Abstract
Abnormal visual experience during the critical period can cause deficits in visual function, such as amblyopia. High magnesium (Mg2+) supplementary can restore ocular dominance (OD) plasticity, which promotes the recovery of amblyopic eye acuity in adults. However, it remains unsolved whether Mg2+ could recover binocular vision in amblyopic adults and what the molecular mechanism is for the recovery. We found that in addition to the recovery of OD plasticity, binocular integration can be restored under the treatment of high Mg2+ in amblyopic mice. Behaviorally, Mg2+-treated amblyopic mice showed better depth perception. Moreover, the effect of high Mg2+ can be suppressed with transient receptor potential melastatin-like 7 (TRPM7) knockdown. Collectively, our results demonstrate that high Mg2+ could restore binocular visual functions from amblyopia. TRPM7 is required for the restoration of plasticity in the visual cortex after high Mg2+ treatment, which can provide possible clinical applications for future research and treatment of amblyopia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-024-01242-x.
Keywords: Amblyopia, Magnesium, TRPM7
Introduction
Amblyopia is a common visual disorder that is closely related to experience-dependent plasticity during development [1–3]. The critical period of heightened plasticity is essential for various neural systems, including the visual system, and plays an important role in the formation of new functions and circuits [4–6]. Monocular deprivation (MD) during this critical period can severely disrupt the ocular dominance (OD) distribution in the primary visual cortex (V1), causing deficits in both the amblyopic eye’s acuity and binocular integration, but is ineffective after the closure of the critical period [7–9]. Thus, promoting synaptic plasticity in V1 is of great significance for the treatment of amblyopia in adults [10].
In the brain, magnesium (Mg2+) is required for many important physiological processes related to the proper function and plasticity of neuronal circuits [11, 12]. For example, it modulates the voltage-dependent blockade of NMDA receptors and regulates their opening during coincident excitation, which is critical for synaptic plasticity [13, 14]. In cultured hippocampal neural networks, elevating Mg2+ concentration in the extracellular fluid leads to permanent enhancement of synaptic plasticity [15]. Notably, it has been demonstrated that an increase in brain Mg2+ promotes learning and memory in rodents by enhancing both short-term synaptic facilitation and long-term potentiation (LTP) [16, 17]. A previous study demonstrated that Mg2+ can enhance the OD plasticity of V1 by upregulating the expression of NR2B-containing NMDA receptors in adult mice [18]. However, it is unknown whether the increase of Mg2+ concentration in V1 can rescue binocular dysfunction caused by amblyopia. Furthermore, the molecular mechanism that caused the upregulation of NR2B expression also remains to be discovered.
In the current study, we combined long-term MD spanning the whole critical period and treatment of a Mg2+ compound (Magnesium-l-Threonate, MgT) to establish the mouse model of amblyopia with high Mg2+. According to the results of in vivo electrophysiological recording, we found the OD distribution and binocular matching of orientation preference were both recovered after Mg2+ treatment, indicating a heightened synaptic plasticity. In addition, we showed that high Mg2+ treatment reinstated the variability and reliability of binocular responses in amblyopic mice. These results were accompanied by the restoration of the depth perception ability. Furthermore, we showed that in adult V1, knocking down the expression of the transient receptor potential melastatin-like 7 (TRPM7) chanzyme could prevent the upregulation of NR2B expression and reverse the recovery of visual function after Mg2+ treatment.
Materials and Methods
Animals
The wild-type mice C57BL/6 were housed with littermates at 24 °C and maintained on a 12 h:12 h light/dark cycle. Food and water were provided ad libitum. The study involved conducting experiments on mice of both genders. All efforts were made to reduce animal potential discomfort. All protocols and procedures followed the instructions of the Animal Care and Use Committee of Fudan University.
Drug Administration
Magnesium-l-Threonate (953 mg/kg per day) [18] or water was treated via intragastric administration for one month starting from P60. The daily water intake of each mouse was measured and then 0.2 mL of MgT solution (143 mg/mL) or water was administrated at 5 pm every day. There was no significant difference in growth trend between Veh and MgT mice. Mg2+ concentration of visual cortices in two groups was quantitated using a magnesium assay kit (MAK026 SIGMA) according to the manufacturer’s instructions.
Monocular Deprivation (MD)
Ophthalmic scissors, forceps, and suture needles used for MD were strictly sterilized. To obtain amblyopic mice, long-term MD from P21 to P35 was used in this study. Animals were anesthetized with isoflurane in air (5% for induction, 1%–2% for maintenance). The upper and lower eyelid margins of one eye were surgically trimmed and subsequently sutured together with three mattress sutures (6-0 silk, Ethicon) [19, 20]. Chloramphenicol Eye Drops were applied to prevent inflammation of the wound after MD. After the surgical procedure, the mice were subjected to daily examinations. Any mice that displayed spontaneous reopening of the sutured eye were excluded from further experimentation. The eyes of long-term MD mice would be reopened on P35 for further experiments.
In Vivo Electrophysiology
Mice were administered urethane (1.25 g/kg, i.p., Sigma-Aldrich) for anesthesia [21]. Body temperature was continuously monitored and maintained at 37 °C using a thermostatic electric blanket (Harvard). Silicon oil and black tap were applied to both eyes to prevent them from drying and light irritation. A small craniotomy (∼ 4 mm2) was performed on the binocular zone of the visual cortex (V1b). In V1b, a linear silicon electrode was inserted perpendicular to the pial surface. To avoid recording neurons with the same receptive field, we set the lambda 0.5 mm forward and 3 mm aside as the first recording site, and then moved 0.3 mm forward, backward, left, and right respectively to record the next site. Cells recorded throughout all layers were included in our recordings for each animal. We recorded the cells at the first penetration depth of 400 μm, followed by moving down more than 80 μm sequentially to record the second and third penetration depths [22]. The depths were confirmed for a subset of recordings through Dil staining and penetration reconstruction. The OmniPlex Neural Recording Data Acquisition System (Plexon Inc., Dallas, USA) was used to collect the signals. Toe-pinch reflex was tested throughout the recordings, and extra urethane was added when necessary. During monocular stimulation, the other eye was covered with adhesive tape. Following each recording session, the animal was sacrificed to conduct further experiments.
Visual Stimuli
Psychopy 3.0 was used to create the visual stimuli, which were displayed on a gamma-corrected Dell monitor (57 × 34 cm, 60 Hz refresh rate, about 100 cd/m2 brightness) that was situated 20 cm in front of the animal. The drifting sinusoidal gratings’ direction was changed randomly between 0 and 330 (12 steps at 30 spacing) to measure orientation selectivity. The stimuli were characterized by a spatial frequency of 0.04 c/deg and a temporal frequency of 2 Hz. Additionally, it was presented with a contrast level of 100%. Each stimulus was presented for a duration of 1.5 s, followed by a 1.5-s interval of a gray screen between stimuli. To calculate the spontaneous firing rate, a gray blank condition (mean luminance) was added.
Cortical Slice Preparation and in Vitro Electrophysiology
Mice were anesthetized with isoflurane before decapitation and the dissected brain was immediately rinsed in ice-cold oxygenated (95% O2, 5% CO2) cutting solution (pH 7.3–7.4, 300–305 mOsm) containing the following (in mmol/L): 110 choline chloride, 2.5 KCl, 4 MgSO4, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 11 d-glucose, 10 Na-ascorbate, 3.1 sodium pyruvate. Coronal slices were sectioned at 300 µm with a vibratome (Leica VT1200) and transferred to oxygenated ACSF (pH 7.3–7.4, 300-305 mOsm) containing the following (in mmol/L): 124 NaCl, 2.5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3,11 D-glucose, 2 MgCl2. After 30 min incubation at 34 °C followed by 1 h incubation at room temperature, slices were transferred to a recording chamber of an upright microscope (Olympus BW51) and submerged in ACSF. Whole-cell patch-clamp was performed in the primary visual cortex using the morphology of the hippocampus and subcortical white matter as primary landmarks according to the atlas (Paxinos and Watson Mouse Brain in Stereotaxic Coordinates, 3rd edition). A Sutter P97 puller was used to pull patch pipettes from borosilicate glass capillaries with filament (1.5 mm outer diameter and 0.86 mm inner diameter, Sutter BF150-86-10) with a resistance of 3 MΩ–8 MΩ. Neurons were classified as pyramidal neurons according to pyramidal-like soma shape, and the presence of an apical dendrite. For recording miniature excitatory and inhibitory postsynaptic currents (mEPSC and mIPSC), the pipette recording solution consisted of 120 mmol/L Cs-methanesulfonate, 15 mmol/L CsCl, 10 mmol/L HEPES, 2 mmol/L Mg-ATP, 0.3 mmol/L Na3-GTP, 1 mmol/L EGTA, 10 mmol/L Na2-phosphocreatine, and 2.5 mmol/L QX-314 (pH 7.3 adjusted with Tris-base, 290 mOsm). mEPSCs in 1 μmol/L TTX were recorded first at −70 mV, followed by recordings of mIPSCs at 10 mV in the same cells. All recordings were performed at 29 °C–31 °C with the chamber perfused with oxygenated ACSF. All voltages reported were not corrected for junction potential. Series resistance was compensated in current clamp mode and was kept below 30 MΩ. Signals were recorded at 10 kHz and filtered at 2 kHz (Digidata 1550A, Molecular Devices). Data were analyzed with Multiclamp 700B (Molecular Devices) using pClamp 10.7.
