and other autoimmune diseases are more common in women. In Japan, 76.3% of patients with rheumatoid arthritis are women [12], and a similar trend was observed in the JADER adverse drug reaction reports. As this study analyzed JAK inhibitors and thromboembolism regardless of the reason for their use, the background of the indicated disease should also be considered when interpreting the results. For example, patients with rheumatoid arthritis are at a higher risk of VTE than nonpatients, and patients with rheumatoid arthritis treated with JAK inhibitors have been reported to be at a higher risk of PE than those treated with disease-modifying antirheumatic drugs [13]. In contrast, adult patients with atopic dermatitis have been reported to be at a higher risk of developing VTE than nonpatients [14]. However, a meta-analysis showed that treatment with JAK inhibitors in patients with atopic dermatitis was not associated with increased venous thromboembolism [15], and although the frequency of thrombosis in Japanese patients with inflammatory bowel disease is reportedly similar to that of other Asians and Westerners [16], the risk of thromboembolism with JAK inhibitors restricted to patients with inflammatory bowel disease is unknown.
In our analysis, two distinct DVT signals were detected using the JAK inhibitors as a whole. Conversely, in the analysis of each drug, we detected two different DVT signals only for baricitinib which indicates that the risk of adverse events with its use may be as high as that in Westerners [4]. Whether the risk of thrombosis for JAK inhibitors is a class effect or varies according to drug type remains debatable. Baricitinib was reported to have a venous thromboembolism incidence rate per 100 patient-year (95% CI) of 0.25 (0.15–0.36) in a 3-year all-case post-marketing surveillance in patients with rheumatoid arthritis in Japan [17]. In this study, no JAK inhibitor expressed two different signals for PE; FAERS analysis reported that ROR and IC signals were detected with baricitinib, upadacitinib, and filgotinib [3]. In addition, venous thromboembolic signals were not detected with ruxolitinib in this study, whereas portal vein thrombosis and thrombosis signals were detected with FAERS analysis. It is unclear whether this discrepancy is due to race or other factors. Unlike other JAK inhibitors, the Japanese package insert for ruxolitinib does not include a warning on thromboembolism, which may introduce a reporting bias.
The limitations of this study include reporting biases due to spontaneous reporting and channeling because the products have not been on the market for a long time [18]. In addition, risk factors for thrombosis, such as age, obesity, concomitant medications, and underlying diseases, were not considered in the reported cases. Furthermore, although the analysis in this study considered Japanese backgrounds, there was no item for race in the report, and the possibility that non-Japanese races were reported cannot be ruled out. However, the results of the imbalance analysis suggest that some JAK inhibitors may be associated with a higher risk of DVT in Japanese patients, indicating the need for future clinical studies. The effects on the coagulation–fibrinolytic system and platelet activation need to be elucidated in the future.
Conclusions
We analyzed the association between JAK inhibitors and DVT and PE using JADER. Although the number of reports in JADER is small and some drugs are under postmarketing surveillance, caution should be exercised due to the development of DVT and PE when using JAK inhibitors. The accumulation of spontaneous adverse event reports is required in the future.
Acknowledgements
We thank Editage (www.editage.jp) for English language editing.
Declarations
Funding
This research was supported by funds from Teikyo University.
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Ethics approval was not required for this study. All results were obtained from data openly available online from the website of the PMDA (http://www.pmda.go.jp). All data from the JADER database were fully anonymized by the regulatory authority before we accessed them.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Data availability statement
The data presented in this study are available upon request from the corresponding author.
Author contributions
T.M. designed the study. T.M. and S.A. performed the analyses. The first draft of the manuscript was written by T.M. and N.O., and M.W. and F.I. reviewed the manuscript. All authors have read and approved the final manuscript and agree to accept responsibility for this work.
References
- 1.FDA. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions; 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death20240216. Accessed 2 Oct 2023.
