Abstract
Background and Objectives
Nintedanib, a tyrosine kinase inhibitor, is integral in slowing pulmonary fibrosis progression in chronic fibrotic interstitial lung disease (ILD). However, the occurrence of adverse drug reactions (ADRs) often limits its use, leading to treatment discontinuation, typically within 3–12 months. Discontinuation adversely affects patient outcomes. The study investigated whether aggressive ADR management can prolong nintedanib therapy and improve patient outcomes.
Methods
This retrospective, single-center study enrolled Taiwanese patients with chronic fibrotic ILD who were treated with nintedanib from January 2016 to December 2022 in Kaohsiung Chang Gung Memorial Hospital. Patients were categorized into those who discontinued treatment within 180 days and those continuing beyond. Management of ADRs was identified through concurrent prescriptions for symptoms such as nausea, vomiting, diarrhea, or hepatic dysfunction. Baseline demographics, comorbidities, pulmonary function tests, and instances of acute exacerbation were analyzed.
Results
The study enrolled 94 patients, with 71 (75.5%) experiencing ADRs. Among these, 41 (43.6%) discontinued nintedanib within 180 days. The administration of medications for managing nausea/vomiting [17 (41.5%) versus 36 (67.9%), p = 0.0103] and diarrhea [12 (29.3%) versus 33 (62.3%), p = 0.0015] was less frequent in the discontinued group compared with the continued group. Additionally, a higher incidence of acute exacerbation was observed in the discontinued group (34.1% versus 20.8%, p = 0.016).
Conclusion
Aggressive management of ADRs may enhance patient tolerance to nintedanib, potentially prolonging treatment duration and improving outcomes in chronic fibrotic ILD.
Key Points
| 1. Higher incidence rate of ADRs was noted in the discontinued group. |
| 2. Medications for ADRs were significantly less frequently prescribed in the discontinued group. |
| 3. Aggressive management of ADRs may help patients tolerate nintedanib prolonging the duration of management and improving outcomes in chronic fibrotic ILD. |
Introduction
Interstitial lung diseases (ILDs) encompass a diverse group of diffuse parenchymal lung disorders that share similar clinical presentations and lung injury patterns. Idiopathic pulmonary fibrosis (IPF) is the predominant chronic fibrotic ILD and is a progressive disease characterized by deteriorating lung function and early mortality [1, 2]. Nintedanib is a tyrosine kinase inhibitor that targets key pathways involved in fibrotic ILDs and is approved for treating IPF [3–6], systemic sclerosis-associated ILD (SSc-ILD) [7], and progressive fibrosing interstitial lung disease (PF-ILD) [8]. Nintedanib has been shown to slow disease progression in IPF, PF-ILD, and SSc-ILD, potentially reducing the risks of lung function decline, acute exacerbations, and respiratory hospitalizations.
Adverse reactions (ADRs) to nintedanib usually involve the gastrointestinal system (e.g., nausea, vomiting, and diarrhea) or liver. Dose adjustments, temporary interruptions, and discontinuation of treatment can be necessary to eliminate these ADRs [3–8]. In the INPULSIS-ON study, nintedanib exhibited an acceptable safety and tolerability profile even over a long period (63 months), although safety concerns led to treatment discontinuation in approximately 10% of the included patients [9]. In a study involving patients with SSc-ILD, patients receiving nintedanib reported a higher incidence of common ADRs [8] than did those not receiving nintedanib.
The prescribing information for nintedanib includes recommendations for the management and monitoring of ADRs, which may include symptom treatment, dose adjustment, or discontinuation of treatment [10]. Given the limited availability of effective ILD treatments, discontinuing nintedanib could lead to rapid disease progression and reduced quality of life. Therefore, aggressively managing ADRs is warranted to prolong the duration of nintedanib use. However, this assumption lacks evidence and requires further investigation.
