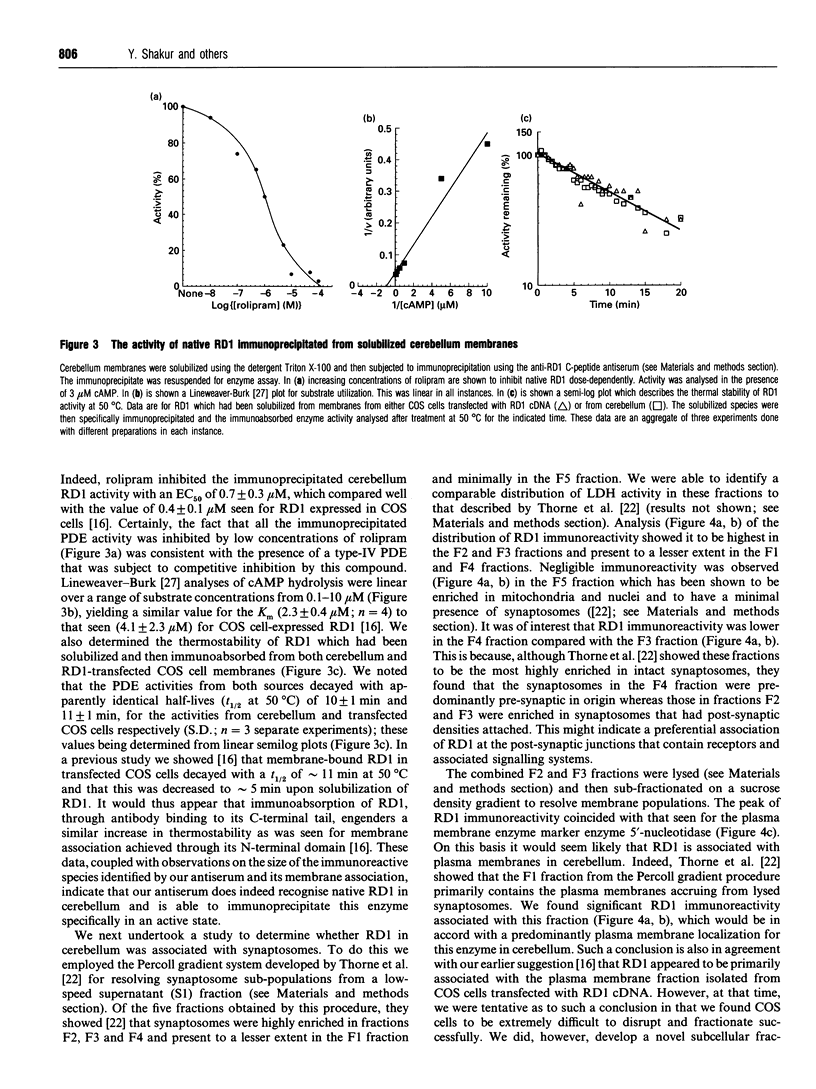
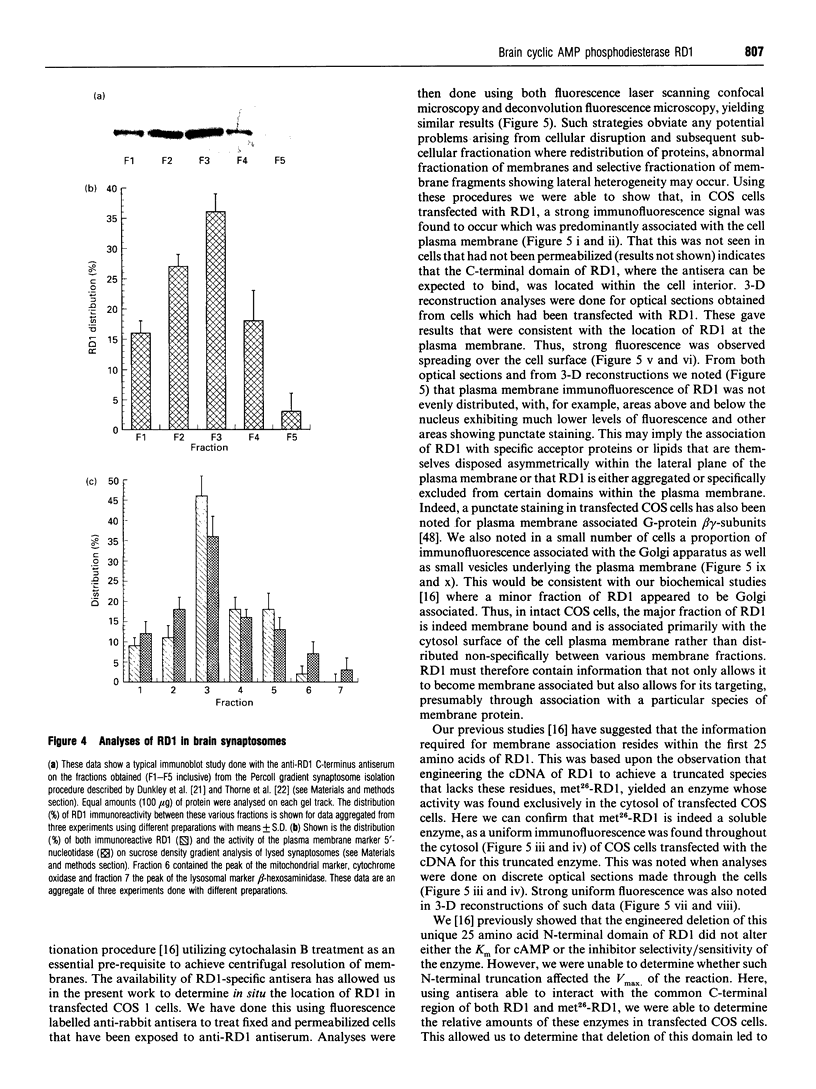
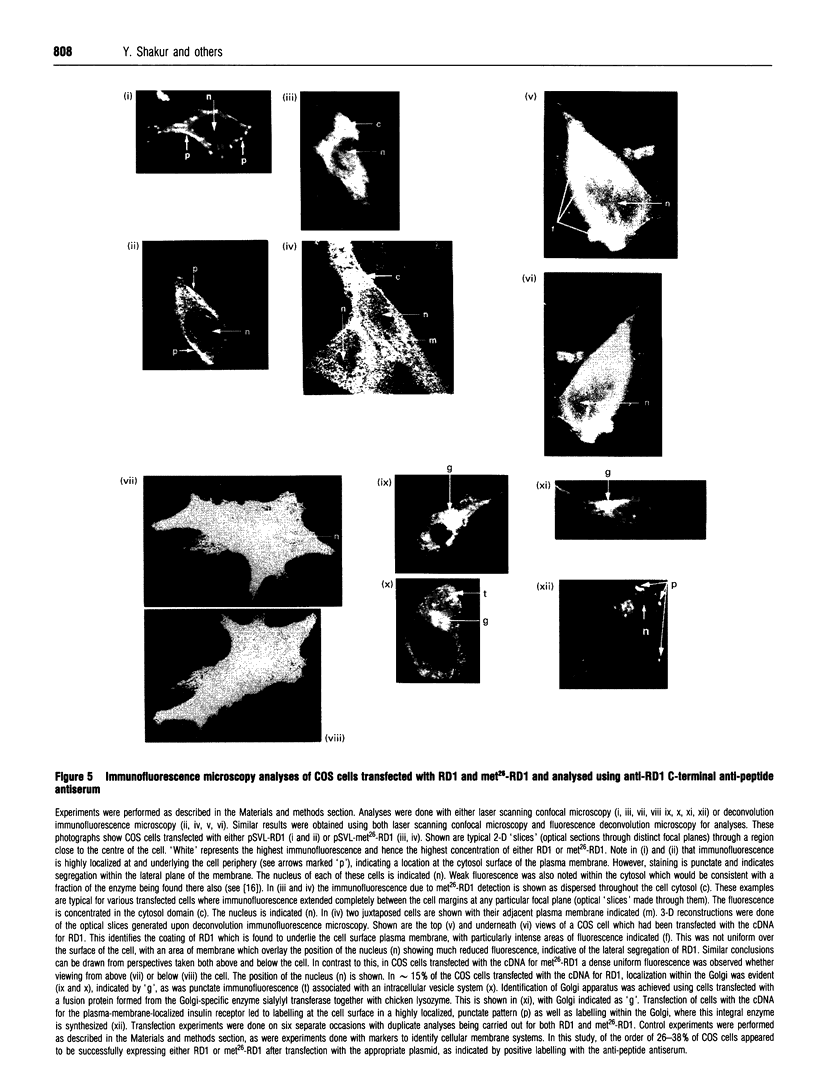
Abstract
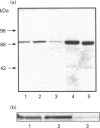
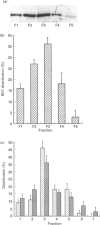
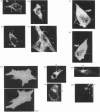
An antiserum was generated against a dodecapeptide whose sequence is found at the C-terminus of a cyclic AMP (cAMP)-specific, type-IVA phosphodiesterase encoded by the rat 'dunc-like' cyclic AMP phosphodiesterase (RD1) cDNA. This antiserum identified a single approximately 73 kDa protein species upon immunoblotting of cerebellum homogenates. This species co-migrated upon SDS/PAGE with a single immunoreactive species observed in COS cells transfected with the cDNA for RD1. Native RD1 in cerebellum was found to be predominantly (approximately 93%) membrane-associated and could be found in isolated synaptosome populations, in particular those enriched in post-synaptic densities. Fractionation of lysed synaptosomes on sucrose density gradients identified RD1 as co-migrating with the plasma membrane marker 5'-nucleotidase. Laser scanning confocal and digital deconvolution immunofluorescence studies done on intact COS cells transfected with RD1 cDNA showed RD1 to be predominantly localized to plasma membranes but also associated with the Golgi apparatus and intracellular vesicles. RD1-specific antisera immunoprecipitated phosphodiesterase activity from solubilized cerebellum membranes. This activity had the characteristics expected of the type-IV cAMP phosphodiesterase RD1 in that it was cAMP specific, exhibited a low Km cAMP of 2.3 microM, high sensitivity to inhibition by 4-[3-(cyclopentoxyl)-4-methoxyphenyl]-2-pyrrolidone (rolipram) (Ki approximately 0.7 microM) and was unaffected by Ca2+/calmodulin and low concentrations of cyclic GMP. The phosphodiesterase activities of RD1 solubilized from both cerebellum and transfected COS cell membranes showed identical first-order thermal denaturation kinetics at 50 degrees C. Native RD1 from cerebellum was shown to be an integral protein in that it was solubilized using the non-ionic detergent Triton X-100 but not by either re-homogenization or high NaCl concentrations. The observation that hydroxylamine was unable to cause the release of RD1 from either cerebellum or COS membranes and that [3H]palmitate was not incorporated into the RD1 protein immunoprecipitated from COS cells transfected with RD1 cDNA, indicated that RD1 was not anchored by N-terminal acylation. The engineered deletion of the 25 