Abstract
Background
Bowel urgency is a highly burdensome symptom among patients with inflammatory bowel disease (IBD).
Objectives
To assess changes in severity of bowel urgency and identify predictors of worsening or improvement among patients with Crohn’s disease (CD) and ulcerative colitis (UC) at 6 months from their enrollment visit.
Methods
Data from patients in the Study of a Prospective Adult Research Cohort with IBD were analyzed. Enrolled patients with CD or UC with 6-month visits were included. Changes and predictors of bowel urgency severity over 6 months in patients with CD or UC were examined using two separate analyses: (a) “worsening” versus “no change” excluding those with moderate-to-severe bowel urgency at enrollment, and (b) “improvement” versus “no change” excluding those with no bowel urgency at enrollment. The enrollment characteristics were compared within these groups.
Results
At baseline, in both CD and UC, use of biologics and/or immunomodulators at enrollment was similar across cohorts. Among patients with CD, 206 of 582 (35.4%) reported worsening, and 195 of 457 (42.7%) reported improvement in bowel urgency. Younger age (P = 0.013) and moderate-to-severe bowel urgency (P < 0.001) were associated with improvement. Moderate bowel urgency (P = 0.026) and bowel incontinence while awake (P = 0.022) were associated with worsening. Among patients with UC, 84 of 294 (28.6%) reported worsening, and 111 of 219 (50.7%) reported improvement in bowel urgency. Higher symptomatic disease severity (P = 0.011) and more severe bowel urgency (P < 0.001) were associated with improvement.
Conclusions
Bowel urgency is an unpredictable and unstable symptom among patients with IBD. Over 50% of patients with CD or UC experienced either worsening or improvement at 6 months postenrollment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-024-00434-1.
Plain Language Summary
What is known about bowel urgency in inflammatory bowel disease (IBD)?
Around six to eight in every ten patients with inflammatory bowel disease suffer from bowel urgency, a sudden need to have bowel movement. Many patients with IBD perceive bowel urgency as a bothersome symptom impacting their everyday activities.
Why did we do this study?
Despite the importance of bowel urgency, the changes in bowel urgency severity among the IBD-affected US population are yet to be fully known. We aimed to assess changes in severity of bowel urgency in patients with Crohn’s disease (CD) or ulcerative colitis (UC) at 6 months.
What have we found from this study?
Bowel urgency is a common and unpredictable symptom among patients with CD and UC. Over 50% of patients reported that the severity of bowel urgency has either worsened or improved at the 6 months postenrollment. While about 40–50% of IBD patients reported improvement, about 30% reported worsening, suggesting a lack of effective therapies to treat bowel urgency.
Future implication
There is a need for advanced therapies to resolve bowel urgency in patients with CD and UC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-024-00434-1.
Key Points
| Bowel urgency is a highly disruptive symptom among patients with inflammatory bowel disease and has a high impact on health-related quality of life. |
| We aimed to assess changes in severity of bowel urgency among patients with Crohn’s disease (CD) or ulcerative colitis (UC) at 6 months postenrollment. |
| 40–50% of CD and UC patients reported improvement in bowel urgency severity; however, over 50% continue to experience some degree of bowel urgency. |
| Although a large proportion of IBD patients reported improvement at 6 months postenrollment, one out of three reported worsening, suggesting a lack of effective therapies to treat bowel urgency. |
Introduction
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are characterized by chronic inflammation of the gastrointestinal tract [1]. Clinical manifestations of IBD include bowel urgency, lack of energy, abdominal pain, and diarrhea [2]. Bowel urgency, defined as a sudden need to have a bowel movement, is reported in 74% of patients with CD and more than 80% of patients with UC [3].
Patients with IBD perceive bowel urgency as a highly burdensome symptom [2, 4], with the highest impact among all other symptoms on health-related quality of life [5, 6]. Patients also report bowel urgency as a major reason for limited participation in physical activity or exercise [7].
Despite increasing real-world evidence on bowel urgency among patients with IBD, data on changes in severity of bowel urgency are limited [5, 8–10]. In a cross-sectional analysis of data from the multicenter longitudinal Study of a Prospective Adult Research Cohort with IBD (SPARC IBD), 31.4% and 28.1% of patients with CD and UC, respectively, reported moderate-to-severe bowel urgency in the past week [10]. However, this study did not compare changes in bowel urgency severity over time. We used real-world data from the SPARC IBD database to assess changes in severity of bowel urgency among patients with CD or UC at 6 months from their enrollment visit.
Methods
Study Design and Patient Population
This observational, longitudinal study utilized the SPARC IBD database with data collected from November 2016 to March 2022. Details about the SPARC IBD registry and its data collection procedures are presented elsewhere [10]. Adults (≥ 18 years) with newly diagnosed or established CD or UC (by standard clinical, radiographic, endoscopic, and histologic criteria) who were patients at any of the participating clinical sites were included in the analysis.
For this study, patients were required to have a mutually exclusive diagnosis of CD or UC during the enrollment window, defined as 7 days from the date of their consent [10]. Patients were also required to have completed the bowel urgency questionnaire at the time of enrollment and during a 6-month follow-up period, which was defined as 6 months (± 30 days) from enrollment. Patients with both CD and UC diagnoses were excluded [10].