Methods
Study Design, Population, and Group Classifications
This study is a retrospective analysis conducted in a single center- Kaohsiung Chang Gung Memorial Hospital. Taiwanese patients aged ≥ 18 years with chronic fibrotic ILD who underwent nintedanib treatment between January 2016 and December 2022 were included. Diagnoses were reassessed in multidisciplinary discussions, and patients who were deemed to in fact not have chronic fibrotic ILD were excluded. Patients were excluded if they had a diagnosis of liver cirrhosis, chronic hepatitis, liver function impairment, or an active gastrointestinal tract disorder before receiving nintedanib or if they received any other antifibrotic agents.
Demographic characteristics, body surface area (BSA), body mass index (BMI), smoking history, comorbidities, fibrosis severity, results of pulmonary function tests, high-resolution computed tomography (HRCT) patterns, and instances of acute exacerbation for each patient were obtained from electronic medical records. Acute exacerbations were defined based on the consensus [11, 12].
Information regarding the prescription, interruption, or permanent discontinuation of nintedanib and the management of ADRs, such as the use of domperidone, mosapride, loperamide, silymarin, or ursodeoxycholic acid, was obtained from the database of hospital pharmacy attached to the medical center. Patients were divided into discontinued and continued groups depending on whether they discontinued nintedanib within 180 days.
Ethical approval for this study was obtained from the Institutional Review Board of Chang Gung Medical Foundation (IRB no.: 202201260B0 and 202201284B0). The study was performed in accordance with the standards of ethics outlined in the Declaration of Helsinki.
Statistical Analysis
Data are expressed as mean ± standard deviation unless otherwise stated. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test, as appropriate. Continuous variables were compared using Student’s t-test or the Mann–Whitney U-test. The multiple logistic regression analysis with stepwise model selection was used to identify potential risk factors associated with nintedanib discontinuation. The regression model included the following variables: age in 10-year increments, gender, BSA (< 1.58 or ≥ 1.58 m2), GAP stage, smoking history, underlying fibrotic ILD (IPF or SSc-ILD), advanced ILD, chronic respiratory disease, and the requirement for additional medication to manage ADRs (nausea/vomiting and diarrhea). Stepwise regression was performed and covariates with a 0.1 significance level entered the model, while a 0.05 significant level was required to stay in the model.. Data analysis was performed using IBM SPSS software version 21.0. A two-sided p value of < 0.05 was considered statistically significant.
Results
In this study, 97 patients undergoing nintedanib therapy were identified from the database of hospital pharmacy. Following multidisciplinary discussions, three patients were excluded, leaving a total of 94 patients, comprising 78 with IPF and 16 with SSC-ILD (Fig. 1).
Fig. 1.
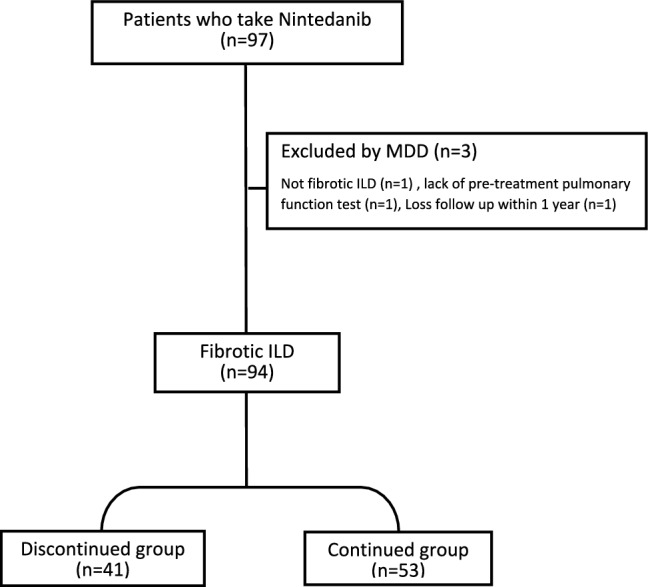
Study flow chart. MMD Multidisciplinary discussions, ILD Interstitial lung disease