- 2.Setyawan J, Azimi N, Strand V, Yarur A, Fridman M. Reporting of thromboembolic events with JAK inhibitors: analysis of the FAERS database 2010–2019. Drug Saf. 2021;44:889–97. 10.1007/s40264-021-01082-y. 10.1007/s40264-021-01082-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Z, Ye X, Chen C, Wang R, Liu D, Xu X, et al. Thromboembolic events in Janus kinase inhibitors: a pharmacovigilance study from 2012 to 2021 based on the Food and Drug Administration’s Adverse Event Reporting System. Br J Clin Pharmacol. 2022;88:4180–90. 10.1111/bcp.15361. 10.1111/bcp.15361 [DOI] [PubMed] [Google Scholar]
- 4.Mytheen S, Varghese A, Joy J, Shaji A, Tom AA. Investigating the risk of deep vein thrombosis with JAK inhibitors: a disproportionality analysis using FDA Adverse Event Reporting System Database (FAERS). Expert Opin Drug Saf. 2023;22:985–94. 10.1080/14740338.2023.2223955. 10.1080/14740338.2023.2223955 [DOI] [PubMed] [Google Scholar]
- 5.Ota S, Matsuda A, Ogihara Y, Yamada N, Nakamura M, Mori T, et al. Incidence, characteristics and management of venous thromboembolism in Japan during 2011. Circ J. 2018;82:555–60. 10.1253/circj.CJ-17-0579. 10.1253/circj.CJ-17-0579 [DOI] [PubMed] [Google Scholar]
- 6.Nicole Tran H, Klatsky AL. Lower risk of venous thromboembolism in multiple Asian ethnic groups. Prev Med Rep. 2019;13:268–9. 10.1016/j.pmedr.2019.01.006. 10.1016/j.pmedr.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suehisa E, Nomura T, Kawasaki T, Kanakura Y. Frequency of natural coagulation inhibitor (antithrombin III, protein C and protein S) deficiencies in Japanese patients with spontaneous deep vein thrombosis. Blood Coagul Fibrinolysis. 2001;12:95–9. 10.1097/00001721-200103000-00002. 10.1097/00001721-200103000-00002 [DOI] [PubMed] [Google Scholar]
- 8.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–23. 10.1002/pds.1001. 10.1002/pds.1001 [DOI] [PubMed] [Google Scholar]
- 9.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54:315–21. 10.1007/s002280050466. 10.1007/s002280050466 [DOI] [PubMed] [Google Scholar]
- 10.Weber J. Epidemiology of adverse reactions to nonsteroidal anti inflammatory drugs. Adv Inflam Res. 1984;6:1–7. [Google Scholar]
- 11.Matsuda S, Aoki K, Kawamata T, Kimotsuki T, Kobayashi T, Kuriki H, et al. Bias in spontaneous reporting of adverse drug reactions in Japan. PLoS ONE. 2015;10: e0126413. 10.1371/journal.pone.0126413. 10.1371/journal.pone.0126413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima A, Sakai R, Inoue E, Harigai M. Prevalence of patients with rheumatoid arthritis and age-stratified trends in clinical characteristics and treatment, based on the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Int J Rheum Dis. 2020;23:1676–84. 10.1111/1756-185X.13974. 10.1111/1756-185X.13974 [DOI] [PubMed] [Google Scholar]
- 13.Molander V, Bower H, Frisell T, Delcoigne B, Di Giuseppe D, Askling J, et al. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82:189–97. 10.1136/ard-2022-223050. 10.1136/ard-2022-223050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen TL, Huang WT, Loh CH, Huang HK, Chi CC. Risk of venous thromboembolism among adults with atopic dermatitis. JAMA Dermatol. 2023;159:720–7. 10.1001/jamadermatol.2023.1300. 10.1001/jamadermatol.2023.1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TL, Lee LL, Huang HK, Chen LY, Loh CH, Chi CC. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254–61. 10.1001/jamadermatol.2022.3516. 10.1001/jamadermatol.2022.3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ando K, Fujiya M, Watanabe K, Hiraoka S, Shiga H, Tanaka S, et al. A nationwide survey concerning the mortality and risk of progressing severity due to arterial and venous thromboembolism in inflammatory bowel disease in Japan. J Gastroenterol. 2021;56:1062–79. 10.1007/s00535-021-01829-5. 10.1007/s00535-021-01829-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eli Lilly Japan. Olumiant® Proper Use Information vol. 2023; 8 Safety of 4720 patients in a specific use-results study for rheumatoid arthritis at 3-year observation (in Japanese) https://medical.lilly.com/jp/answers/194368. Accessed 16 Nov 2023.
- 18.Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10:577–81. 10.1002/sim.4780100409. 10.1002/sim.4780100409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.