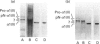Abstract
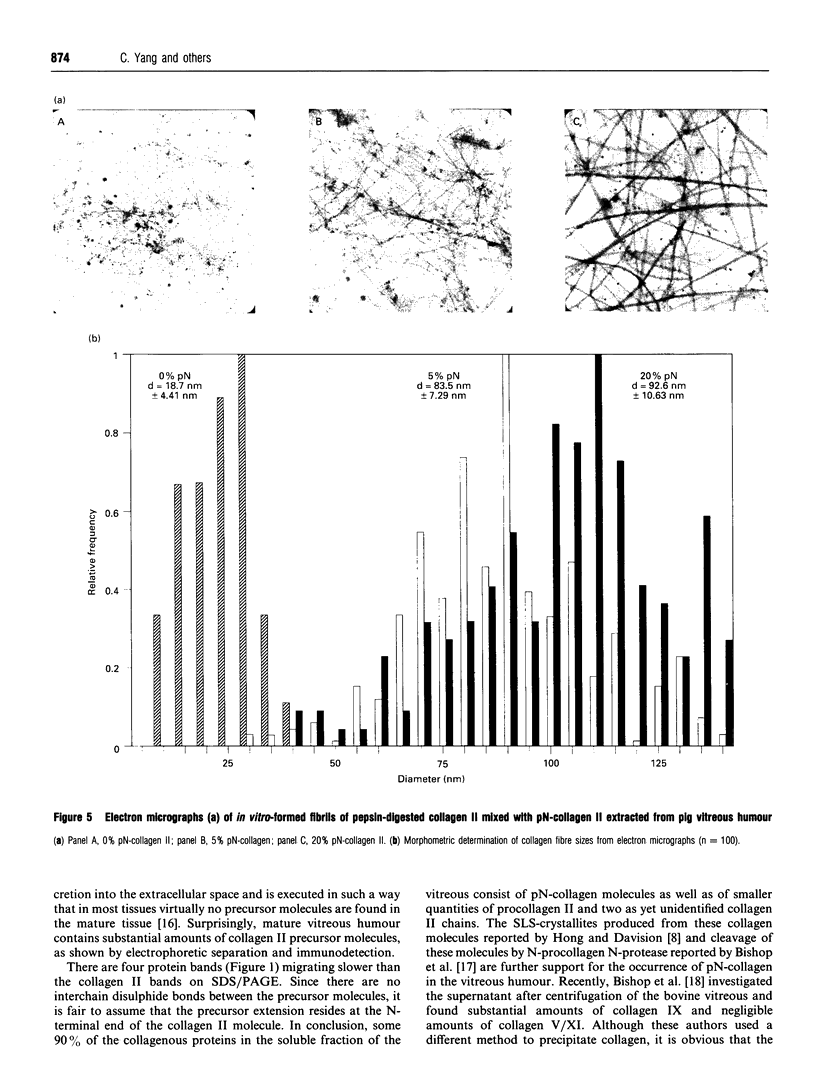
Collagen from pig vitreous humour was fractionated into a soluble and an insoluble fraction by centrifugation. Most of the collagen II in the soluble fraction was present as pN-collagen II (procollagen II without the C-terminal propeptide), besides smaller quantities of procollagen II, collagen II and two as yet unidentified alpha-chains of collagen II. Other collagen types may be present only in trace amounts. Collagen II of the insoluble fraction, which is mostly deposited in fibrillar aggregates, consists of both pN-collagen II and collagen II. To determine the possible role of collagen II precursors in the formation of the extracellular matrix of the vitreous humour these collagen molecules were purified and in vitro fibrillogenesis was used to demonstrate that pN-collagen II could form fibrils in mixtures with collagen II. These fibrils have a reduced mass per unit length depending on the content of pN-collagen in the mixture. Cross-sections of the newly formed fibrillar aggregates revealed a flattened shape. The incomplete processing of the precursors of collagen II may be part of regulatory mechanisms possibly controlling the formation of a translucent scaffold as is required in the vitreous humour.