Patient baseline characteristics are presented in Table 1. The mean age was 70.7 years, with 34 (36.2%) of the patients being older than 75 years. Most patients (n = 67, 71.3%) were men, and 57 (60.6%) patients had a history of smoking. The most common radiological finding on HRCT was a usual interstitial pneumonia pattern (88.3%). Patients were categorized into gender, age, and physiology (GAP) stages, with 27 (28.7%), 45 (47.9%), and 22 (23.4%) patients being categorized into stages I, II, and III, respectively. Advanced ILD [defined as diffusing capacity of the lung for carbon monoxide (DLco) ≤ 35% or forced vital capacity (FVC) ≤ 50%] was present in 22 (23.4%) patients, and 39 (41.5%) patients had chronic respiratory disease. Inhaled medications, including long-acting bronchodilators (LABA), long-acting muscarinic antagonists (LAMA), and inhaled corticosteroids, were prescribed for 67 (71.3%) patients, and 46(48.9%) patients undergoing oral corticosteroid therapy at baseline. Nintedanib was initiated at a low starting dose in 5 (5.3%) patients. Acute exacerbation within 12 months occurred in 25 (26.6%) patients.
Table 1.
Basic characteristics of the study population.
| Characteristics | All (n = 94) | Discontinued group (n = 41) | Continued group (n = 53) | p-value |
|---|---|---|---|---|
| Age, years | 70.7 ± 9.9 | 72.3 ± 9.9 | 69.5 ± 9.9 | 0.1759 |
| < 75 years | 60 (63.9) | 25 (61.0) | 35 (66.0) | 0.6125 |
| ≥ 75 years | 34 (36.2) | 16 (39.0) | 18 (34.0) | |
| Sex | ||||
| Female | 27 (28.7) | 15 (36.6) | 12 (22.6) | 0.1384 |
| Male | 67 (71.3) | 26 (63.4) | 41 (77.4) | |
| BMI (kg/m2) | 23.1 ± 3.9 | 23.0 ± 3.1 | 23.1 ± 4.4 | 0.8988 |
| BSA (m2) | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 0.0852 |
| < 1.58 | 66 (70.2) | 22 (53.7) | 44 (83.0) | 0.0842 |
| ≥ 1.58 | 28 (29.8) | 19 (46.3) | 9 (17.0) | |
| Smoking history | ||||
| Never | 38 (40.4) | 17 (41.5) | 31 (58.5) | 0.1015 |
| Current/former | 56 (59.65) | 24 (58.54 | 22 (41.5) | |
| Fibrotic ILD | ||||
| IPF | 78 (83.0) | 33 (80.5) | 45 (84.9) | 0.5719 |
| SSC-ILD | 16 (17.0) | 8 (19.5) | 8 (15.1) | |
| HRCT pattern | ||||
| UIP | 83 (88.3) | 34 (82.9) | 49 (92.5) | 0.1542 |
| Others | 11 (11.7) | 7 (17.1) | 4 (7.5) | |
| GAP index | 0.0408 | |||
| Stage I (0–3) | 27 (28.7) | 12 (29.3) | 13 (24.5) | |
| Stage II (4–5) | 45 (47.9) | 15 (36.5) | 32 (60.4) | |
| Stage III (6–8) | 22 (23.4) | 14 (34.2) | 8 (15.1) | |
| Advanced ILDa | 22 (23.4) | 7 (17.1) | 15 (28.3) | 0.2023 |
| Chronic respiratory diseaseb | 39 (41.5) | 14 (34.2) | 25 (47.2) | 0.2038 |
| Inhaled medicine | 67 (71.3) | 29 (70.7) | 38 (71.7) | 0.3642 |
| LAMA alone | 6 (6.4) | 4 (9.8) | 2 (3.8) | |
| LABA + LAMA | 38 (40.4) | 14 (34.2) | 24 (45.3) | |
| LABA + ICS | 12 (12.8) | 4 (9.8) | 8 (15.1) | |
| LABA + LAMA + ICS | 11 (11.7) | 7 (17.1) | 4 (7.6) | |
| Oral corticosteroidc | 51 (54.3) | 19 (46.3) | 32 (60.4) | 0.1942 |
| Starting dose of nintedanib, n (%) | ||||
| 300 mg/day | 89 (94.7) | 39 (95.1) | 50 (94.3) | 0.8669 |
| < 300 mg/day | 5 (5.3) | 2 (4.9) | 3 (5.7) | |
| Acute exacerbation during follow-up, n (%) | 25 (26.6) | 14 (34.1) | 11 (20.8) | 0.0160 |
Values are displayed as number (%), mean ± standard deviation