Compliance with Ethics Guidelines
This study is based on previously existing de-identified observational data. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and are consistent with Good Pharmacoepidemiology Practices and applicable local laws and regulations, as appropriate. The University of Pennsylvania Institutional Review Board approved the study as part of the all-inclusive approval for observational studies conducted using SPARC IBD data.
Study Measures
Demographics included age at enrollment, gender, and age at diagnosis; clinical characteristics included disease duration, presence or absence of fatigue, average number of bowel movements, stool frequency (compared with normal), stool description, rectal bleeding, abdominal pain, and bowel incontinence. Bowel urgency was assessed via an electronic case report form that asked patients, “Over the last week, how much urgency have you had before bowel movements?” Response options were none (i.e., can wait 15 minutes or longer to have a bowel movement), mild (i.e., need to get to the bathroom within 5–15 min), moderate (i.e., need to get to the bathroom within 2–5 min), moderately severe (i.e., need to get to the bathroom in less than 2 min), and severe (i.e., sometimes unable to make it to the bathroom in time). Patients with a change in urgency severity status from enrollment to the 6-month visit were categorized into the “improvement” cohort, “no change” cohort, or “worsening” cohort.
Disease activity measures in SPARC IBD included Physician’s Global Assessment (PGA), six-point and nine-point UC Disease Activity Index (UCDAI), and short CD Activity Index (sCDAI) scores. Medications assessed at baseline included immunomodulators (methotrexate, mercaptopurine, azathioprine, and cyclosporine), 5-aminosalicylates (balsalazide, mesalamine, olsalazine, and sulfasalazine), biologic tumor necrosis factor inhibitors (TNFi: adalimumab, certolizumab, golimumab, and infliximab), biologic non-TNFi (ustekinumab and vedolizumab), other advanced therapies (tofacitinib), steroids, and antibiotics (ciprofloxacin, metronidazole, rifaximin, sulfamethoxazole and trimethoprim, and vancomycin). We assessed which of the variables measured at baseline were associated with worsening or improvement in bowel urgency among UC And CD patients.
A detailed list of patient-reported outcomes is presented in Table S1.
Statistical Analysis
Descriptive statistics and contingency tables were created to summarize enrollment clinical characteristics, disease activity, and medication status stratified by the changing status of bowel urgency severity. To examine changes in bowel urgency severity over the study period among patients with CD or UC, two separate analyses were performed: (a) “worsening” versus “no change” excluding those with moderate-to-severe bowel urgency at enrollment and (b) “improvement” versus “no change” excluding those with no bowel urgency at enrollment. The enrollment characteristics were compared within these two groups. Categorical variables were presented using frequencies and percentages and compared using Chi-square tests or Fisher’s exact tests (when any cell count ≤ 5). Continuous measures were summarized with means and standard deviations (SD) and compared using t tests. A two-sided alpha = 0.05 was used for statistical comparisons. Baseline variables which were significantly different between (a) “worsening” versus “no change” cohorts and (b) “improvement” versus “no change” cohorts.
Results
Patient attrition and the number of patients with bowel urgency data at 6 months categorized by bowel urgency at enrollment are presented in Fig. S1 and Table S2, respectively. Participants with missing 6-month urgency data who were excluded from this analysis were more likely to have moderate-to-severe urgency at baseline in both CD and UC (Table S2).