Open Field Test
The mice in open field tests were adult males of the same age (P90). Open field tests were carried out using a method similar to what was reported previously, with minor modifications [23]. Each mouse was placed in an open field apparatus (40 cm × 40 cm × 40 cm). Distance traveled and time spent in the central region (defined as 25% of the total area) were recorded and calculated automatically over a 15-min period using Image OFCR software (EthoVision XT 14).
Visual Cliff Test
In the visual cliff task, the apparatus (15 cm × 30 cm × 30 cm) consists of a shallow side and a deep side [24]. Each mouse was positioned on a cylindrical platform with a diameter of 3 cm, located at the center. The mice were allowed to move freely and had the option to step onto either side of the platform. Their behavior was monitored for 1 min via a camera placed above the apparatus [25]. Each mouse performed 6 trials. In each trial, the mouse stepped down from the stage and onto the platform. The trials were classified as “depth perceived” when the mice placed their paws on the “shallow side” of the platform, whereas they were categorized as “depth not perceived” when the mice placed their paws on the “deep side” [26]. The mouse head was kept as far between the sides as possible before the trial began. Trials of mice that did not come down from the stage for more than 10 min or jump out of the apparatus were not included in the statistics. The percentage of mice in the two groups that went to the safe side was compared in this test.
Western Blot
Western blotting was conducted routinely. After the end of the in vivo electrophysiology, the experimental mice in each group were placed on ice and decapitated. The bilateral visual cortices were carefully dissected and each side was one sample. The method of protein extraction and concentration determination were in the same way as previously described [27]. The primary antibodies we used included GRIN2B (rabbit polyclonal, 1:2,000, ABclonal), AMPA Receptor 1 (GluA1, rabbit monoclonal,1:1,000, Cell Signaling Technology), GABAA Receptor a1 (rabbit polyclonal, 1:5000, Millipore), TRPM7 (rabbit monoclonal, 1:500, Alomone) and GAPDH (mouse monoclonal, 1:2,000, Abcam). Western blot results were repeated multiple times, and ImageJ was used to measure the light density in each strip. All protein levels were standardized by normalizing them to the levels of GAPDH.
Virus Injection
Mice were anesthetized with isoflurane in air (5% for induction, 1%–2% for maintenance) and secured in a stereotaxic frame (RWD Instruments, Shenzhen, China). Body temperature was continuously monitored and maintained at 37 °C using a thermostatic electric blanket (Harvard). Each side of V1b (2.5 mm–3.3 mm lateral from the midline and 0.5 mm–0.8 mm anterior from the lambda suture) was infused with 560 nL virus (CtrlSCR or T7shRNA) for over 5 min at a rate of 0.12 μL/min. The mice’s scalps were sutured after the end of the injection and the mice were left in their home cages for three weeks post-surgery to recover.
Electrophysiological Data Analysis
The recording protocols were refined based on methods previously published [27]. Single units were isolated from the multiunit activity using Offline Sorter (Plexon) and custom Python scripts were then utilized for data quantification and statistical analysis.
Global Orientation Selective Index (gOSI) Calculation
We calculated the response magnitude (R) by subtracting the spontaneous rate determined by the presentation of the blank condition from the average spike rate during the 1.5 s of each direction and spatial frequency combination. The responses at the direction of θ, R(θ), were used to calculate gOSI = which is the normalized vector sum of the responses in the 180° orientation space [28]. We calculated the difference in the preferred orientation between the two eyes in previously described methods [21, 22, 29]. The absolute value of this interocular difference in preferred orientation, which is referred to as ΔO (Abs (Pref_contra - Pref_ipsi)), was used in all quantifications [25].
ODI and CBI Measurements
We assigned neurons to OD categories according to Hubel and Wiesel’s seven-category scheme [9, 30]. ODI was computed as ODI = (contra − ipsi response)/(contra + ipsi response). The ODI has a range of − 1 to 1, with positive values denoting contralateral bias and negative values denoting ipsilateral bias [19]. Responses were derived by computing the average firing rate during visual stimulation, surpassing the spontaneous baseline rate. The contralateral bias index (CBI) was used to calculate the contralateral bias in V1b of each mouse as follows: [(n1–n7) + 2/3(n2–n6)+1/3(n3–n5) + N]/2 N, where N = the total number of cells and nx = the number of cells corresponding to an OD score of x.
Narrow and Broad Units Classification
The units were subsequently classified as narrow or broad spiking neurons based on the properties of their average waveforms by means of through-to-peak time and off-end slope of the spike waveform [31–33]. The ratio of the firing rates of broad neurons and narrow neurons was calculated in contralateral and ipsilateral eyes in two groups, respectively.
Calculation of Response Variability and Stability
There are two calculation formulas for response variability and stability. The Fano factor: (the ratio between the variance and the mean of firing rates with repeated stimulation) was calculated to measure the response variability [34–36]. F >1 was considered a significant variability in the underlying firing rate of a neuron [37]. The reliability index was calculated for grating stimulation (gRI):, where variable T represents the quantity of repetitions of gratings in the optimal direction, is the distribution histogram of the neuronal firing rates during a 1.5-s stimulation period, with each bin representing a 100 ms interval, is the Pearson correlation coefficient [38, 39].
Statistical Analysis
The statistical analyses were conducted using GraphPad Prism software (version 8.0) and custom Python scripts. Prior to analysis, all data were assessed for normality using the Shapiro-Wilk test, and subsequent statistical testing was performed accordingly. Differences between two independent groups were assessed with the student’s unpaired t-test or Mann-Whitney U test. To compare the cumulative distributions, the Kolmogorov Smirnov (K-S) test was performed. One-way ANOVA was used to assess the differences between multiple groups. Differences were considered significant when the P value < 0.05.
Results
High Mg2+ Treatment Restored OD Distribution in Amblyopic Adult Mice After Long-Term MD
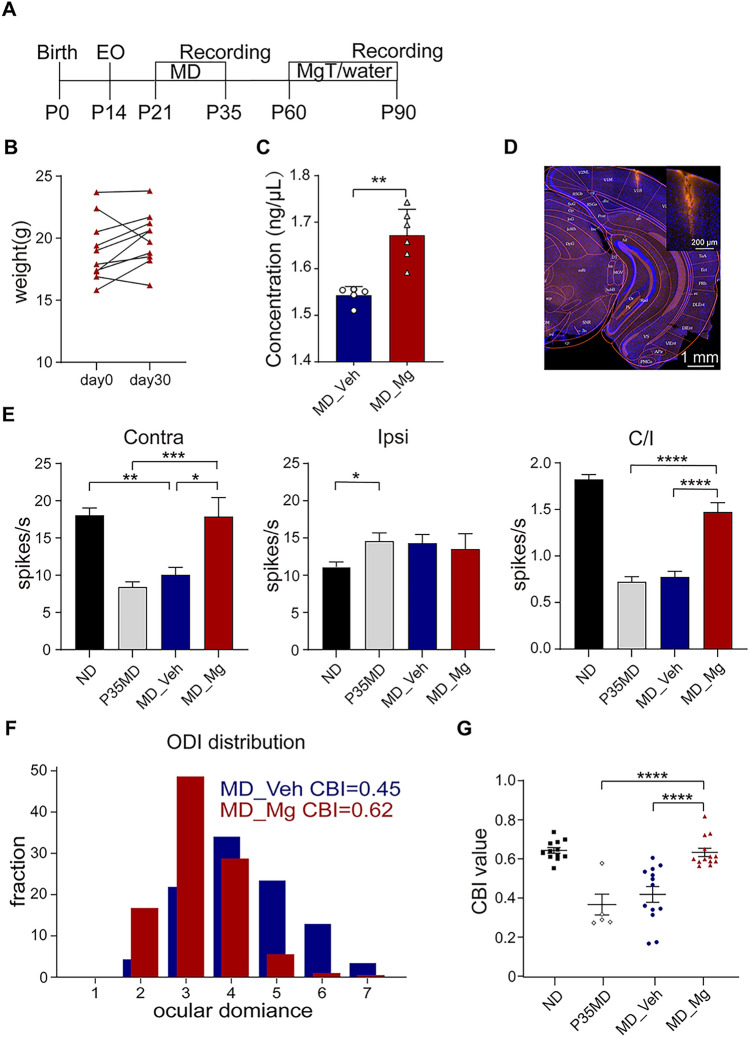
Long-term MD can cause severe deficits in OD distribution in V1, which cannot be spontaneously recovered due to the decline of synaptic plasticity in the adult brain. Previous studies showed that Mg2+ can improve synaptic plasticity in the hippocampus and V1 [16, 18]. To investigate whether abnormal OD distribution in adult mice after long-term MD can be recovered by high Mg2+ under normal visual experience, we first performed a 2-week MD on C57BL/6 mice from postnatal day (P)21 to P35, which spans the whole critical period of V1 development. On P35, we assessed the OD distribution in a subset of mice after 14 days MD (P35MD), and the remaining mice were randomly assigned into two groups after the deprived eye was opened (Fig. 1A). The experimental group was supplied with high Mg2+ (953 mg/kg per day), while the vehicle group was supplied with normal water, both from P60 to P90 via gavage. There was no significant difference in body weight pre- and 30 days post-Mg2+ treatment, and no obvious health problems were seen in the Mg2+-treated mice (Fig. 1B). We measured the concentration of Mg2+ in V1 and found a significant increase in Mg2+-treated mice (Fig. 1C). Next, we assessed OD distribution in MD_Veh and MD_Mg mice through in vivo electrophysiological recordings from the binocular area of V1 (V1b) contralateral to the deprived eye (Fig. 1D). OD was quantitatively attributed to each unit based on the eye-specific responses to a modified Hubel and Wiesel classification [7]. Notably, the neuronal responses driven by deprived eyes in P35MD and MD_Veh were lower than that in ND (no MD) mice, and the OD distribution was shifted to the non-deprived eye. In contrast, MD_Mg mice exhibited a significant recovery of stimulation-evoked firing rates for the deprived eye and the normal OD distribution that biased the deprived eye (Fig. 1E, F). In addition, the contralateral bias index (CBI) was significantly higher in MD_Mg mice compared to MD_Veh and P35MD mice (Fig. 1G). Considering many works of literature suggest that residual visual plasticity still exists during P35-P90 [40, 41], to demonstrate that the previously deprived eye response is restored primarily due to Mg2+ treatment but not the residual plasticity after P35, we extended the duration of MD to P90 and then measured the CBI of mice in both groups after 30 days of MgT/water treatment (Fig. S1A). It was shown that even with MD remaining between P35-P90, the OD of amblyopia mice was still restored by Mg2+ (Fig. S1B). Thus, elevated Mg2+ in V1 with normal visual experience can restore the OD distribution in amblyopic mice. Interestingly, we discovered by short-term MD that Mg2+ may be responsible for promoting the expression of adult-like plasticity, which could lead to the restoration of ocular dominance shift in adults (Fig. S2).