BMI body mass index, BSA body surface area, DLco diffusion capacity of carbon monoxide, FVC forced vital capacity, GAP gender–age–physiology, ICS inhaled corticosteroid, ILD interstitial lung disease, LABA long-acting β2 agonist, LAMA long-acting muscarinic antagonist
aDLco pred ≤ 35% or FVC pred ≤ 50%
bChronic obstructive pulmonary disease or asthma
cBaseline using oral steroid before nintedanib management
Baseline characteristics between the discontinued and continued groups displayed no significant differences, except in the incidence of acute exacerbations (34.1% versus 20.8%, p = 0.0160) and GAP index (p = 0.0408; Table 1). FVC (% of predicted value) and DLco (% of predicted value) remained stable within the first 180 days and exhibited no significant differences between the two groups (Table 2).
Table 2.
Pulmonary function test at baseline, 24 weeks and difference between 0 and 24 weeks
| All (n = 94) | Discontinued group (n = 41) | Continued group (n = 53) | p-value | |
|---|---|---|---|---|
| FVC, % of predicted value, baseline | 69.2 ± 19.9 | 69.3 ± 17.3 | 69.2 ± (15.1) | 0.9893 |
| Category, n (%) | ||||
| < 70% | 46 (48.9) | 22 (53.7) | 25 (47.2) | 0.5327 |
| ≥ 70% | 48 (51.1) | 19 (46.3) | 28 (52.8) | |
| < 50% | 9 (9.6) | 5 (12.2) | 4 (7.5) | 0.4476 |
| ≥ 50% | 85 (90.4) | 36 (87.8) | 49 (92.5) | |
| < 50% | 9 (9.6) | 5 (12.2) | 4 (7.6) | 0.4725 |
| ≥ 50% and < 80% | 64 (68.1) | 29 (70.7) | 35 (66.0) | |
| ≥ 80% | 21 (22.3) | 7 (17.1) | 14 (26.4) | |
| FVC, % of predicted value, at 24 weeks | 66.7 ± 16.3 | 65.4 ± 14.9 | 67.5 ± 17.0 | 0.6539 |
| Change FVC between 0 and 24 weeks | − 1.7 ± 8.6 | − 1.3 ± 1.5 | − 0.1 ± 1.0 | 0.5076 |
| FEV1,% of predicted value, at baseline | 76.1 ± 17.1 | 78.3 ± 18.0 | 74.5 ± 16.3 | 0.2900 |
| FEV1,% of predicted value, at 24 weeks | 73.4 ± 17.8 | 74.5 ± 16.7 | 73.0 ± 18.4 | 0.7824 |
| DLco, % of predicted value, at baseline | 48.0 ± 15.5 | 51.3 ± 14.4 | 46.0 ± 15.9 | 0.1711 |
| DLco, % of predicted value, at 24 weeks | 44.3 ± 15.9 | 42.0 ± 32.1 | 45.1 ± 39.0 | 0.5866 |
| Change DLco between 0 and 24 weeks | − 0.7 ± 9.2 | − 4.0 ± 6.4 | 0.47 ± 9.8 | 0.1724 |
Values are displayed as number (%), mean ± standard deviation
DLco diffusion capacity of carbon monoxide, FEV1 forced expiratory volume in one second, FVC forced vital capacity
ADRs were experienced by 71 (75.5%) patients, with a higher incidence in the discontinued group (82.9% versus 69.8%, p = 0.0132; Table 3). Five patients in the discontinued group died, with their deaths suspected to be due to ADR-related sepsis. ADR-related dose reductions occurred in 14 (14.9%) patients, and 24 (25.5%) needed to discontinue nintedanib. Most discontinuations (23.4%) were temporary, but two patients in the discontinued group permanently stopped treatment. Medical therapy for ADRs, such as nausea/vomiting, diarrhea, and hepatitis, was prescribed in both groups. Medications specifically for nausea/vomiting, and diarrhea were significantly less frequently prescribed in the discontinued group compared with the continued group. The figures were 17 (41.5%) and 12 (29.3%) in the discontinued group versus 36 (67.9%) and 33 (62.3%) in the continued group for nausea/vomiting and diarrhea, respectively (p = 0.0103 and 0.0015, respectively; Table 3).