Demographics and Other Variables at Enrollment by Change of Urgency at 6 Months in Patients with CD
Detailed demographics and clinical characteristics at enrollment by change in urgency severity at the 6-month visit are presented for patients with CD in Table 1.
Table 1.
Demographics and clinical characteristics of patients with CD at enrollment by change in urgency severity at 6 months
| Improvement a (N = 195) |
No change a (N = 262) |
Improvement versus no change P-value | Worsening b (N = 206) |
No change b (N = 376) |
Worsening versus no change P-value | |
|---|---|---|---|---|---|---|
| Age at enrollment (years), mean (SD) | 40.5 (13.7) | 43.9 (15.0) | 0.013 | 42.5 (15.3) | 41.0 (14.1) | 0.251 |
| Gender, n (%) | 0.216 | 0.002 | ||||
| Female | 125 (64.1) | 153 (58.4) | 133 (64.6) | 193 (51.3) | ||
| Male | 70 (35.9) | 109 (41.6) | 73 (35.4) | 183 (48.7) | ||
| Disease duration at enrollment (years), mean (SD) | 13.8 (11.12) | 15.4 (11.9) | 0.144 | 14.0 (11.49) | 14.3 (11.00) | 0.776 |
| Age at diagnosis (years), mean (SD) | 27.0 (12.97) | 28.5 (13.8) | 0.246 | 28.6 (15.20) | 26.6 (13.02) | 0.122 |
| sCDAI total score, mean (SD) | 140.0 (88.90) | 148.8 (98.74) | 0.403 | 115.3 (69.28) | 102.1 (68.66) | 0.055 |
| Fatigue, n (%) | 59 (48.8) | 67 (57.8) | 0.165 | 46 (42.6) | 74 (40.9) | 0.776 |
| Enrollment number of daily bowel movement, mean (SD) | 2.8 (2.20) | 3.1 (2.30) | 0.133 | 2.6 (1.83) | 2.5 (1.84) | 0.511 |
| Current average number of daily bowel movement, mean (SD) | 4.1 (3.31) | 4.5 (3.18) | 0.212 | 3.5 (2.87) | 3.2 (2.23) | 0.183 |
| Current average number of daily liquid bowel movement, mean (SD) | 2.5 (3.22) | 2.8 (3.57) | 0.420 | 1.7 (2.59) | 1.4 (2.27) | 0.217 |
| Daily stool frequency, n (%) | 0.732 | 0.521 | ||||
| Normal | 99 (51.6) | 127 (48.8) | 130 (63.4) | 240 (64.7) | ||
| 1–2 stools per day more than normal | 44 (22.9) | 64 (24.6) | 47 (22.9) | 74 (19.9) | ||
| 3–4 stools per day more than normal | 27 (14.1) | 32 (12.3) | 10 (4.9) | 31 (8.4) | ||
| 5 or more stools per day more than normal | 20 (10.4%) | 30 (11.5%) | 11 (5.4%) | 15 (4.0%) | ||
| Stool description, n (%) | 0.739 | 0.623 | ||||
| Formed | 36 (23.4) | 35 (20.6) | 63 (41.7) | 106 (42.2) | ||
| Soft or semi-formed | 77 (50.0) | 92 (54.1) | 63 (41.7) | 112 (44.6) | ||
| Mostly or all liquid | 41 (26.6) | 43 (25.3) | 25 (16.6) | 33 (13.1) | ||
| Blood in stool, n (%) | 0.467 | 0.844 | ||||
| No blood seen | 156 (80.0) | 197 (75.2) | 170 (82.5) | 304 (80.9) | ||
| Blood less than 50% of the time | 28 (14.4) | 48 (18.3) | 26 (12.6) | 54 (14.4) | ||
| Blood passed 50% or more or blood passed alone | 11 (5.6) | 17 (6.5) | 10 (4.9) | 18 (4.8) | ||
| Abdominal pain, n (%) | 0.640 | 0.289 | ||||
| None | 83 (42.6) | 103 (39.3) | 109 (52.9) | 216 (57.4) | ||
| Mild | 64 (32.8) | 97 (37.0) | 65 (31.6) | 118 (31.4) | ||
| Moderate or severe | 48 (24.6) | 62 (23.7) | 32 (15.5) | 42 (11.2) | ||
| Bowel urgency, n (%) | < 0.001 | 0.026 | ||||
| None | – | – | 113 (54.9) | 169 (44.9) | ||
| Mild | 69 (35.4) | 153 (58.4) | 61 (29.6) | 153 (40.7) | ||
| Moderate | 76 (39.0) | 54 (20.6) | 32 (15.5) | 54 (14.4) | ||
| Moderately severe to severe | 50 (25.6) | 55 (21.0) | – | – | ||
| General well-being, n (%) | 0.844 | 0.201 | ||||
| Generally well | 95 (48.7) | 133 (50.8) | 127 (62.0) | 260 (69.1) | ||
| Slightly under par | 65 (33.3) | 87 (33.2) | 61 (29.8) | 93 (24.7) | ||
| Poor to terrible | 35 (17.9) | 42 (16.0) | 17 (8.3) | 23 (6.1) | ||
| Physician’s Global Assessment of current disease status, n (%) | 0.295 | 0.413 | ||||
| Quiescent | 67 (44.1) | 92 (52.6) | 99 (61.9) | 170 (65.9) | ||
| Mild | 39 (25.7%) | 40 (22.9%) | 33 (20.6) | 55 (21.3) | ||
| Moderate or severe | 46 (30.3) | 43 (24.6) | 28 (17.5) | 33 (12.) | ||
| Bowel incontinence while awake in the last month, n (%) | 28 (18.5) | 41 (24.6) | 0.194 | 25 (16.6) | 22 (8.9) | 0.022 |