Fig. 1.
High Mg2+ treatment restored OD distribution in adult MD mice. A Schematic of the experimental procedure (P0: birth, P14: eye-opening, P21-P35: long-term MD, P60-P90: MgT/water treatment via gavage, P90: in vivo single-unit recording). B Mg2+-treated mice (n = 10) did not show significant changes in body weight within 1 month (P = 0.1277, Paired t-test). C The concentration of Mg2+ in V1 of Vehicle (5 mice) and Mg group (6 mice, P = 0.0043, Mann-Whitney U test). D Image showing traces of electrode in V1b. E Stimulation-evoked firing rates for contralateral eye in ND, P35MD, MD_Veh, and MD_Mg group (left, ND vs. MD_Veh: P = 0.0018; MD_Veh vs. MD_Mg: P = 0.0224; P35MD vs. MD_Mg: P = 0.0002, one way ANOVA with Tukey’s multiple comparisons test). Stimulation-evoked firing rates for ipsilateral eye in ND, P35MD, MD_Veh, and MD_Mg group (middle, ND vs. P35MD: P = 0.0386, one way ANOVA with Tukey’s multiple comparisons test). The ratio of contralateral eye-evoked response to ipsilateral eye-evoked response in four groups (right, P < 0.0001, one-way ANOVA with Tukey’s multiple comparisons test). F OD distribution for MD_Veh and MD_Mg. G Contralateral bias index (CBI) value for ND (non-deprived, 459 cells, 12 mice), MD_Veh (470 cells,13 mice), MD_Mg (396 cells,13 mice) and P35MD (100 cells, 5 mice) animals (MD_Veh vs. MD_Mg: P < 0.0001; ND vs. MD_Mg: P = 0.9948; MD_Mg vs. P35MD: P < 0.0001, one way ANOVA with Tukey’s multiple comparisons test). Error bars, SEM. Scale bars, 200 μm, and 1 mm.
High Mg2+ Treatment Partially Recovered Binocular Integration in Amblyopic Adult Mice
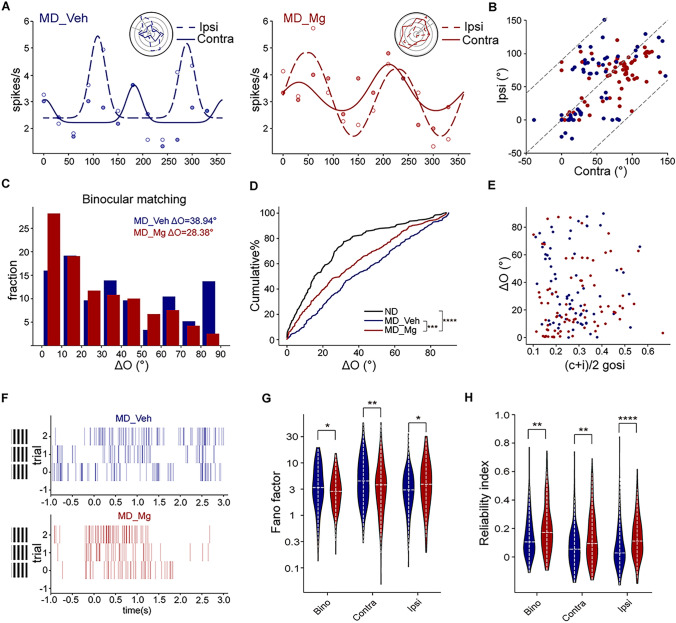
Amblyopia not only affects OD distribution but also disrupts binocular matching of eye-specific orientation preference, which is closely related to binocular integrative function [42]. To answer if high Mg2+ can restore binocular matching in amblyopic mice, we did single-unit recordings in V1b of Veh and Mg2+ treated amblyopic mice at P90 and investigated the specific tuning properties of individual neurons in relation to the visual stimuli of drifting sinusoidal gratings with 12 different orientations. We compared the eye-specific preferred orientations between the two eyes, and their difference (“ΔO”) was used to quantify the degree of binocular matching [21]. We found that the difference in preferred orientation between two eyes in MD_Mg mice was significantly lower than that in MD_Veh mice, showing that the neurons were tuned to similar orientations through the two eyes in MD_Mg mice, but not in MD_Veh mice (Fig. 2A–D). However, the rescue was partial since it was not fully recovered to a normal level, different from the recovery of OD distribution that we showed above. It has previously been demonstrated that an increase in the orientation selectivity of individual neurons may be responsible for the development of binocular matching of orientation preference [21]. To gain a deeper comprehension of the correlation between binocular matching and orientation selectivity, we conducted a comparison between the level of ΔO and the orientation selectivity of individual cells and found no obvious correlation in both groups (Fig. 2E). These results indicated that high Mg2+ partially restored the matching of the two streams of eye-specific inputs in V1 while keeping monocular tuning properties intact.
Fig. 2.
High Mg2+ treatment restored binocular integration in adult MD mice. A Orientation tuning curve of a visually responsive neuron in two groups: MD_Veh (left) and MD_Mg (right) mice. Orientation-tuned responses from the contralateral (solid line) and ipsilateral (dashed line) eyes are shown in representative polar plots (top right). B The correlation of preferred orientation of two eyes in MD_Veh and MD_Mg group. C Distribution of the disparity between the two eyes’ preferred orientations in the MD_Veh and MD_Mg mice. D Cumulative distribution of ΔO for ND (116 cells, 12 mice, ΔO = 17.01 ± 1.581°), MD_Veh (68 cells, 13 mice, ΔO = 36.63 ± 3.70°) and MD_Mg (83 cells, 13 mice, ΔO = 26.12 ± 2.65°). ΔO distribution for MD_Mg was statistically different from MD_Veh (P < 0.001, Kolmogorov-Smirnov (K-S) test). ΔO distribution for MD_Mg was statistically different from ND (P < 0.0001, K-S test). E Scatter plots of ΔO as a function of orientation selectivity for individual cells for MD_Veh and MD_Mg mice. F Schematic diagram of example spiking train of MD_Veh and MD_Mg mice. G Distribution of the V1b responses’ Fano factor in MD_Veh and MD_Mg mice. The significance between MD_Veh and MD_Mg: bino: P = 0.0408; contra: P = 0.0099; ipsi: P = 0.017, Mann-Whitney U-test. H Distribution of the V1b responses’ reliability index in MD_Veh and MD_Mg mice. The significance between MD_Veh and MD_Mg: bino: P = 0.0034; contra: P = 0.0046; ipsi: P < 0.0001, Mann-Whitney U-test.