Table 3.
Adverse drug reactions and the medication for ADRs in the study groups
| All (n = 94) | Discontinued group (n = 41) | Continued group (n = 53) | p-value | |
|---|---|---|---|---|
| Any ADRs | 71 (75.5) | 34 (82.9) | 37 (69.8) | 0.0132 |
| ADRs leading to: | ||||
| Death | 5 (5.3) | 5 (12.2) | 0 | < 0.0001 |
| Lost follow-up | 8 (8.5) | 4 (9.8) | 4 (7.6) | 0.6715 |
| Dose reduction | 14 (14.9) | 5 (12.2) | 9 (17.0) | 0.6864 |
| Discontinuation | 24 (25.5) | 17 (41.5) | 7 (13.2) | < 0.0001 |
| Temporary | 22 (23.4) | 15 (36.6) | 7 (13.2) | < 0.0001 |
| Permanent | 2 (2.1) | 2 (4.9) | 0 (0) | 0.0453 |
| Medication for ADRs: | ||||
| For nausea/vomiting | 53 (56.4) | 17 (41.5) | 36 (67.9) | 0.0103 |
| For diarrhea | 45 (47.9) | 12 (29.3) | 33 (62.3) | 0.0015 |
| For hepatic dysfunction | 21 (22.3) | 8 (19.5) | 13 (24.5) | 0.5626 |
Values are displayed as number (%)
ADR adverse drug reactions
Multivariable analysis considered potential factors influencing discontinuation (each 10-year age increase, BSA < 1.58 m2, GAP index, and medications for ADRs; Table 4). Older age (in 10-year increments) and low BSA (< 1.58 m2) were independently associated with discontinuation. A lower GAP index (stage II versus III) and aggressive medication management for ADRs were associated with less discontinuation (Table 4).
Table 4.
Multivariate analysis of factors associated with discontinuation of nintedanib
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age in years, 10-year increments | 1.851 (1.049–3.013) | 0.0365 | 1.562 (1.011–3.262) | 0.0449 |
| Gender (F versus M) | 2.172 (0.960–5.019) | 0.0650 | ||
| BSA, (< 1.58 m2 versus ≥ 1.58 m2) | 2.172 (1.160–6.019) | 0.0253 | 2.660 (1.198–7.205) | 0.0343 |
| GAP stage, (I versus III) | 0.581 (0.179–1.834) | 0.8727 | ||
| GAP stage, (II versus III) | 0.289 (0.102–0.781) | 0.0188 | 0.203 (0.059–0.622) | 0.0072 |
| Smoking history (never versus current/former) | 0.503 (0.219–1.135) | 0.1006 | ||
| Fibrotic ILD (IPF versus SSc-ILD) | 1.671 (0.743–3.814) | 0.2171 | ||
| Advanced ILD (N versus Y) | 1.186 (0.528–2.677) | 0.6802 | ||
| Chronic respiratory disease (without versus with) | 1.533 (0.682–3.487) | 0.3033 | ||
| Medication for ADRs: | ||||
| For nausea/vomiting | 0.321 (0.136–0.735) | 0.0081 | 0.302 (0.110–0.779) | 0.0154 |
| For diarrhea | 0.266 (0.111–0.615) | 0.0024 | 0.226 (0.082–0.582) | 0.0028 |
ADR adverse drug reactions, BSA body surface area, GAP gender–age–physiology.