| Nighttime bowel movement in the last month, n (%) | 66 (43.7) | 68 (40.5) | 0.559 | 35 (23.3) | 56 (22.7) | 0.879 |
| Leakage of stool during sleep in the last month, n (%) | 13 (8.5) | 29 (17.2) | 0.021 | 14 (9.3) | 22 (8.8) | 0.883 |
| Number of days of work/school missed due to illness in the past 12 months, mean (SD) | 2.0 (8.24) | 2.0 (6.93) | 0.967 | 0.4 (1.81) | 1.1 (4.05) | 0.120 |
| Fecal calprotectin (μg/g), mean (SD) | 437.7 (639.23) | 290.2 (529.58) | 0.134 | 346.5 (553.22) | 229.2 (467.83) | 0.099 |
| Biologics, n (%) | 116 (77.3) | 152 (74.5) | 0.540 | 132 (78.6) | 206 (71.0) | 0.077 |
| TNFi | 76 (50.7) | 91 (44.6) | 0.259 | 81 (48.2) | 137 (47.2) | 0.841 |
| Non-TNFi | 40 (26.7) | 61 (29.9) | 0.505 | 51 (30.4) | 69 (23.8) | 0.124 |
| 5-aminosalicylates, n (%) | 13 (8.7) | 12 (5.9) | 0.312 | 17 (10.1) | 30 (10.3) | 0.939 |
| Antibiotics, n (%) | 7 (4.7) | 11 (5.4) | 0.759 | 4 (2.4) | 10 (3.4) | 0.588 |
| Immunomodulators, n (%) | 46 (30.7) | 62 (30.4) | 0.956 | 41 (24.4) | 93 (32.1) | 0.082 |
| Steroids, n (%) | 14 (9.3) | 17 (8.3) | 0.742 | 14 (8.3) | 16 (5.5) | 0.240 |
CD Crohn’s disease, sCDAI short CD Activity Index, SD standard deviation, TNFi tumor necrosis factor inhibitor, N total number of patients, n number of patients reporting the information
aPatients with CD, excluding those with no urgency at enrollment
bPatients with CD, excluding those with severe urgency at enrollment
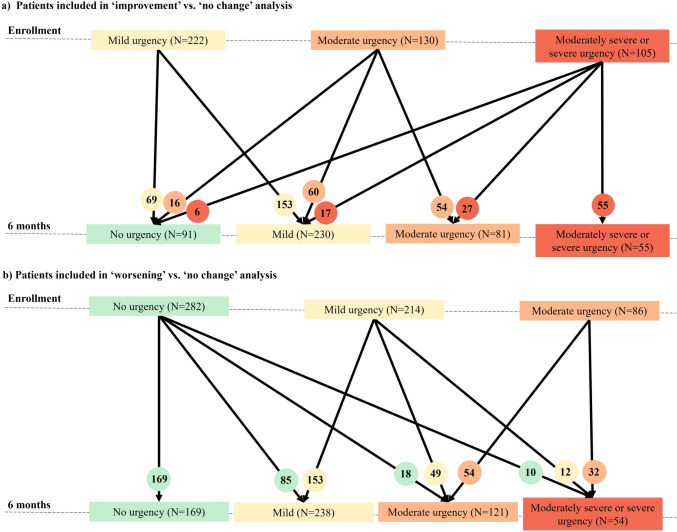
Of the 457 patients with CD who reported bowel urgency at enrollment, 195 (42.7%) reported an improvement, and 262 (57.3%) showed no change (Fig. 1a). Patients with CD in the “improvement” cohort were younger (40.5 years versus 43.9 years; P = 0.013) and more likely to have moderate, moderately severe, and severe bowel urgency (64.6% versus 41.6%; P < 0.001) compared with those in the “no change” cohort (Table 1).
Fig. 1.
Disposition of patients with Crohn's disease based on bowel urgency severity. a Patients included in the “improvement” versus “no change” analysis. b Patients included in the “worsening” versus “no change” analysis. Microsoft PowerPoint presentation was used to create these figures.
Out of 582 patients included in the “worsening” versus “no change” analysis, 206 (35.4%) reported worsening, and 376 (64.6%) reported no change in bowel urgency severity at 6 months (Fig. 1b). Compared with patients with no change in urgency, patients who reported worsening were more often females (64.6% versus 51.3%; P = 0.002) and have had bowel incontinence while awake (16.6% versus 8.9%; P = 0.022; Table 1). There was a significant difference in bowel urgency severity between the “worsening” and “no change” cohorts (P = 0.026).
Overall, most patients with CD received biologics and/or immunomodulators at enrollment. Treatment use was not significantly different between the “improvement” versus “no change” and “worsening” versus “no change” cohorts (Table 1).
Demographics and Other Variables at Enrollment by Change of Urgency Severity at 6 Months in Patients with UC
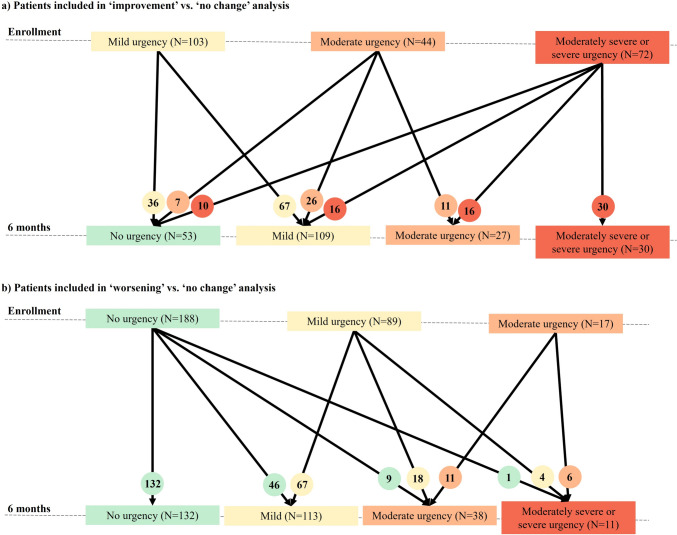
Detailed demographics and characteristics at enrollment by change in urgency severity at 6 months for patients with UC are presented in Table 2. Out of 219 patients with UC who reported bowel urgency at enrollment, 111 (50.7%) reported improvement, and 108 (49.3%) reported no change at 6 months (Fig. 2a). In the improvement cohort, patients reported higher mean UCDAI six-point score (2.4 versus 1.7; P = 0.011), greater number of daily bowel movements (5.3 versus 4.1; P = 0.016), higher proportion of patients with ≥ 5 stools frequency daily (21.1% versus 14.8%; P = 0.026), and were more likely to have moderate, moderately severe, and severe bowel urgency at enrollment (67.5% versus 38.0%; P < 0.001) compared with those in the no change cohort (Table 2).