Full text
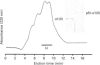
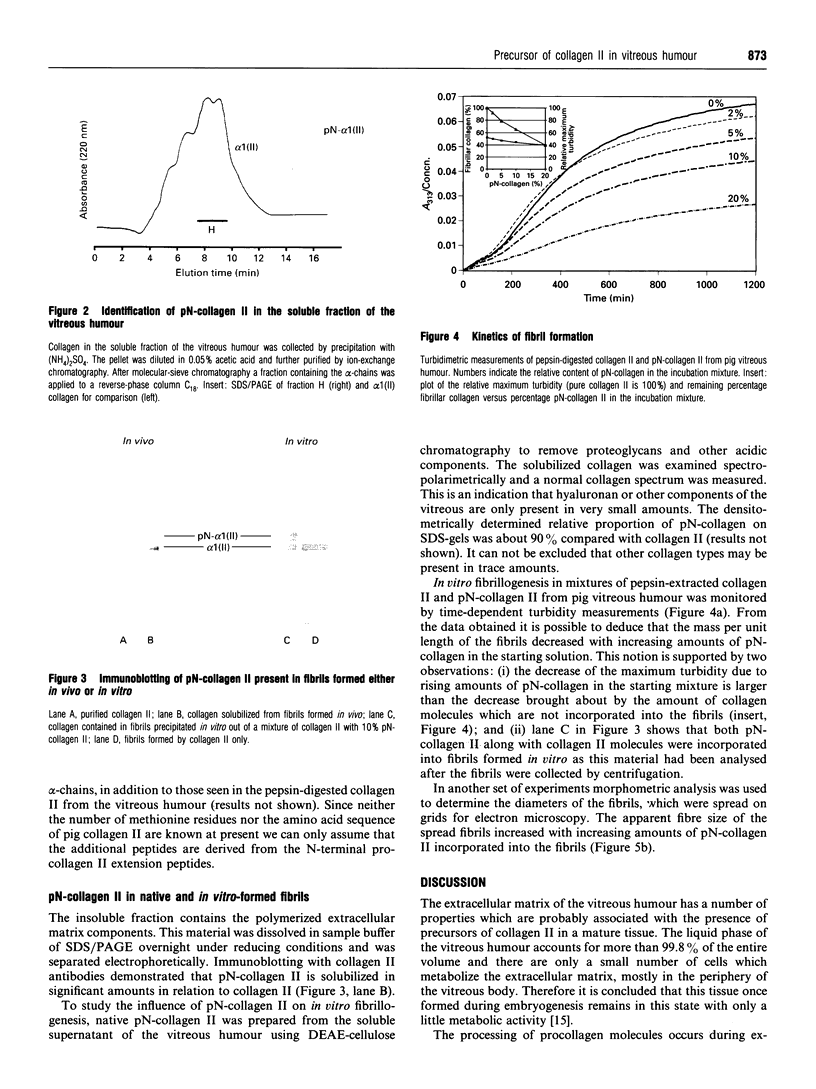
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S., Weiss J. B. A new look at vitreous-humour collagen. Biochem J. 1984 Mar 15;218(3):835–840. doi: 10.1042/bj2180835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. N., Crossman M. V., McLeod D., Ayad S. Extraction and characterization of the tissue forms of collagen types II and IX from bovine vitreous. Biochem J. 1994 Apr 15;299(Pt 2):497–505. doi: 10.1042/bj2990497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. N., Reardon A. J., McLeod D., Ayad S. Identification of alternatively spliced variants of type II procollagen in vitreous. Biochem Biophys Res Commun. 1994 Aug 30;203(1):289–295. doi: 10.1006/bbrc.1994.2180. [DOI] [PubMed] [Google Scholar]
- Brewton R. G., Wright D. W., Mayne R. Structural and functional comparison of type IX collagen-proteoglycan from chicken cartilage and vitreous humor. J Biol Chem. 1991 Mar 15;266(8):4752–4757. [PubMed] [Google Scholar]
- Chapman J. A. The regulation of size and form in the assembly of collagen fibrils in vivo. Biopolymers. 1989 Aug;28(8):1367–1382. doi: 10.1002/bip.360280803. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Timpl R., Olsen B. R. Procollagen intermediates during tendon fibrillogenesis. J Histochem Cytochem. 1988 Nov;36(11):1425–1432. doi: 10.1177/36.11.3049791. [DOI] [PubMed] [Google Scholar]
- Holmes D. F., Watson R. B., Steinmann B., Kadler K. E. Ehlers-Danlos syndrome type VIIB. Morphology of type I collagen fibrils formed in vivo and in vitro is determined by the conformation of the retained N-propeptide. J Biol Chem. 1993 Jul 25;268(21):15758–15765. [PubMed] [Google Scholar]
- Hong B. S., Davison P. F. Identification of type II procollagen in rabbit vitreous. Ophthalmic Res. 1985;17(3):162–167. doi: 10.1159/000265368. [DOI] [PubMed] [Google Scholar]
- Kohno K., Martin G. R., Yamada Y. Isolation and characterization of a cDNA clone for the amino-terminal portion of the pro-alpha 1(II) chain of cartilage collagen. J Biol Chem. 1984 Nov 25;259(22):13668–13673. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane J. M., Weiss C. Review of articular cartilage collagen research. Arthritis Rheum. 1975 Nov-Dec;18(6):553–562. doi: 10.1002/art.1780180605. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Martin G. R., Kohn L. D., Byers P. H., McKusick V. A. Defect in conversion of procollagen to collagen in a form of Ehlers-Danlos syndrome. Science. 1973 Oct 19;182(4109):298–300. doi: 10.1126/science.182.4109.298. [DOI] [PubMed] [Google Scholar]