Discussion
This study highlights the importance of aggressive management ADRs in enhancing patient tolerance to nintedanib, prolonging the duration of management, and improving outcomes for those with chronic fibrotic ILD.
Our findings align with relevant clinical trials and real-world studies that show a high incidence of ADRs [3–,10,13–15]. Notably, Asian patients appear to experience ADRs at a higher rate compared with their counterparts in Europe and the USA [16–24]; the reasons for this discrepancy are unclear. Efforts to reduce ADRs are crucial for enhancing drug tolerability and maximizing long-term benefits for patients receiving nintedanib. Early intervention is key to reducing drug discontinuation. Although our study did not find an association between baseline or 24-week FVC and DLco levels and nintedanib discontinuation (Table 2), a deterioration in FVC was observed in a Japanese study [15] when nintadanib was discontinued within 3 months. This aligns with several studies indicating that early discontinuation of nintedanib is associated with a higher risk of acute exacerbation [3–8], a finding also supported by our study (Table 1). Patients discontinuing nintedanib within 180 days had a higher risk of acute exacerbations.
The primary ADRs associated with nintedanib include nausea, vomiting, diarrhea, and hepatic dysfunction [3–10, 13–15], each of which can negatively affect the nutritional status of patients. Japanese real-world data [15] indicated that a BSA of less than 1.58 m2 was associated with early discontinuation of nintedanib within 3 months. Our study corroborates these findings, indicating the necessity for strategies to counteract the nutritional effects. These gastrointestinal ADRs also reduce the willingness of patients to continue nintedanib, thereby influencing their compliance and adherence. Although our study associated ADR management with continued nintedanib use, direct evidence linking aggressive ADR management to enhanced drug toleration and patient compliance remains limited and requires further investigation. Notably, this association applies to all ADRs except for those related to hepatic dysfunction. This study is among the first to explore this concept, although further prospective investigations are warranted to confirm these findings and provide stronger evidence.
The INPULSIS-ON study [9] revealed that patients who continued nintedanib beyond 52 weeks rarely discontinued it due to diarrhea, suggesting that longer treatment durations may enhance tolerance to ADRs and improve patient outcomes. Our data, alongside the INPULSIS-ON study results, reinforce the necessity of optimal care for patients on nintedanib. Aggressive management of ADRs contributes to the continued use of nintedanib and superior patient outcomes.
Limitations
Our study demonstrates the advantages of early intervention in managing ADRs, which aids in maintaining the continuity of nintedanib treatment. However, acknowledging the study’s limitations is crucial. These include the study being retrospective, conducted at a single center, involving a relatively small patient cohort, and lacking detailed data on the incidence and severity of nausea, vomiting, diarrhea, and hepatic dysfunction. Additionally, the study lacks information on the severity of acute exacerbations and the incidence rate of acute exacerbation is notably high although similar results were observed [25, 26].
Conclusions
Our study underscores the significance of aggressive management of ADRs in helping patients tolerate nintedanib, thereby prolonging the duration of management and improving outcomes in chronic fibrotic ILD. The findings suggest that further prospective studies are necessary to validate these observations and to provide more comprehensive insights into this area of treatment.