Table 2.
Demographics and clinical characteristics of patients with UC at enrollment by change in urgency severity at 6 months
| Improvement a (N = 111) |
No change a (N = 108) |
Improvement versus no change P-value | Worsening b (N = 84) |
No change b (N = 210) |
Worsening versus no change P-value | |
|---|---|---|---|---|---|---|
| Age at enrollment (years), mean (SD) | 43.9 (14.21) | 42.0 (14.01) | 0.318 | 46.3 (13.90) | 40.6 (13.85) | 0.002 |
| Gender, n (%) | 0.117 | 0.911 | ||||
| Female | 53 (47.7) | 63 (58.3) | 47 (56.0) | 116 (55.2) | ||
| Male | 58 (52.3) | 45 (41.7) | 37 (44.0) | 94 (44.8) | ||
| Disease duration at enrollment (years), mean (SD) | 11.4 (11.13) | 10.4 (8.08) | 0.479 | 13.0 (10.73) | 11.0 (8.97) | 0.135 |
| Age at diagnosis (years), mean (SD) | 32.5 (12.92) | 31.8 (14.50) | 0.721 | 32.8 (13.16) | 29.7 (12.86) | 0.065 |
| UCDAI 6-point score, mean (SD) | 2.4 (2.01) | 1.7 (1.78) | 0.011 | 0.9 (1.30) | 0.7 (1.24) | 0.382 |
| UCDAI 9-point score mean (SD) | 3.4 (2.58) | 3.0 (2.46) | 0.280 | 1.6 (2.07) | 1.1 (1.80) | 0.120 |
| Fatigue, n (%) | 24 (46.2) | 26 (49.1) | 0.766 | 21 (44.7) | 35 (33.3) | 0.180 |
| Enrollment number of daily bowel movement, mean (SD) | 2.5 (1.86) | 2.6 (2.32) | 0.727 | 2.1 (1.63) | 2.1 (1.84) | 0.881 |
| Current average number of daily bowel movement, mean (SD) | 5.3 (4.00) | 4.1 (3.34) | 0.016 | 3.2 (2.76) | 2.7 (2.06) | 0.170 |
| Current average number of daily liquid bowel movement, mean (SD) | 3.3 (4.21) | 2.7 (3.68) | 0.281 | 1.1 (1.85) | 1.0 (2.02) | 0.889 |
| Daily stool frequency, n (%) | 0.026 | 0.513 | ||||
| Normal | 29 (26.6) | 47 (43.5) | 59 (70.2) | 154 (73.3) | ||
| 1–2 stools per day more than normal | 32 (29.4) | 32 (29.6) | 12 (14.3) | 35 (16.7) | ||
| 3–4 stools per day more than normal | 25 (22.9) | 13 (12.0) | 7 (8.3) | 10 (4.8%) | ||
| 5 or more stools per day more than normal | 23 (21.1) | 16 (14.8) | 6 (7.1) | 9 (4.3%) | ||
| Stool description, n (%) | 0.726 | 0.278 | ||||
| Formed | 27 (32.1) | 24 (30.0) | 27 (43.5) | 81 (55.1) | ||
| Soft or semi-formed | 37 (44.0) | 40 (50.0) | 30 (48.4) | 58 (39.5) | ||
| Mostly or all liquid | 20 (23.8) | 16 (20.0) | 5 (8.1) | 8 (5.4) | ||
| Blood in stool, n (%) | 0.083 | 0.798 | ||||
| No blood seen | 55 (49.5) | 61 (56.5) | 64 (76.2) | 165 (78.9) | ||
| Blood less than 50% of the time | 26 (23.4) | 31 (28.7) | 14 (16.7) | 33 (15.8) | ||
| Blood passed 50% or more or blood passed alone | 30 (27.0) | 16 (14.8) | 6 (7.1%) | 11 (5.3) | ||
| Abdominal pain, n (%) | 0.101 | 0.005 | ||||
| None | 46 (41.4) | 53 (49.1) | 44 (52.4) | 148 (70.5) | ||
| Mild | 47 (42.3) | 31 (28.7) | 28 (33.3) | 50 (23.8) | ||
| Moderate or severe | 18 (16.2) | 24 (22.2) | 12 (14.3) | 12 (5.7) | ||
| Bowel urgency, n (%) | < 0.001 | 0.560 | ||||
| None | – | – | 56 (66.7) | 132 (62.9) | ||
| Mild | 36 (32.4) | 67 (62.0) | 22 (26.2) | 67 (31.9) | ||
| Moderate | 33 (29.7) | 11 (10.2%) | 6 (7.1) | 11 (5.2) | ||
| Moderately severe to severe | 42 (37.8) | 30 (27.8) | – | – | ||
| General well-being, n (%) | 0.409 | 0.315 | ||||
| Generally well | 52 (46.8) | 59 (54.6) | 60 (71.4) | 166 (79.0) | ||
| Slightly under par | 36 (32.4) | 33 (30.6) | 19 (22.6) | 37 (17.6) | ||
| Poor to terrible | 23 (20.7) | 16 (14.8) | 5 (6.0) | 7 (3.3) | ||
| Physician's Global Assessment of current disease status, n (%) | 0.382 | 0.099 | ||||
| Quiescent | 28 (34.1) | 33 (40.7) | 39 (60.0) | 116 (73.9) | ||
| Mild | 26 (31.7) | 18 (22.2) | 14 (21.5) | 19 (12.1) | ||
| Moderate or severe | 28 (34.1) | 30 (37.0) | 12 (18.5) | 22 (14.0) | ||
| Bowel incontinence while awake in the last month, n (%) | 16 (19.3) | 15 (18.8) | 0.932 | 8 (13.1) | 9 (6.2) | 0.097 |
| Nighttime bowel movement in the last month, n (%) | 30 (36.1) | 32 (40.0) | 0.612 | 15 (24.6) | 21 (14.4) | 0.077 |
| Leakage of stool during sleep in the last month, n (%) | 4 (4.8) | 4 (5.0) | 1.000 | 2 (3.3) | 0 (0.0) | 0.086 |