residues forming the unique N-terminal domain of RD1 caused both a profound increase in its activity (approximately 2-fold increase in Vmax) and a profound change in intracellular distribution. Thus, immunofluorescence studies identified the N-terminal truncated species as occurring exclusively ion the cytosol of transfected COS cells. The cDNA for RD1 thus appears to encode a native full-length type-IVA phosphodiesterase that is expressed in cerebellum.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsony J., Marx S. J. Immunocytology on microwave-fixed cells reveals rapid and agonist-specific changes in subcellular accumulation patterns for cAMP or cGMP. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1188–1192. doi: 10.1073/pnas.87.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger G., Michaeli T., Martins T., St John T., Steiner B., Rodgers L., Riggs M., Wigler M., Ferguson K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993 Oct;13(10):6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brunton L. L., Hayes J. S., Mayer S. E. Functional compartmentation of cyclic AMP and protein kinase in heart. Adv Cyclic Nucleotide Res. 1981;14:391–397. [PubMed] [Google Scholar]
- Bushfield M., Murphy G. J., Lavan B. E., Parker P. J., Hruby V. J., Milligan G., Houslay M. D. Hormonal regulation of Gi2 alpha-subunit phosphorylation in intact hepatocytes. Biochem J. 1990 Jun 1;268(2):449–457. doi: 10.1042/bj2680449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushfield M., Pyne N. J., Houslay M. D. Changes in the phosphorylation state of the inhibitory guanine-nucleotide-binding protein Gi-2 in hepatocytes from lean (Fa/Fa) and obese (fa/fa) Zucker rats. Eur J Biochem. 1990 Sep 11;192(2):537–542. doi: 10.1111/j.1432-1033.1990.tb19258.x. [DOI] [PubMed] [Google Scholar]
- Chen C. N., Denome S., Davis R. L. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M., Jin S. L., Monaco L., Repaske D. R., Swinnen J. V. Hormonal regulation of cyclic nucleotide phosphodiesterases. Endocr Rev. 1991 Aug;12(3):218–234. doi: 10.1210/edrv-12-3-218. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Takayasu H., Eberwine M., Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley P. R., Heath J. W., Harrison S. M., Jarvie P. E., Glenfield P. J., Rostas J. A. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain Res. 1988 Feb 16;441(1-2):59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Hayes J. S., Bowling N., King K. L., Boder G. B. Evidence for selective regulation of the phosphorylation of myocyte proteins by isoproterenol and prostaglandin E1. Biochim Biophys Acta. 1982 Jan 12;714(1):136–142. doi: 10.1016/0304-4165(82)90135-0. [DOI] [PubMed] [Google Scholar]