Abbreviations
- ADRs
Adverse drug reactions
- BMI
Body mass index
- BSA
Body surface area
- DLco
Diffusing capacity of the lung for carbon monoxide
- FVC
Forced vital capacity
- ILDs
Interstitial lung diseases
- IPF
Idiopathic pulmonary fibrosis
- PF-ILD
Progressive fibrosing interstitial lung disease
- SSc-ILD
Systemic sclerosis-associated ILD
Declarations
Funding
This study did not receive financial support from any public, commercial, or nonprofit funding agencies.
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethics Approval
Ethical approval for this study was obtained from the Institutional Review Board of Chang Gung Medical Foundation (IRB no.: 202201260B0 and 202201284B0). The study was performed in accordance with the standards of ethics outlined in the Declaration of Helsinki.
Consent to Participate
We anonymized the data retrospectively. Taiwan medical ethics guidelines do not require informed consent be obtained for this type of research.
Consent for Publication
Not applicable.
Data Availability
Data will be made available on reasonable request.
Code Availability
Not applicable.
Author Contributions
Yu-Wen Chang: Data collection, Statistic analysis and Manuscription. Meng-Yun Tsai: Data collection, Statistic analysis and Manuscription. Yu-Ping Chang: Data collection. Chien-Chang Liao: Data collection, multidisciplinary discussions. Yu-Ting Lin: Data collection, multidisciplinary discussions. Chien-Hao Lai: Data collection, multidisciplinary discussions. Meng-Chih Lin: Study design and consultation, multidisciplinary discussions. Kuo-Tung Huang: Study design and consultation, multidisciplinary discussions, and Corresponding author.
References
- 1.Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cottin V, Wollin L, Fischer A, Quaresma M, Stowasser S, Harari S. Fibrosing interstitial lung diseases: knowns and unknowns. Eur Respir Rev. 2019;28(151):180100. [DOI] [PMC free article] [PubMed]
- 3.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 4.Richeldi L, Cottin V, du Bois RM, Selman M, Kimura T, Bailes Z, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the TOMORROW and INPULSIS(®) trials. Respir Med. 2016;113:74–9. 10.1016/j.rmed.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Richeldi L, Kreuter M, Selman M, Crestani B, Kirsten AM, Wuyts WA, et al. Long-term treatment of patients with idiopathic pulmonary fibrosis with nintedanib: Results from the TOMORROW trial and its open-label extension. Thorax. 2018;73(6):581–3. 10.1136/thoraxjnl-2016-209701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87. 10.1056/NEJMoa1103690 [DOI] [PubMed] [Google Scholar]
- 7.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–28. 10.1056/NEJMoa1903076 [DOI] [PubMed] [Google Scholar]
- 8.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–27. 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 9.Crestani B, Huggins JT, Kaye M, Costabel U, Glaspole I, Ogura T, et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: results from the open-label extension study, INPULSIS-ON. Lancet Respir Med. 2019;7(1):60–8. 10.1016/S2213-2600(18)30339-4 [DOI] [PubMed] [Google Scholar]
- 10.US Food & Drug Administration, label OFEV (nintedanib). September 2019. Reference ID 4572087.