| Number of days of work/school missed due to illness in past 12 months, mean (SD) | 10.5 (58.47) | 0.9 (2.10) | 0.311 | 1.3 (4.11) | 1.0 (3.73) | 0.727 |
| Fecal calprotectin (μg/g), mean (SD) | 555.7 (750.06) | 294.7 (455.20) | 0.084 | 323.5 (505.46) | 187.4 (371.54) | 0.157 |
| Biologics, n (%) | 47 (50.5) | 35 (40.7) | 0.187 | 34 (50.0) | 74 (43.5) | 0.365 |
| TNFi | 25 (26.9) | 21 (24.4%) | 0.706 | 20 (29.4) | 51 (30.0) | 0.929 |
| Non-TNFi | 22 (23.7) | 14 (16.3) | 0.219 | 14 (20.6) | 23 (13.5) | 0.175 |
| 5-aminosalicylates, (%), n (%) | 44 (47.3) | 42 (48.8) | 0.838 | 29 (42.6%) | 84 (49.4) | 0.345 |
| Antibiotics, n (%) | 1 (1.1) | 2 (2.3) | 0.609 | 0 (0.0) | 3 (1.8) | 0.560 |
| Immunomodulators, n (%) | 17 (18.3) | 17 (19.8) | 0.800 | 16 (23.5) | 46 (27.1) | 0.575 |
| Steroids, n (%) | 21 (22.6) | 20 (23.3) | 0.914 | 6 (8.8) | 11 (6.5) | 0.524 |
SD standard deviation, TNFi tumor necrosis factor inhibitor, UC ulcerative colitis, UCDAI UC Disease Activity Index, N otal number of patients, n number of patients reporting the information
aPatients with UC, excluding those with no urgency at enrollment
bPatients with UC, excluding those with severe urgency at enrollment
Fig. 2.
Disposition of patients with ulcerative colitis based on urgency severity. a Patients included in “improvement” versus “no change” analysis. b Patients included in the “worsening” versus “no change” analysis. Microsoft PowerPoint presentation was used to create these figures.
Out of 294 patients with UC included in the “worsening” versus “no change” analysis, 84 (28.6%) patients reported worsening, and 210 (71.4%) had no change in bowel urgency (Fig. 2b). Patients with UC who reported worsening of urgency severity were older than those with no change in bowel urgency severity (46.3 years versus 40.6 years; P = 0.002; Table 2). There was a difference in abdominal pain between the “worsening” and “no change” cohorts (P = 0.005; Table 2).
About 50% of patients in both analyses were on biologics at enrollment. No significant differences were reported for treatment use between the “improvement” versus “no change” and “worsening” versus “no change” cohorts (Table 2).
Discussion
Patients with CD or UC consider bowel urgency a more relevant and important symptom than abdominal pain, blood in stools, or stool frequency [2, 4, 11]. In this retrospective analysis of patients enrolled in the SPARC IBD, we found that bowel urgency is common among patients with CD and UC. The course of bowel urgency is dynamic. Despite that approximately 40–50% of patients showed improvement in bowel urgency 6 months postenrollment, about one out of three patients continue to experience worsening. Although we have previously demonstrated a cross-sectional association of bowel urgency with the cardinal symptoms of CD and UC [10], in this longitudinal study, there were surprisingly few factors measured at enrollment that were associated with changes in bowel urgency at 6 months.
Younger patients with CD were more likely to show improvement. On the other hand, older patients with UC were more likely to experience worsening of bowel urgency. UC patients with severe bowel frequency and bowel urgency reported improvement in urgency by 6 months, suggesting that bowel inflammation may have contributed to the urgency. However, this was less evident for CD. This highlights the dynamic and somewhat unpredictable nature of bowel urgency among these patients.
Bowel urgency is a key element of patient burden in IBD and is known to have the highest impact on quality of life [5, 12, 13]. In a survey-based study by Hibi et al. on patients with UC, when asked “what symptoms do you want to improve,” the most common answer was bowel urgency [6]. Despite evidence supporting the importance of bowel urgency for patients with both CD and UC, clinical guidelines in the USA mention assessment of bowel urgency only for UC and not CD [12, 14].