The firing patterns of a fully mature cortical neuron frequently demonstrate consistency across multiple trials, with minimal variability observed during repeated stimulation. This characteristic is essential for the reliable encoding of large amounts of sensory information in signal processing [43] (Fig. 2F). A previous study has shown that the maturation of binocular variability and reliability was disrupted in amblyopic mice, and this defect could be partially rescued by enhancing cortical plasticity [39]. To test if Mg2+ treatment can show similar effects, we recorded binocular, ipsilateral, and contralateral responses of V1b neurons in MD_Veh and MD_Mg mice at P90. To assess the variability of spike rates and the reliability of spike timing in response to repeated stimulation in the preferred orientation, we employed the Fano factors (FFs) and reliability indexes (RIs) as quantitative measures respectively (see section “Materials and Methods” for details). According to our results, of all cortical neurons that can respond significantly to various types of stimulus input (including binocular, ipsilateral, and contralateral input), binocular and contralateral responses were less variable in MD_Mg mice than in MD_Veh mice, whereas ipsilateral responses were more variable in MD_Mg mice than in MD_Veh mice (Fig. 2G). Meanwhile, binocular, ipsilateral, and contralateral responses in MD_Mg mice exhibited higher reliability compared to MD_Veh mice (Fig. 2H). Thus, our results indicated that binocular variability and reliability can also be reduced by high Mg2+ treatment.
High Mg2+ Treatment Restored Depth Perceptive Ability in Amblyopic Adult Mice
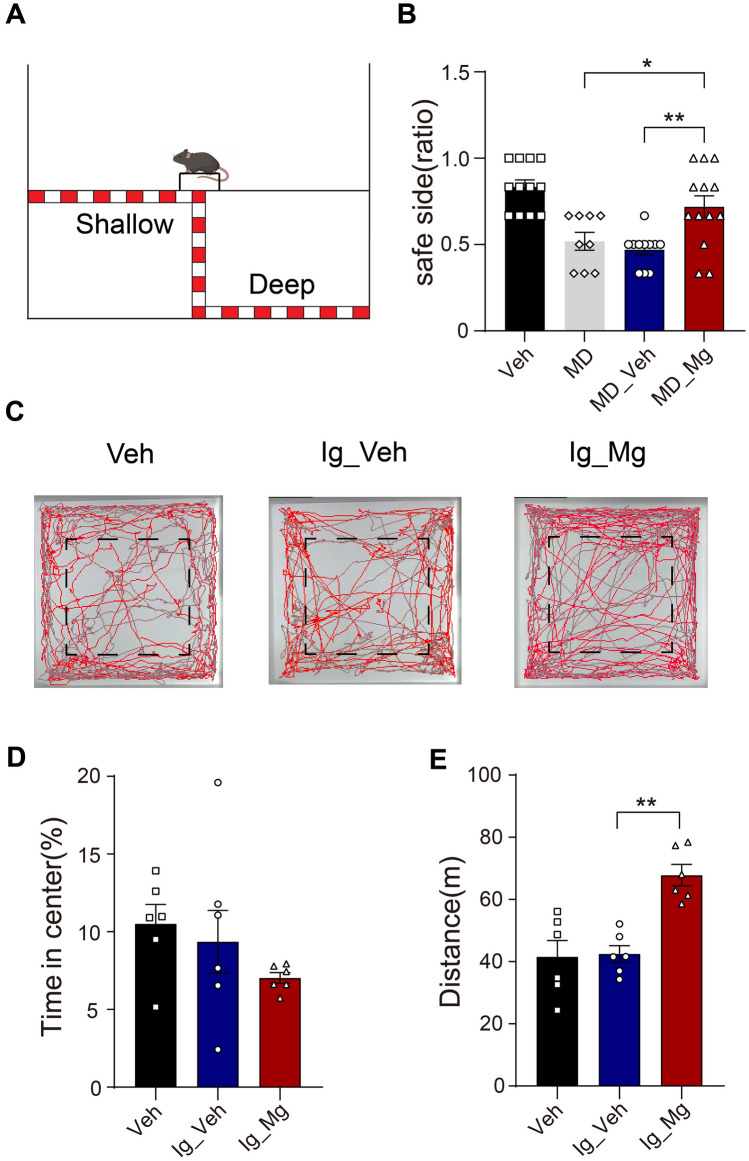
Binocular matching is proposed to be the basis for the formation of depth perception, and MD during the critical period suppresses both binocular matching and depth perception ability [20, 44, 45]. To test if depth perception is also restored together with the recovery of binocular matching after Mg2+ treatment, we applied the well-known paradigm “visual cliff” test, which is used to measure depth perception in both rodents and humans [46, 47] (Fig. 3A). We conducted a comparison of the performance among Veh, MD (mice were performed with 3 days MD at P60 and maintained monocular vision), MD_Veh, and MD_Mg mice. It was observed that Veh and MD_Mg mice consistently exhibited a preference for stepping onto the shallow side of the platform, whereas MD and MD_Veh mice demonstrated an inability to differentiate between the shallow and deep sides (Fig. 3B).
Fig. 3.
Mg2+-treated amblyopic mice displayed better depth perception. A Schematic diagram of the visual cliff tests. B Quantification of depth perception comparing Veh (12 mice), MD (9 mice), MD_Veh (11 mice) and MD_Mg group (13 mice). MD_Veh vs. MD_Mg: P = 0.0043; MD vs. MD_Mg: P = 0.0422, one-way ANOVA with Tukey’s multiple comparisons test. C Automated tracking in the large open field. Representative figures of one mouse in Veh, Ig_Veh, and Ig_Mg group. The black dotted pane is the central region. D No significant difference was observed in the time spent in the central area (Ig_Veh vs. Ig_Mg: P = 0.4737, Veh vs. Ig_Mg: P = 0.2075, one-way ANOVA with Tukey’s multiple comparisons test). E Ig_Mg mice traveled significantly longer than Ig_Veh mice (P = 0.001, one-way ANOVA with Tukey’s multiple comparisons test). Error bars, SEM.
Stress experiences can have an impact on the plasticity of the visual cortex. To detect whether continuous intragastric administration of MgT for 30 days would cause adverse emotions such as prolonged stress in mice, we performed open field tests in Veh (normally fed with water for 30 days), Ig_Veh (intragastric administration of water for 30 days) and Ig_Mg (intragastric administration of MgT for 30 days) groups (Fig. 3C). We calculated the percentage of time spent in the central area of the field, which is used as an index of anxiety. Our results showed that there was no significant difference observed in the percentage of time spent in the central area between Ig_Veh and Ig_Mg mice (Fig. 3D). By comparing the distance that the mice traveled in the open field, we found that Mg2+-treated mice exhibited higher locomotion activity in the open field test compared to other groups (Fig. 3E). Previous research showed that Mg2+ administration could alleviate motor deficits after traumatic brain injury [48]. Thus, we hypothesize that Mg2+ treatment may enhance the motor ability of mice without affecting their emotion. Together, these results demonstrated that high Mg2+ promoted the recovery of depth perception after amblyopia.
Possible Cellular and Molecular Mechanism that Enhanced Plasticity After High Mg2+ Treatment
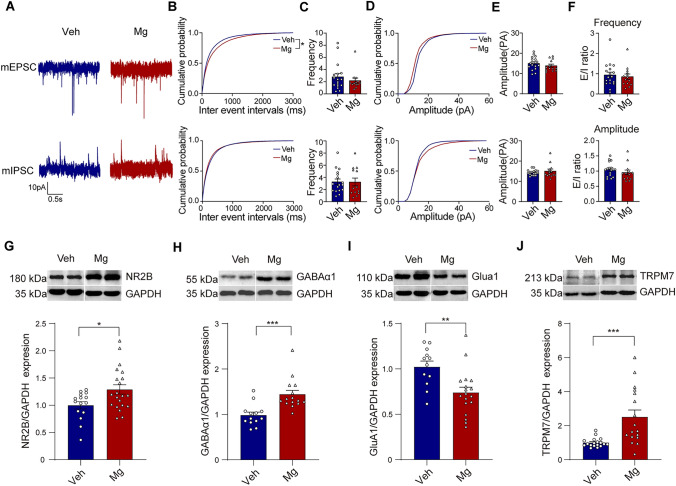
The regulation of the critical period for OD plasticity is thought to be influenced by changes in the balance between excitation and inhibition (E/I balance) [1, 46]. To further explore if E/I balance was changed in V1 after high Mg2+ treatment, whole-cell recording was used to measure the miniature excitatory and inhibitory postsynaptic currents (mEPSCs and mIPSCs, respectively) in V1 layer 2/3/4 pyramidal neurons in acute brain slices of Veh and Mg groups after one month (P60-P90) gavage treatment (Fig. 4A). We observed no significant differences in the frequency and amplitude of mEPSC and mIPSC between the two groups (Fig. 4B–E). By comparing the ratio of mean frequency and amplitude between mEPSC and mIPSC in both groups, we found that Mg2+ treatment had no effect on E/I balance (Fig. 4F). In addition, we classified the neurons recorded in vivo as putative excitatory (broad-spiking) or inhibitory (narrow-spiking) based on the characteristics of their waveforms (Fig. S3A–C, see section “Materials and Methods” for details). Our results revealed that there was no significant difference in the proportion of excitatory or inhibitory neurons between the two groups (Fig. S3D). In addition, we compared the ratio of broad/narrow spiking in Veh and Mg mice and found no significant difference (Fig. S3E, F). These in vitro and in vivo results indicated that E/I balance may not be the major regulator of plasticity after high Mg2+ treatment.