- 11.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, Behr J, Brown KK, Cottin V, Flaherty KR, Fukuoka J, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kolb M, Lynch DA, Myers JL, Raghu G, Richeldi L, Taniguchi H, Martinez FJ. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194(3):265–75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 12.Luppi F, Sebastiani M, Salvarani C, Bendstrup E, Manfredi A. Acute exacerbation of interstitial lung disease associated with rheumatic disease. Nat Rev Rheumatol. 2022;18(2):85–96. 10.1038/s41584-021-00721-z [DOI] [PubMed] [Google Scholar]
- 13.Song JW, Ogura T, Inoue Y, Xu Z, Quaresma M, Stowasser S, et al. Long-term treatment with nintedanib in Asian patients with idiopathic pulmonary fibrosis: Results from INPULSIS®-ON. Respirology. 2020;25(4):410–6. 10.1111/resp.13647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng SL, Sheu CC, Chian CF, Hsu JY, Kao KC, Hang LW, et al. NICEFIT–A prospective, non-interventional, and multicentric study for the management of idiopathic pulmonary fibrosis with antifibrotic therapy in Taiwan. Biomedicines. 2022;10(10):2362. [DOI] [PMC free article] [PubMed]
- 15.Ogura T, Inoue Y, Azuma A, Homma S, Kondoh Y, Tanaka K, et al. Real-world safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: Interim report of a post-marketing surveillance in Japan. Adv Ther. 2023;40(4):1474–93. 10.1007/s12325-022-02411-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato M, Sasaki S, Tateyama M, Arai Y, Motomura H, Sumiyoshi I, et al. Clinical significance of continuable treatment with nintedanib over 12 months for idiopathic pulmonary fibrosis in a real-world setting. Drug Des Devel Ther. 2021;15:223–30. 10.2147/DDDT.S284819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon HY, Park S, Kim DS, Song JW. Efficacy and safety of nintedanib in advanced idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):203. 10.1186/s12931-018-0907-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobashi M, Tanaka H, Taima K, Itoga M, Ishioka Y, Shiratori T, et al. The efficacy of nintedanib in 158 patients with idiopathic pulmonary fibrosis in real-world settings: a multicenter retrospective study. SAGE Open Med. 2021;9:20503121211023356. 10.1177/20503121211023357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toellner H, Hughes G, Beswick W, Crooks MG, Donaldson C, Forrest I, et al. Early clinical experiences with nintedanib in three UK tertiary interstitial lung disease centres. Clin Transl Med. 2017;6(1):41. 10.1186/s40169-017-0172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniou K, Markopoulou K, Tzouvelekis A, Trachalaki A, Vasarmidi E, Organtzis J, et al. Efficacy and safety of nintedanib in a Greek multicentre idiopathic pulmonary fibrosis registry: a retrospective, observational, cohort study. ERJ Open Res. 2020;6(1):00172–2019. [DOI] [PMC free article] [PubMed]
- 21.Bonella F, Kreuter M, Hagmeyer L, Neurohr C, Keller C, Kohlhaeufl MJ, et al. Insights from the German compassionate use program of nintedanib for the treatment of idiopathic pulmonary fibrosis. Respiration. 2016;92(2):98–106. 10.1159/000448288 [DOI] [PubMed] [Google Scholar]
- 22.Galli JA, Pandya A, Vega-Olivo M, Dass C, Zhao H, Criner GJ. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: Tolerability and adverse drug reactions. Respirology. 2017;22(6):1171–8. 10.1111/resp.13024 [DOI] [PubMed] [Google Scholar]
- 23.Tzouvelekis A, Karampitsakos T, Kontou M, Granitsas A, Malliou I, Anagnostopoulos A, et al. Safety and efficacy of nintedanib in idiopathic pulmonary fibrosis: a real-life observational study in Greece. Pulm Pharmacol Ther. 2018;49:61–6. 10.1016/j.pupt.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Fletcher SV, Jones MG, Renzoni EA, Parfrey H, Hoyles RK, Spinks K, et al. Safety and tolerability of nintedanib for the treatment of idiopathic pulmonary fibrosis in routine UK clinical practice. ERJ Open Res. 2018;4(4):00049–2018. [DOI] [PMC free article] [PubMed]
- 25.Huang TH, Kuo CW, Chen CW, Tseng YL, Wu CL, Lin SH. Baseline plasma KL-6 level predicts adverse outcomes in patients with idiopathic pulmonary fibrosis receiving nintedanib: a retrospective real-world cohort study. BMC Pulm Med. 2021;21(1):165. 10.1186/s12890-021-01530-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng CM, Chen MY, Kao CY, Tao CW. Investigation of clinical predictors of survival in idiopathic pulmonary fibrosis patients: a cohort study in Taiwan. J Chin Med Assoc. 2022;85(5):578–83. 10.1097/JCMA.0000000000000719 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request.