Approximately two-thirds or more of patients with IBD reported some degree of bowel urgency [3, 9, 15]. A retrospective study found bowel urgency to be a common symptom among patients with CD (67%) and UC (84%) [15]. Given the importance and prevalence of bowel urgency, we sought to determine the dynamics of this symptom over time. We leveraged a five-point bowel urgency scale that we have previously demonstrated to be strongly associated with general well-being in the SPARC IBD cohort [10]. We found that a significantly greater proportion of patients with CD or UC who had moderate-to-severe bowel urgency at enrollment reported improvement in the urgency severity at 6 months compared with those with mild bowel urgency. Moreover, the improvement in urgency was attained to the level of mild symptoms, while very few patients reported complete resolution of this symptom, about 30% continue to experience worse urgency. These data indicate the lack of effective therapies to resolve bowel urgency completely.
Although clinically important, the literature on change in bowel urgency in patients with CD and UC is limited. To our knowledge, only two real-world studies have previously reported changes in bowel urgency severity among patients with UC over 6 or more months [16, 17]. Wolf et al. identified urgency among UC patients as a symptom of great concern and is reflective of inadequate therapy in a real-world setting [16]. Another longitudinal study emphasized the importance of bowel urgency as a patient-reported outcome to capture quality of life and risk of clinical decompensation [17]. Sninsky et al. found that increased levels of bowel urgency (hurry, immediately, and incontinence) in patients with UC are proportionally associated with greater odds of social impairment, depression, anxiety, and fatigue [17].
Our study has some limitations. The data for our study are largely derived from the population in tertiary care academic centers and may limit the generalizability of the results to the broader IBD population. However, the SPARC IBD cohort captures a range of disease activity. Patients in SPARC IBD were not selected for enrollment based on their level of IBD or urgency severity. Not all participants in SPARC IBD had a follow-up visit at 6 months. Patients who were relatively healthier or those who managed their condition better might have less engagement with healthcare providers and/or less frequent visits. Surprisingly, those without data at 6 months had more severe urgency at baseline. It is possible that these patients had earlier follow-up such that their cadence of follow-up visits missed the 6-month window or transferred to another provider. If this were the case, we may have underestimated the proportion of patients with improvement. It is also possible to have underestimated the proportion of patients with less urgency as these patients may be less likely to seek follow-up care.
Conclusions
Bowel urgency is a common, unstable, and somewhat unpredictable symptom among patients with CD and UC and should be assessed regularly in clinical practice. While a relatively large proportion of patients achieved improvement in bowel urgency at 6 months postenrollment, more than half of the patients continued to have some degree of bowel urgency. Therapies specifically targeting bowel urgency may be needed to achieve complete resolution of symptoms.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participants of the SPARC IBD study. The authors also thank Payal Jha and Keerthana Muthiah, employees of Eli Lilly Services India Pvt. Ltd., for providing medical writing support.
Declarations
Funding
This study was sponsored by Eli Lilly and Company, Indianapolis, USA.
Competing interests
Dr. James D. Lewis—Research grants: Nestle Health Science, Takeda, Janssen, and AbbVie. Consulting fees: Entasis Therapeutics, Bridge Biotherapeutics, AbbVie, Scipher Medicine, Celgene/BMS, Merck, and Janssen. Participation on a Data Safety Monitoring Board or advisory board: Gilead, Galapagos, Arena Pharmaceuticals, Protagonist Therapeutics, Sanofi, Amgen, and Pfizer. Payment for expert testimony: Manufacturers of generic ranitidine. Dr. Ghadeer K. Dawwas—Research grants: National Institutes of Health and American Society of Hematology. Honoria: Valley Health Winchester Medical Center and BMS-Pfizer alliance. Dr. Ghadeer K. Dawwas—Employment and stockholder: Vanderbilt University Medical Center. Dr. Xian Zhoa—Employment and stockholder: Syneos Health. Drs. Theresa H. Gibble, Mingyang Shan, and April N. Naegeli—Employees and stockholders: Eli Lilly and Company.
Data availability statement
All the data from this study are presented in this manuscript, including the supplementary material. People interested in accessing SPARC IBD data for research should contact the Crohn’s & Colitis Foundation.
Ethics approval
This study is based on previously existing de-identified observational data. The study was conducted using the ethical principles that have their origin in the Declaration of Helsinki and are consistent with Good Pharmacoepidemiology Practices and applicable local laws and regulations, as appropriate. The University of Pennsylvania Institutional Review Board approved the study as part of the all-inclusive approval for observational studies conducted using SPARC IBD data (approval number: 823980).
Consent to participate
Consent was not obtained, as the data used by the research team were de-identified.
Consent to publication
Not applicable to this study owing to its design.
Code availability
Not applicable.
Author contributions
The authors confirm contributions to the paper as follows: Drs. Hunter contributed to the conception of the study. Drs. Hunter, Lewis, Shan, and Zhoa designed the study. Zhou analyzed the data. Dr. Lewis contributed to the acquisition of data. Drs. Dawwas, Hunter, Lewis, Shan, Naegeli, and Zhou contributed to the interpretation of the data. Drs. Dawwas, Hunter, Lewis, Shan, Naegeli, and Zhou contributed to the critical revision of the paper for important intellectual content. All authors reviewed the results and approved the final version of the manuscript.