Fig. 4.
Cellular and molecular mechanism of enhanced plasticity with high Mg2+. A Representative traces of mEPSC (top) and mIPSC (bottom) were recorded from layer 2/3/4 pyramidal neurons in V1 of Veh and Mg group. Scale bar, 10 PA and 0.5s. B, C Comparison of mEPSC and mIPSC frequency among Veh (17 cells, 5 mice) and Mg (13 cells, 5 mice) group (P = 0.0337 for mEPSC ISI, P = 0.176 for mIPSC ISI, K-S test; P = 0.1891 for mEPSC frequency, P = 0.5917 for mIPSC frequency, Mann-Whitney test). D, E Comparison of mEPSC and mIPSC amplitude among Veh and Mg group (P = 0.06667 for mEPSC amplitude cumulative probability, P = 0.2667 for mIPSC amplitude cumulative probability, K-S test; P = 0.2812 for mEPSC amplitude, P = 0.4516 for mIPSC amplitude, Mann-Whitney test). F The ratio of mean frequency between mEPSC and mIPSC in both groups of mice (top, P = 0.6666, Mann-Whitney test). The ratio of mean amplitude between mEPSC and mIPSC in both groups of mice (bottom, P = 0.1448, Mann-Whitney test). G Cropped images of immunoblotting for NR2B on protein extracts from P90 V1 of Veh and Mg mice (top). Quantification of NR2B expression in Veh and Mg mice (bottom, P = 0.0166, Unpaired t-test). H Cropped images of immunoblotting for GABAα1 on protein extracts from P90 V1 of Veh and Mg mice (top). Quantification of GABAα1 expression levels in Veh and Mg mice (bottom, P = 0.0004, Unpaired t-test). I Cropped images of immunoblotting for GluA1 on protein extracts from P90 V1 of Veh and Mg mice (top). Quantification of GluA1 expression levels in Veh and Mg mice (bottom, P = 0.0032, Unpaired t-test). J Cropped images of immunoblotting for TRPM7 on protein extracts from P90 V1 of Veh and Mg mice (top). Quantification of TRPM7 expression levels in Veh and Mg mice (bottom, P = 0.0005, Unpaired t-test). Error bars, SEM.
There is empirical evidence indicating a strong association between the NMDA receptor and the plasticity of both excitatory and inhibitory synapses. Furthermore, it is postulated that the NMDA receptor plays a crucial role in the formation of visually evoked responses that are dependent on experience, particularly during the critical period of development [15, 49–53]. NR2A and NR2B are two major NR2 subunits of NMDA receptors in the brain. Notably, NR2B is the predominant subunit in juvenile V1, and its upregulation is related to the heightened plasticity in the adult brain [18]. In line with prior research, we found that the expression of the NR2B subunits in V1b of Mg mice was significantly higher than in Veh mice (Fig. 4G). To detect whether high Mg2+ changed the expression of other excitatory and inhibitory receptors, we examined the expression of GABAα1 and GluA1 in V1 of Veh mice and Mg mice. We found that the expression of GABAα1 in Mg mice was higher than that in Veh, while the expression of GluA1 in Mg mice was lower than that in Veh mice (Fig. 4H, I).
It is not known how high Mg2+ leads to the specific upregulation of NR2B, considering that the system has different ways to maintain the homeostatic balance of ion concentrations in and out of the cell. One possibility is that the Mg2+ flux across the cell membrane can directly initiate a signaling pathway, which requires the participation of several ion modulation factors and kinases. Among more than 300 mammalian ion channels, only TRPM6 and its homolog TRPM7 are coupled with the α-kinase domain, which has been suggested to play a major role in synapse density, synaptic plasticity, and learning and memory [54–57]. Although both genes are selective for divalent cations and highly sensitive to Mg2+, TRPM6 was not investigated in this study because it is mainly expressed in kidney and colon cells [56, 58]. TRPM7 coenzyme is an ion channel that possesses a distinctive structure, characterized by the presence of a serine-threonine-α-kinase domain on its C terminus [59, 60] and is able to transport divalent cations such as zinc (Zn2+), magnesium (Mg2+), and calcium (Ca2+) [61]. Previous studies revealed that brain TRPM7 plays a crucial role in maintaining normal synaptic and cognitive functions under physiological (non-pathological) conditions. TRPM7 knockdown in the hippocampus of adult rats would reduce synaptic density and plasticity and impair learning and memory [57]. Thus, we compared TRPM7 expression in V1 between Mg and Veh mice and found that the expression of TRPM7 in Mg mice was significantly higher than that in Veh mice (Fig. 4J). This result suggests that TRPM7 could be involved in the regulation after high Mg2+ treatment in V1 of amblyopic mice.
TRPM7 is Required for the Restoration of Visual Function After High Mg2+ Treatment
To test if TRPM7 is required for the restoration of plasticity after high Mg2+ treatment, we knocked down TRPM7 in the V1 neurons of Mg2+-treated amblyopic mice. Specifically, we injected TRPM7shRNA (T7shRNA, a small interfering RNA hairpin sequence (shRNA), which packaged in adeno-associated virus (AAV) that included tdTomato fluorescent protein) into V1b of amblyopic mice at P39. Control groups were injected with AAV carrying scrambled shRNA (referred to as CtrlSCR) (Fig. 5A, B). After the injection of CtrlSCR and TRPM7shRNA virus, we supplemented both groups of mice with MgT or water from P60 to P90 as before and performed cliff test, single unit recording, and western blot at P90 (Fig. 5A). We found that TRPM7shRNA transfected V1 exhibited significantly lower TRPM7 expression compared to CtrlSCR transfected V1 (Fig. 5C). Notably, the expression of NR2B in TRPM7shRNA was significantly lower than that in CtrlSCR (Fig. 5D), indicating the upregulation of NR2B is dependent on TRPM7. According to the data of single-unit recordings at P90, the ocular dominance distribution and binocular matching in the T7shRNA were not well restored in comparison to the CtrlSCR group after Mg2+ treatment (Fig. 5E–H). In the visual cliff test, CtrlSCR+Mg mice preferred to step onto the shallow side, whereas T7shRNA+Mg mice failed to distinguish between the shallow and deep sides of the platform (Fig. 5I). Collectively, these findings indicated that TRPM7 plays a key role in the restoration of OD plasticity and binocular integrative functions after high Mg2+ treatment in amblyopic mice.
Fig. 5.
TRPM7 plays a key role in the restoration of binocular visual functions. A Schematic of the experimental procedure. B Image of the virus expression in V1b. C Cropped images of immunoblotting for TRPM7 on protein extracts from P90 V1 of CtrlSCR and T7shRNA mice (up). Quantification of TRPM7 expression levels in CtrlSCR and T7shRNA mice (down, P < 0.0001, Unpaired t-test). D Cropped images of immunoblotting for NR2B on protein extracts from P90 V1 of CtrlSCR and T7shRNA mice (up). Quantification of NR2B expression levels in CtrlSCR and T7shRNA mice (down, P = 0.0002, Unpaired t-test). E OD distribution for CtrlSCR+Mg and T7shRNA+Mg mice. F Contralateral bias index (CBI) value for Veh (470cells, 13 mice), CtrlSCR+Mg (194 cells, 7 mice), T7shRNA (288 cells, 7 mice) and T7shRNA+Mg (334 cells, 6 mice) animals (CtrlSCR+Mg vs. T7shRNA+Mg: P = 0.0262; Veh vs. T7shRNA: P = 0.3414, one way ANOVA with Tukey’s multiple comparisons test). G Distribution of difference in preferred orientations through the two eyes in CtrlSCR+Mg and T7shRNA+Mg mice. H Cumulative distribution of ΔO for Veh (68 cells, ΔO = 36.63 ± 3.70°), CtrlSCR+Mg (64 cells, ΔO = 29.65 ±3.75°), T7shRNA (56 cells, ΔO = 45.9 ± 4.70°) and T7shRNA+Mg (73 cells, ΔO = 39.54 ± 3.12°) mice (CtrlSCR+Mg vs. T7shRNA+Mg: P < 0.0001; Veh vs. T7shRNA: P = 0.4024, K-S test). I Quantification of depth perception comparing CtrlSCR (7 mice) and T7shRNA group (6 mice, P = 0.0055, Unpaired t-test). Error bars, SEM. Scale bar, 1 mm.