- Mayne R., Brewton R. G., Mayne P. M., Baker J. R. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem. 1993 May 5;268(13):9381–9386. [PubMed] [Google Scholar]
- Müller P. K., Jamhawi O. The biosynthesis of a precursor of cartilage collagen by embryonic chicken sterna in the presence of alpha, alpha'-dipyridyl. Biochim Biophys Acta. 1974 Sep 13;365(1):158–168. doi: 10.1016/0005-2795(74)90260-8. [DOI] [PubMed] [Google Scholar]
- Notbohm H., Mosler S., Müller P. K., Brinckmann J. In vitro formation and aggregation of heterotypic collagen I and III fibrils. Int J Biol Macromol. 1993 Oct;15(5):299–304. doi: 10.1016/0141-8130(93)90030-p. [DOI] [PubMed] [Google Scholar]
- Ren Z. X., Brewton R. G., Mayne R. An analysis by rotary shadowing of the structure of the mammalian vitreous humor and zonular apparatus. J Struct Biol. 1991 Feb;106(1):57–63. doi: 10.1016/1047-8477(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Romanic A. M., Adachi E., Hojima Y., Engel J., Prockop D. J. Polymerization of pNcollagen I and copolymerization of pNcollagen I with collagen I. A kinetic, thermodynamic, and morphologic study. J Biol Chem. 1992 Nov 5;267(31):22265–22271. [PubMed] [Google Scholar]
- Ryan M. C., Sandell L. J. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J Biol Chem. 1990 Jun 25;265(18):10334–10339. [PubMed] [Google Scholar]
- Swann D. A., Sotman S. S. The chemical composition of bovine vitreous-humour collagen fibres. Biochem J. 1980 Mar 1;185(3):545–554. doi: 10.1042/bj1850545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Allan R. E., Polak K. L. Conversion of type II procollagen to collagen. Extracellular removal of the amino-terminal and carboxy-terminal extensions without a preferential sequence. Eur J Biochem. 1979 Aug 15;99(1):97–103. doi: 10.1111/j.1432-1033.1979.tb13236.x. [DOI] [PubMed] [Google Scholar]
- Watson R. B., Wallis G. A., Holmes D. F., Viljoen D., Byers P. H., Kadler K. E. Ehlers Danlos syndrome type VIIB. Incomplete cleavage of abnormal type I procollagen by N-proteinase in vitro results in the formation of copolymers of collagen and partially cleaved pNcollagen that are near circular in cross-section. J Biol Chem. 1992 May 5;267(13):9093–9100. [PubMed] [Google Scholar]
- Yang C. L., Bodo M., Notbohm H., Peng A., Müller P. K. Fulvic acid disturbs processing of procollagen II in articular cartilage of embryonic chicken and may also cause Kashin-Beck disease. Eur J Biochem. 1991 Dec 18;202(3):1141–1146. doi: 10.1111/j.1432-1033.1991.tb16482.x. [DOI] [PubMed] [Google Scholar]