References
- 1.Mulder DJ, Noble AJ, Justinich CJ, Duffin JM. A tale of two diseases: the history of inflammatory bowel disease. J Crohns Colitis. 2014;8(5):341–8. 10.1016/j.crohns.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 2.Farrell D, McCarthy G, Savage E. Self-reported symptom burden in individuals with inflammatory bowel disease. J Crohn’s Colitis. 2016;10(3):315–22. 10.1093/ecco-jcc/jjv218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nóbrega VG, Silva INN, Brito BS, Silva J, Silva M, Santana GO. The onset of clinical manifestations in inflammatory bowel disease patients. Arq Gastroenterol. 2018;55(3):290–5. 10.1590/s0004-2803.201800000-73 [DOI] [PubMed] [Google Scholar]
- 4.van Deen WK, Obremskey A, Moore G, van den Akker-van Marle ME, Doctor JN, Hwang C. An assessment of symptom burden in inflammatory bowel diseases to develop a patient preference-weighted symptom score. Qual Life Res. 2020;29(12):3387–96. 10.1007/s11136-020-02606-2 [DOI] [PubMed] [Google Scholar]
- 5.Rubin DT, Sninsky C, Siegmund B, Sans M, Hart A, Bressler B, Bouhnik Y, Armuzzi A, Afzali A. International perspectives on management of inflammatory bowel disease: opinion differences and similarities between patients and physicians from the IBD GAPPS Survey. Inflamm Intest Dis. 2021;27(12):1942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibi T, Ishibashi T, Ikenoue Y, Yoshihara R, Nihei A, Kobayashi T. Ulcerative colitis: disease burden, impact on daily life, and reluctance to consult medical professionals: results from a Japanese internet survey. Inflamm Intest Dis. 2020;5(1):27–35. 10.1159/000505092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tew GA, Jones K, Mikocka-Walus A. Physical activity habits, limitations, and predictors in people with inflammatory bowel disease: a large cross-sectional online survey. Inflamm Bowel Dis. 2016;22(12):2933–42. 10.1097/MIB.0000000000000962 [DOI] [PubMed] [Google Scholar]
- 8.Newton L, Randall JA, Hunter T, Keith S, Symonds T, Secrest RJ, Komocsar WJ, Curtis SE, Abetz-Webb L, Kappelman M, et al. A qualitative study exploring the health-related quality of life and symptomatic experiences of adults and adolescents with ulcerative colitis. J Patient Rep Outcomes. 2019;3(1):66. 10.1186/s41687-019-0154-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulai PS, Jairath V, Khanna R, Ma C, McCarrier KP, Martin ML, Parker CE, Morris J, Feagan BG, Sandborn WJ. Development of the symptoms and impacts questionnaire for Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2020;51(11):1047–66. 10.1111/apt.15726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawwas GK, Jajeh H, Shan M, Naegeli AN, Hunter T, Lewis JD. Prevalence and factors associated with fecal urgency among patients with ulcerative colitis and Crohn’s disease in the study of a prospective adult research cohort with inflammatory bowel disease. Crohn’s Colitis 360. 2021;3(3):otab046. 10.1093/crocol/otab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis E, Ramos-Goñi JM, Cuervo J, Kopylov U, Barreiro-de Acosta M, McCartney S, Rosenfeld G, Bettenworth D, Hart A, Novak K, et al. A qualitative research for defining meaningful attributes for the treatment of inflammatory bowel disease from the patient perspective. Patient. 2020;13(3):317–25. 10.1007/s40271-019-00407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413. 10.14309/ajg.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 13.Carpio D, López-Sanromán A, Calvet X, Romero C, Cea-Calvo L, Juliá B, Argüelles-Arias F. Perception of disease burden and treatment satisfaction in patients with ulcerative colitis from outpatient clinics in Spain: UC-LIFE survey. Eur J Gastroenterol Hepatol. 2016;28(9):1056–64. 10.1097/MEG.0000000000000658 [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517. 10.1038/ajg.2018.27 [DOI] [PubMed] [Google Scholar]
- 15.Petryszyn PW, Paradowski L. Stool patterns and symptoms of disordered anorectal function in patients with inflammatory bowel diseases. Adv Clin Exp Med. 2018;27(6):813–8. 10.17219/acem/68986 [DOI] [PubMed] [Google Scholar]
- 16.Wolf DC, Naegeli AN, Moore PC, Janak JC, Crabtree MM, Shan M, Hunter TM, Sontag A, Cross RK. P187 Change in urgency status among ulcerative colitis patients: understanding the impact of treatment changes from the Corrona Inflammatory Bowel Disease Registry. J Crohn’s Colitis. 2021;15(Supplement_1):S256–7. 10.1093/ecco-jcc/jjab076.314 [DOI] [Google Scholar]
- 17.Sninsky JA, Barnes EL, Zhang X, Long MD. Urgency and its association with quality of life and clinical outcomes in patients with ulcerative colitis. Am J Gastroenterol. 2022;117(5):769–76. 10.14309/ajg.0000000000001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data from this study are presented in this manuscript, including the supplementary material. People interested in accessing SPARC IBD data for research should contact the Crohn’s & Colitis Foundation.