Discussion
In this study, using in vivo electrophysiological recordings and visual cliff behavioral tests, we demonstrated the importance of high Mg2+ treatment in the recovery of the binocular integrative function of V1b neurons and the depth perception ability of adult amblyopic mice. In addition, on the molecular mechanism of synaptic plasticity, we found that TRPM7 is required for heightened plasticity by upregulating the expression of NR2B in adult V1.
Binocular Integration May Have a Higher Threshold of Recovery than OD Distribution
A previous study revealed that binocular matching of orientation preference takes place during a similar time window to the formation of OD distribution in early life, which is the critical period for visual development [21]. MD during this period can lead to both a shift of OD towards the non-deprived eye and a binocular mismatch of orientation preference [21]. Our result demonstrated that Mg2+ treatment can restore both the OD distribution and the matching of the two streams of eye-specific inputs in adult amblyopic V1. However, compared to OD distribution, although the binocular matching was recovered, it was not completely returned to normal level. It should be noted that the formation of binocular matching of orientation preference and OD distribution is mechanistically different. OD distribution is accomplished by a competitive process that involves changes in the relative strength of eye-specific inputs [52]. Changes in OD distribution are induced by modulation of the feedforward balance between eye-specific inputs. However, according to the previously proposed models [62–64], binocular matching could be mediated by synaptic changes of both feedforward and feedback connections. Notably, the modulation of changes in synaptic connections is crucially dependent on the synchronized responses from both eyes during normal visual experience, requiring precise and delicate control [21]. Therefore, compared to OD distribution, the formation of binocular matching may be a more refined process and may have a higher threshold of recovery from amblyopia.
The Restoration of OD Plasticity with High Mg2+ Treatment May Not be Caused by Regulating E/I Balance
The modulation of the balance between excitatory and inhibitory inputs is widely recognized as the primary mechanism governing visual plasticity and its reactivation during the developmental process. Resetting the E/I balance to mimic the critical period onset, such as increasing excitation or decreasing inhibition in V1, is thought to be an effective way to restart the critical period in adulthood. However, we found high Mg2+ treatment would not affect the E/I balance in the adult visual cortex (Fig. 4A–F), indicating it may not be the major regulator of plasticity after high Mg2+ treatment. A previous study suggested that the decrease in the neuronal response driven by the deprived eye was usually caused by juvenile-like plasticity after short-term MD, whereas adult plasticity led to an increase in the neuronal response driven by the non-deprived eye [52]. To verify whether the recovered plasticity is juvenile or adult-like, we performed 4d MD from P90 to P94 after normal water or Mg2+ treatment and then assessed CBI value and stimulation-evoked firing rates for two eyes in Veh_ND, Veh_MD, and Mg_MD (Fig. S2A). Our results showed that although there was a significant decrease in CBI value in the Mg_MD group after short-term MD, this was mainly due to an increase in the neuronal response driven by the non-deprived eye (Fig. S2B, C). Thus, we supposed that Mg2+ treatment did not recover the juvenile-like plasticity but facilitated the expression of adult plasticity, which can also promote the recovery of adult amblyopic mice.
In our study, we measured the expression of excitatory or inhibitory neurotransmitter receptors associated with synaptic plasticity in V1, including GluA1, GABAα1, and NR2B, which are involved in OD plasticity and binocular integration [21, 65]. NR2B is the predominant NR2 subunit of NMDA receptors in juvenile V1, which is involved in multiple physiological processes that are essential to visual plasticity [66–68]. During the development, NR2B-domination is gradually switched with NR2A-domination, which is important for closing the critical period [69]. Our result showed that high Mg2+ treatment led to the upregulation of NR2B and GABAα1 and the downregulation of GluA1 (Fig. 4D–F). The upregulation of NR2B is consistent with the previous study showing high Mg2+ can result in enhanced activation of NMDA receptors and facilitation of synaptic potentiation in response to stimulation [70]. However, the downregulation of GluA1 and upregulation of GABAα1 is inconsistent with the notion that reactivating plasticity in adulthood through increasing excitation or decreasing inhibition, suggests other mechanisms are involved in modulating OD plasticity. It is indeed shown that the effects of GluA1 in regulating plasticity are limited to the postnatal critical period but not later in adulthood [71]. Furthermore, there is no significant change in GABAα1 expression in bumetanide-treated mice, whose critical period was delayed [27]. Homeostatic synaptic plasticity interacts with Hebbian forms of synaptic plasticity to allow the brain to work stably and flexibly [72]. LTP induction and synaptic strength increase may be beneficial to the recovery of many functions, such as learning and memory. However, if this process is unconstrained, it can lead to an overexcited state of neuronal synaptic activity in the brain [73, 74]. Therefore, for normal brain function, a dynamic equilibrium response is required to keep neuronal activity within the functional range. It has been demonstrated that there were a number of molecules involved in the homeostatic plasticity of excitatory synapses, including Ca2+-dependent protein phosphatase calcineurin (CN), which can target the GluA1 subunit for dephosphorylation and mediate the expression of Hebbian plasticity [72, 75]. Thus, we propose that the recovery of OD distribution and binocular matching caused by high Mg2+ treatment is mainly due to the activation of NMDA receptors, while the decrease of GluA1 and the increase of GABAα1 could be speculated due to a homeostatic regulation in response to the upregulation of NR2B.
The Role of TRPM7 in Restoring V1 Plasticity
TRPM7 chanzyme is a unique ion channel that can transport divalent cations, including Ca2+, Zn2+, and Mg2+ [59–61]. It is involved in the regulation of many important physiological processes, especially synaptic plasticity and learning and memory [57]. In this study, we examined whether exogenous supplementation of Mg2+ causes changes in TRPM7 expression in V1 and how these changes affect binocular visual function. According to our results, TRPM7 expression was significantly increased after Mg2+ treatment (Fig. 4G). Knocking down the expression of TRPM7 in adult V1 could interfere with the upregulation of NR2B and reverse the recovery of visual function after Mg2+ treatment (Fig. 5).
What is the possible mechanism that is involved in this process? We hypothesize that TRPM7 can modulate plasticity in two ways. First, TRPM7 is involved in the regulation of Mg2+ homeostasis in many types of cells [76–79]. This demonstrates the increase of TRPM7 expression in Mg2+-treated mice. The elevation of TRPM7 in V1 allows more Mg2+ to enter the cells, thereby improving synaptic plasticity. Thus, it can regulate OD plasticity by modulating ion concentration homeostasis. Second, previous studies showed that the activation of p38 MAPK, which is dependent on the NMDA receptor, successfully restored long-term potentiation (LTP) in adolescent mice lacking the Ras-GRF protein [80]. The α and β isoforms of p38 MAPK have been shown to promote the activation of MAP kinase and act on specific transcription factors to induce changes in the expression of the key proteins involved in synaptic plasticity, such as brain-derived neurotrophic factor (BDNF) [81]. Notably, silencing TRPM7 reduced the phosphorylation of signal molecules (P38, ERK, and JNK), ultimately resulting in a significant inactivation of MAPKs [82]. Thus, TRPM7 can modulate OD plasticity by activating downstream signaling pathways to cause changes in the transcription or translation of molecules related to heightened plasticity. TRP is a large family of cation channels, and several subtypes of different forms of TRP (C, M, or V) in neurons have been reported to participate in the processes of regulating neuron survival, synaptic structure, function, and plasticity [83–85]. Nonetheless, both the TRPC and TRPV families are Ca2+-permeable nonselective cation channels, which regulate synaptic functions primarily by mediating Ca2+-influx and then activating downstream pathways [84, 86]. Thus, we speculate that TRPC and TRPV may not play a major role in the increase of synaptic plasticity induced by Mg2+ treatment. It cannot be ruled out that Mg2+ indirectly affects the activity of other channels by affecting the intracellular potential, but this effect may be limited.
Conclusions
Our in vivo results pointed out that Mg2+ treatment facilitates the expression of adult plasticity (Fig. S2). In addition, the results of ex vivo recording suggested that Mg2+ treatment didn’t change the E/I balance in adult V1 (Fig. 4A–F). A previous study, however, found that Mg2+ treatment can restore ocular dominance in adult amblyopic mice by resetting the E/I balance and restoring juvenile-like plasticity [18]. These apparent contradictions might stem from the different protocols used to establish the mouse model of amblyopia with high Mg2+. Mice were given MgT by regular quantitative gavage in our study, whereas the previous study added MgT to the daily drinking water of mice. Thus, even at the same concentration, intragastric administration may cause a transient increase of Mg2+ in mice, while adding MgT to daily drinking water may lead to a stable increase. In addition to this, mice were not fed with MgT during short-term MD (Fig. S2A), while previous studies kept Mg2+ treatment until in vivo electrophysiological recording [18]. It has been demonstrated that changes in Mg2+ concentration affect synaptic density in the hippocampus [16]. By comparing these results, we hypothesized that in our study, Mg2+ concentration in V1 has not been maintained at a consistently high level, which is not sufficient to change the E/I balance, and changes in Mg2+ concentration may induce a different mode of plasticity recovery in visual cortex.