- JOHNSON M. K. The intracellular distribution of glycolytic and other enzymes in rat-brain homogenates and mitochondrial preparations. Biochem J. 1960 Dec;77:610–618. doi: 10.1042/bj0770610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J Biol Chem. 1990 Mar 15;265(8):4534–4540. [PubMed] [Google Scholar]
- Kaumann A. J., Birnbaumer L. Prostaglandin E1 action on sinus pacemaker and adenylyl cyclase in kitten myocardium. Nature. 1974 Oct 11;251(5475):515–517. doi: 10.1038/251515a0. [DOI] [PubMed] [Google Scholar]
- Kilgour E., Anderson N. G., Houslay M. D. Activation and phosphorylation of the 'dense-vesicle' high-affinity cyclic AMP phosphodiesterase by cyclic AMP-dependent protein kinase. Biochem J. 1989 May 15;260(1):27–36. doi: 10.1042/bj2600027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavan B. E., Lakey T., Houslay M. D. Resolution of soluble cyclic nucleotide phosphodiesterase isoenzymes, from liver and hepatocytes, identifies a novel IBMX-insensitive form. Biochem Pharmacol. 1989 Nov 15;38(22):4123–4136. doi: 10.1016/0006-2952(89)90694-1. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin Y., PasMantier R., Fleischer N., Erlichman J. Hormonal activation of the cAMP-dependent protein kinases in AtT20 cells. Preferential activation of protein kinase I by corticotropin releasing factor, isoproterenol, and forskolin. J Biol Chem. 1984 Aug 25;259(16):10296–10302. [PubMed] [Google Scholar]
- Livesey S. A., Kemp B. E., Re C. A., Partridge N. C., Martin T. J. Selective hormonal activation of cyclic AMP-dependent protein kinase isoenzymes in normal and malignant osteoblasts. J Biol Chem. 1982 Dec 25;257(24):14983–14987. [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Gutierrez L., McKay I. A., Marshall C. J., Hall A. Dynamic fatty acylation of p21N-ras. EMBO J. 1987 Nov;6(11):3353–3357. doi: 10.1002/j.1460-2075.1987.tb02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbanks R. M. The osmotically sensitive potassium and sodium compartments of synaptosomes. Biochem J. 1967 Jul;104(1):148–157. doi: 10.1042/bj1040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Sequences within and adjacent to the transmembrane segment of alpha-2,6-sialyltransferase specify Golgi retention. EMBO J. 1991 Dec;10(12):3577–3588. doi: 10.1002/j.1460-2075.1991.tb04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C., Luzio