To sum up, we demonstrated that Mg2+ plays an important role in the recovery of binocular integrative function in amblyopia mice and further investigated the molecular mechanism involved in this process. Binocular integrative functions are the foundation of binocular stereo vision and allow us to perceive spatial position, depth, and distance more accurately, which is important for our daily lives and various activities. Thus, it is meaningful to further explore the mechanism involved in binocular integration, which may be helpful in the treatment of amblyopia. A previous study has shown that environmental enrichment in adulthood can fully restore disrupted binocular matching by reactivating V1 plasticity after MD [22], similar to what we observed with high Mg2+ treatment. The investigation on TRPM7 may result in the findings of a novel clinical therapeutic target for amblyopia. We expect to see more effective methods to restore binocular integration to be proposed in the future, thereby facilitating the treatment of amblyopia to normal visual function in adults.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We gratefully acknowledge Nashat Abumaria (Fudan University) for providing the TRPM7 antibody, CtrlSCR, and T7shRNA virus. This work is supported by the National Natural Science Foundation of China (31872764 and 82171090), Shanghai Science and Technology Committee Rising-Star Program (19QA1401600), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), ZJLab, Shanghai Center for Brain Science and Brain-Inspired Technology.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Hensch TK, Quinlan EM. Critical periods in amblyopia. Vis Neurosci 2018, 35: E014. 10.1017/S0952523817000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maconachie GD, Gottlob I. The challenges of amblyopia treatment. Biomed J 2015, 38: 510–516. 10.1016/j.bj.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res 2013, 33: 67–84. 10.1016/j.preteyeres.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005, 6: 877–888. 10.1038/nrn1787 [DOI] [PubMed] [Google Scholar]
- 5.Cisneros-Franco JM, Voss P, Thomas ME, de Villers-Sidani E. Critical periods of brain development. Handb Clin Neurol 2020, 173: 75–88. 10.1016/B978-0-444-64150-2.00009-5 [DOI] [PubMed] [Google Scholar]
- 6.Adams GGW, Sloper JJ. Update on squint and amblyopia. J R Soc Med 2003, 96: 3–6. 10.1177/014107680309600102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 1970, 206: 419–436. 10.1113/jphysiol.1970.sp009022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morishita H, Hensch TK. Critical period revisited: Impact on vision. Curr Opin Neurobiol 2008, 18: 101–107. 10.1016/j.conb.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 9.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 1996, 16: 3274–3286. 10.1523/JNEUROSCI.16-10-03274.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science 2010, 327: 1145–1148. 10.1126/science.1183962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamanaka R, Shindo Y, Oka K. Magnesium is a key player in neuronal maturation and neuropathology. Int J Mol Sci 2019, 20: 3439. 10.3390/ijms20143439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altura BM, Altura BT. Role of magnesium in patho-physiological processes and the clinical utility of magnesium ion selective electrodes. Scand J Clin Lab Invest Suppl 1996, 224: 211–234. 10.3109/00365519609088642 [DOI] [PubMed] [Google Scholar]
- 13.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984, 309: 261–263. 10.1038/309261a0 [DOI] [PubMed] [Google Scholar]
- 14.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307: 462–465. 10.1038/307462a0 [DOI] [PubMed] [Google Scholar]
- 15.Slutsky I, Sadeghpour S, Li B, Liu G. Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. Neuron 2004, 44: 835–849. 10.1016/j.neuron.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 16.Slutsky I, Abumaria N, Wu LJ, Huang C, Zhang L, Li B. Enhancement of learning and memory by elevating brain magnesium. Neuron 2010, 65: 165–177. 10.1016/j.neuron.2009.12.026 [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Huang Z, Zhang J, Chen JL, Yao PW, Mai CL, et al. Chronic oral administration of magnesium-L-threonate prevents oxaliplatin-induced memory and emotional deficits by normalization of TNF-α/NF-κB signaling in rats. Neurosci Bull 2021, 37: 55–69. 10.1007/s12264-020-00563-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Li Y, Wang Y, Wang X, An X, Wang S, et al. The distinct role of NR2B subunit in the enhancement of visual plasticity in adulthood. Mol Brain 2015, 8: 49. 10.1186/s13041-015-0141-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cang J, Kalatsky VA, Löwel S, Stryker MP. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vis Neurosci 2005, 22: 685–691. 10.1017/S0952523805225178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baroncelli L, Braschi C, Maffei L. Visual depth perception in normal and deprived rats: Effects of environmental enrichment. Neuroscience 2013, 236: 313–319. 10.1016/j.neuroscience.2013.01.036 [DOI] [PubMed] [Google Scholar]
- 21.Wang BS, Sarnaik R, Cang J. Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 2010, 65: 246–256. 10.1016/j.neuron.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine JN, Chen H, Gu Y, Cang J. Environmental enrichment rescues binocular matching of orientation preference in the mouse visual cortex. J Neurosci 2017, 37: 5822–5833. 10.1523/JNEUROSCI.3534-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katano T, Takao K, Abe M, Yamazaki M, Watanabe M, Miyakawa T, et al. Distribution of Caskin1 protein and phenotypic characterization of its knockout mice using a comprehensive behavioral test battery. Mol Brain 2018, 11: 63. 10.1186/s13041-018-0407-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felgerolle C, Hébert B, Ardourel M, Meyer-Dilhet G, Menuet A, Pinto-Morais K, et al. Visual behavior impairments as an aberrant sensory processing in the mouse model of fragile X syndrome. Front Behav Neurosci 2019, 13: 228. 10.3389/fnbeh.2019.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang BS, Bernardez Sarria MS, An X, He M, Alam NM, Prusky GT, et al. Retinal and callosal activity-dependent chandelier cell elimination shapes binocularity in primary visual cortex. Neuron 2021, 109: 502-515.e7. 10.1016/j.neuron.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han KS, Cooke SF, Xu W. Experience-dependent equilibration of AMPAR-mediated synaptic transmission during the critical period. Cell Rep 2017, 18: 892–904. 10.1016/j.celrep.2016.12.084 [DOI] [PubMed] [Google Scholar]
- 27.Chan J, Hao X, Liu Q, Cang J, Gu Y. Closing the critical period is required for the maturation of binocular integration in mouse primary visual cortex. Front Cell Neurosci 2021, 15: 749265. 10.3389/fncel.2021.749265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: Diversity and laminar dependence. J Neurosci 2002, 22: 5639–5651. 10.1523/JNEUROSCI.22-13-05639.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazziotti R, Baroncelli L, Ceglia N, Chelini G, Sala GD, Magnan C, et al. MiR-132/212 is required for maturation of binocular matching of orientation preference and depth perception. Nat Commun 2017, 8: 15488. 10.1038/ncomms15488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol 1962, 160: 106–154. 10.1113/jphysiol.1962.sp006837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 2004, 92: 600–608. 10.1152/jn.01170.2003 [DOI] [PubMed] [Google Scholar]
- 32.Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci 2002, 22: 10966–10975. 10.1523/JNEUROSCI.22-24-10966.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 2007, 55: 131–141. 10.1016/j.neuron.2007.06.018 [DOI] [PubMed] [Google Scholar]
- 34.Gur M, Snodderly DM. High response reliability of neurons in primary visual cortex (V1) of alert, trained monkeys. Cereb Cortex 2006, 16: 888–895. 10.1093/cercor/bhj032 [DOI] [PubMed] [Google Scholar]
- 35.Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 2009, 12: 1594–1600. 10.1038/nn.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghanbari A, Lee CM, Read HL, Stevenson IH. Modeling stimulus-dependent variability improves decoding of population neural responses. J Neural Eng 2019, 16: 066018. 10.1088/1741-2552/ab3a68 [DOI] [PubMed] [Google Scholar]
- 37.Litwin-Kumar A, Doiron B. Slow dynamics and high variability in balanced cortical networks with clustered connections. Nat Neurosci 2012, 15: 1498–1505. 10.1038/nn.3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rikhye RV, Sur M. Spatial correlations in natural scenes modulate response reliability in mouse visual cortex. J Neurosci 2015, 35: 14661–14680. 10.1523/JNEUROSCI.1660-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao X, Liu Q, Chan J, Li N, Shi X, Gu Y. Binocular visual experience drives the maturation of response variability and reliability in the visual cortex. iScience 2022, 25: 104984. 10.1016/j.isci.2022.104984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wandell BA, Smirnakis SM. Plasticity and stability of visual field maps in adult primary visual cortex. Nat Rev Neurosci 2009, 10: 873–884. 10.1038/nrn2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron 2012, 75: 230–249. 10.1016/j.neuron.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: Structure, suppression and plasticity. Ophthalmic Physiol Opt 2014, 34: 146–162. 10.1111/opo.12123 [DOI] [PubMed] [Google Scholar]