J. P., Hales C. N. The properties and extracellular location of 5'-nucleotidase of the rat fat-cell plasma membrane. Biochem J. 1975 Mar;146(3):625–633. doi: 10.1042/bj1460625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Soos M. A., Siddle K. Monoclonal antibodies to the insulin receptor stimulate the intrinsic tyrosine kinase activity by cross-linking receptor molecules. EMBO J. 1987 Dec 20;6(13):4003–4010. doi: 10.1002/j.1460-2075.1987.tb02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. Modification of proteins with covalent lipids. Prog Lipid Res. 1988;27(3):177–197. doi: 10.1016/0163-7827(88)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N. J., Cooper M. E., Houslay M. D. Identification and characterization of both the cytosolic and particulate forms of cyclic GMP-stimulated cyclic AMP phosphodiesterase from rat liver. Biochem J. 1986 Mar 1;234(2):325–334. doi: 10.1042/bj2340325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakur Y., Pryde J. G., Houslay M. D. Engineered deletion of the unique N-terminal domain of the cyclic AMP-specific phosphodiesterase RD1 prevents plasma membrane association and the attainment of enhanced thermostability without altering its sensitivity to inhibition by rolipram. Biochem J. 1993 Jun 15;292(Pt 3):677–686. doi: 10.1042/bj2920677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A. M., Backlund P. S., Jr, Butrynski J. E., Jones T. L., Simonds W. F. The G protein connection: molecular basis of membrane association. Trends Biochem Sci. 1991 Sep;16(9):338–341. doi: 10.1016/0968-0004(91)90139-m. [DOI] [PubMed] [Google Scholar]
- Swinnen J. V., Joseph D. R., Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Thorne B., Wonnacott S., Dunkley P. R. Isolation of hippocampal synaptosomes on Percoll gradients: cholinergic markers and ligand binding sites. J Neurochem. 1991 Feb;56(2):479–484. doi: 10.1111/j.1471-4159.1991.tb08175.x. [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Berrios M., Malbon C. C. Indirect immunofluorescence localization of beta-adrenergic receptors and G-proteins in human A431 cells. Biochem J. 1989 Oct 15;263(2):519–532. doi: 10.1042/bj2630519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M., Subramani S. Presence of cytoplasmic factors functional in peroxisomal protein import implicates organelle-associated defects in several human peroxisomal disorders. J Clin Invest. 1993 Nov;92(5):2462–2468. doi: 10.1172/JCI116854. [DOI] [PMC free article] [PubMed] [Google Scholar]