- 43.Shadlen MN, Newsome WT. The variable discharge of cortical neurons: Implications for connectivity, computation, and information coding. J Neurosci 1998, 18: 3870–3896. 10.1523/JNEUROSCI.18-10-03870.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker AJ. Binocular depth perception and the cerebral cortex. Nat Rev Neurosci 2007, 8: 379–391. 10.1038/nrn2131 [DOI] [PubMed] [Google Scholar]
- 45.Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, et al. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A 2003, 100: 12486–12491. 10.1073/pnas.1934836100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox MW. The visual cliff test for the study of visual depth perception in the mouse. Anim Behav 1965, 13: 232–233. 10.1016/0003-3472(65)90040-0 [DOI] [PubMed] [Google Scholar]
- 47.Gibson EJ, Walk RD. The “visual cliff.” Sci Am 1960, 202: 64–71. 10.1038/scientificamerican0460-64 [DOI] [PubMed] [Google Scholar]
- 48.Young JM, Hoane MR. Magnesium administration after experimental traumatic brain injury improves decision-making skills. Brain Res Bull 2018, 139: 182–189. 10.1016/j.brainresbull.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 49.Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci 1999, 2: 352–357. 10.1038/7263 [DOI] [PubMed] [Google Scholar]
- 50.Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron 2003, 38: 977–985. 10.1016/S0896-6273(03)00323-4 [DOI] [PubMed] [Google Scholar]
- 51.Daw NW, Gordon B, Fox KD, Flavin HJ, Kirsch JD, Beaver CJ, et al. Injection of MK-801 affects ocular dominance shifts more than visual activity. J Neurophysiol 1999, 81: 204–215. 10.1152/jn.1999.81.1.204 [DOI] [PubMed] [Google Scholar]
- 52.Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci 2008, 28: 10278–10286. 10.1523/JNEUROSCI.2451-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanold PO, Kim YA, GrandPre T, Shatz CJ. Co-regulation of ocular dominance plasticity and NMDA receptor subunit expression in glutamic acid decarboxylase-65 knock-out mice. J Physiol 2009, 587: 2857–2867. 10.1113/jphysiol.2009.171215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan J, Li Z, Li J, Hulse RE, Santa-Cruz A, Valinsky WC, et al. Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc Natl Acad Sci USA 2018, 115: E8201–E8210. 10.1073/pnas.1810719115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell 2001, 7: 1047–1057. 10.1016/S1097-2765(01)00256-8 [DOI] [PubMed] [Google Scholar]
- 56.Ferioli S, Zierler S, Zaißerer J, Schredelseker J, Gudermann T, Chubanov V. TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg2+ and Mg·ATP. Sci Rep 2017, 7: 8806. 10.1038/s41598-017-08144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Chen C, Liu Y, Li W, Wang Z, Sun Q, et al. TRPM7 is required for normal synapse density, learning, and memory at different developmental stages. Cell Rep 2018, 23: 3480–3491. 10.1016/j.celrep.2018.05.069 [DOI] [PubMed] [Google Scholar]
- 58.Chubanov V, Gudermann T. TRPM6. Handb Exp Pharmacol 2014, 222: 503–520. 10.1007/978-3-642-54215-2_20 [DOI] [PubMed] [Google Scholar]
- 59.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411: 590–595. 10.1038/35079092 [DOI] [PubMed] [Google Scholar]
- 60.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291: 1043–1047. 10.1126/science.1058519 [DOI] [PubMed] [Google Scholar]
- 61.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 2003, 121: 49–60. 10.1085/jgp.20028740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adorján P, Levitt JB, Lund JS, Obermayer K. A model for the intracortical origin of orientation preference and tuning in macaque striate cortex. Vis Neurosci 1999, 16: 303–318. 10.1017/S0952523899162114 [DOI] [PubMed] [Google Scholar]
- 63.Ben-Yishai R, Hansel D, Sompolinsky H. Traveling waves and the processing of weakly tuned inputs in a cortical network module. J Comput Neurosci 1997, 4: 57–77. 10.1023/A:1008816611284 [DOI] [PubMed] [Google Scholar]
- 64.Somers DC, Nelson SB, Sur M. An emergent model of orientation selectivity in cat visual cortical simple cells. J Neurosci 1995, 15: 5448–5465. 10.1523/JNEUROSCI.15-08-05448.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su LD, Shen Y. PQBP1: A new player in metabotropic glutamate receptor signaling and synaptic plasticity. Neurosci Bull 2021, 37: 1637–1638. 10.1007/s12264-021-00720-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A 2011, 108: 5855–5860. 10.1073/pnas.1012676108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, et al. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 2003, 40: 775–784. 10.1016/S0896-6273(03)00645-7 [DOI] [PubMed] [Google Scholar]
- 68.Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron 2005, 46: 745–760. 10.1016/j.neuron.2005.04.031 [DOI] [PubMed] [Google Scholar]
- 69.Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J Neurosci 2003, 23: 5208–5218. 10.1523/JNEUROSCI.23-12-05208.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, et al. Genetic enhancement of learning and memory in mice. Nature 1999, 401: 63–69. 10.1038/43432 [DOI] [PubMed] [Google Scholar]
- 71.Ranson A, Sengpiel F, Fox K. The role of GluA1 in ocular dominance plasticity in the mouse visual cortex. J Neurosci 2013, 33: 15220–15225. 10.1523/JNEUROSCI.2078-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandes D, Carvalho AL. Mechanisms of homeostatic plasticity in the excitatory synapse. J Neurochem 2016, 139: 973–996. 10.1111/jnc.13687 [DOI] [PubMed] [Google Scholar]
- 73.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol 2000, 10: 358–364. 10.1016/S0959-4388(00)00091-X [DOI] [PubMed] [Google Scholar]
- 74.Turrigiano GG. The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell 2008, 135: 422–435. 10.1016/j.cell.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 2007, 23: 613–643. 10.1146/annurev.cellbio.23.090506.123516 [DOI] [PubMed] [Google Scholar]
- 76.Flannagan RS, Jaumouillé V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol 2012, 7: 61–98. 10.1146/annurev-pathol-011811-132445 [DOI] [PubMed] [Google Scholar]
- 77.Dati LM, Ulrich H, Real CC, Feng ZP, Sun HS, Britto LR. Carvacrol promotes neuroprotection in the mouse hemiparkinsonian model. Neuroscience 2017, 356: 176–181. 10.1016/j.neuroscience.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 78.Kim Y, Oh HG, Cho YY, Kwon OH, Park MK, Chung S. Stress hormone potentiates Zn(2+)-induced neurotoxicity via TRPM7 channel in dopaminergic neuron. Biochem Biophys Res Commun 2016, 470: 362–367. 10.1016/j.bbrc.2016.01.041 [DOI] [PubMed] [Google Scholar]
- 79.Low SE, Amburgey K, Horstick E, Linsley J, Sprague SM, Cui WW, et al. TRPM7 is required within zebrafish sensory neurons for the activation of touch-evoked escape behaviors. J Neurosci 2011, 31: 11633–11644. 10.1523/JNEUROSCI.4950-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan J, Gladding CM, Wang L, Zhang LY, Kaufman AM, Milnerwood AJ, et al. P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease. Neurobiol Dis 2012, 45: 999–1009. 10.1016/j.nbd.2011.12.019 [DOI] [PubMed] [Google Scholar]
- 81.Falcicchia C, Tozzi F, Arancio O, Watterson DM, Origlia N. Involvement of p38 MAPK in synaptic function and dysfunction. Int J Mol Sci 2020, 21: 5624. 10.3390/ijms21165624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng X, Cai C, Wu J, Cai S, Ye C, Chen H, et al. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett 2013, 333: 96–102. 10.1016/j.canlet.2013.01.031 [DOI] [PubMed] [Google Scholar]
- 83.Ramírez-Barrantes R, Cordova C, Poblete H, Muñoz P, Marchant I, Wianny F, et al. Perspectives of TRPV1 function on the neurogenesis and neural plasticity. Neural Plast 2016, 2016: 1568145. 10.1155/2016/1568145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwarz Y, Oleinikov K, Schindeldecker B, Wyatt A, Weißgerber P, Flockerzi V, et al. TRPC channels regulate Ca2+-signaling and short-term plasticity of fast glutamatergic synapses. PLoS Biol 2019, 17: e3000445. 10.1371/journal.pbio.3000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tai Y, Jia Y. TRPC channels and neuron development, plasticity, and activities. Adv Exp Med Biol 2017, 976: 95–110. 10.1007/978-94-024-1088-4_9 [DOI] [PubMed] [Google Scholar]
- 86.Kärki T, Tojkander S. TRPV protein family-from mechanosensing to cancer invasion. Biomolecules 2021, 11: 1019. 10.3390/biom11071019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.