Abstract
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 2, 2008 and previously updated in 2010.
Tonsillectomy continues to be one of the most common surgical procedures performed in children and adults. Despite improvements in surgical and anaesthetic techniques, postoperative morbidity, mainly in the form of pain, remains a significant clinical problem. Postoperative bacterial infection of the tonsillar fossa has been proposed as an important factor causing pain and associated morbidity, and some studies have found a reduction in morbid outcomes following the administration of perioperative antibiotics.
Objectives
To determine whether perioperative antibiotics reduce pain and other morbid outcomes following tonsillectomy.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ICTRP and additional sources for published and unpublished trials. The date of the most recent search was 20 March 2012.
Selection criteria
All randomised controlled trials examining the impact of perioperative administration of systemic antibiotics on post‐tonsillectomy morbidity in children or adults.
Data collection and analysis
Two authors independently collected data. Primary outcomes were pain, consumption of analgesia and secondary haemorrhage (defined as significant if patient re‐admitted, transfused blood products or returned to theatre, and total (any documented) haemorrhage). Secondary outcomes were fever, time taken to resume normal diet and activities and adverse events. Where possible, we generated summary measures using random‐effects models.
Main results
Ten trials, comprising a pooled total of 1035 participants, met the eligibility criteria. Most did not find a significant reduction in pain with antibiotics. Similarly, antibiotics were mostly not shown to be effective in reducing the need for analgesics. Antibiotics were not associated with a reduction in significant secondary haemorrhage rates (risk ratio (RR) 0.49, 95% CI 0.08 to 3.11, P = 0.45) or total secondary haemorrhage rates (RR 0.90, 95% CI 0.56 to 1.44, P = 0.66). With regard to secondary outcomes, antibiotics reduced the proportion of patients with fever (RR 0.63, 95% CI 0.46 to 0.85, P = 0.002).
Authors' conclusions
The present systematic review, including meta‐analyses for select outcomes, suggests that although individual studies vary in their findings, there is no evidence to support a consistent, clinically important impact of antibiotics in reducing the main morbid outcomes following tonsillectomy (i.e. pain, need for analgesia and secondary haemorrhage rates). The limited benefit apparent with antibiotics may be a result of positive bias introduced by several important methodological shortcomings in the included trials. Based on existing evidence, therefore, we would advocate against the routine prescription of antibiotics to patients undergoing tonsillectomy. Whether a subgroup of patients who might benefit from selective administration of antibiotics exists is unknown and needs to be explored in future trials.
Keywords: Adult; Child; Humans; Analgesics; Analgesics/administration & dosage; Anti‐Bacterial Agents; Anti‐Bacterial Agents/adverse effects; Anti‐Bacterial Agents/therapeutic use; Antibiotic Prophylaxis; Bacterial Infections; Bacterial Infections/drug therapy; Convalescence; Fever; Fever/drug therapy; Pain, Postoperative; Pain, Postoperative/drug therapy; Pain, Postoperative/prevention & control; Postoperative Hemorrhage; Postoperative Hemorrhage/drug therapy; Postoperative Hemorrhage/etiology; Randomized Controlled Trials as Topic; Tonsillectomy; Tonsillectomy/adverse effects
Plain language summary
Antibiotics to reduce pain and improve recovery following tonsillectomy
Tonsillectomy is a commonly performed operation in children and adults. Following the operation nearly all patients experience significant pain, need regular painkillers and are unable to resume normal diet and activities for several hours. Rarer but more dangerous complications, such as bleeding from the operated area, also occur. Antibiotics are commonly prescribed to reduce some or all of these undesirable consequences of tonsillectomy.
The present review, however, suggests that antibiotics do not reduce pain, the need for painkillers or bleeding. They do, however, appear to reduce fever. This relatively minor benefit is more likely to be due to weaknesses in the studies themselves than any direct antibiotic effect. The risk of adverse events, such as skin rash and diarrhoea, is also slightly higher in patients who were prescribed antibiotics. Therefore, in the absence of clear‐cut and significant benefit, and with the potential for harm, we advocate against prescribing antibiotics routinely for patients undergoing tonsillectomy.
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 2, 2008 and previously updated in 2010.
Tonsillectomy continues to be one of the most common surgical procedures performed in children and adults. Despite improvements in surgical and anaesthetic techniques, postoperative morbidity remains a significant clinical problem. The most common problems encountered in the postoperative period include pain, which is almost universal, and the consequent need for analgesics, as well as the inability to resume normal diet and activity for a few hours to several days. Post‐tonsillectomy pain has been estimated to last well beyond the first week in the majority of patients, and the resultant economic and social costs are considerable (Salonen 2002). Furthermore, the incidence of postoperative haemorrhage in various studies ranges from 2% to 40% depending on the definition of haemorrhage (Evans 2003; Lowe 2004; Wei 2000) and this may incur additional morbidity in the form of readmission, blood transfusion and return to theatre for haemostasis. Several adjuvant techniques such as administration of potent systemic analgesics, local infiltration with anaesthetic and topical analgesic sprays have all been studied, but their efficacy remains to be proven (Dhiwakar 2005; Hollis 1999).
After tonsillectomy the tonsillar bed heals by secondary intention and is contaminated by bacteria normally present as commensals in the oropharyngeal mucosa (Telian 1986). Several authors argue that this predisposes the patient to an inflammatory reaction and infection and contributes to postoperative morbidity. They therefore recommend prophylactic antibiotics to reduce the morbidity (Colreavy 1999; Grandis 1992; Telian 1986); their routine perioperative administration is frequent and has been well established for decades in otolaryngology practice (Kay 2003; Krishna 2004). However, there is considerable variation in practice worldwide: a recent study from the UK showed that only 12% of otolaryngologists routinely prescribe antibiotics (Dhiwakar 2005), while another study showed a figure of 79% among American otolaryngologists (Krishna 2004). Those who favour routine administration of antibiotics cite decreased pain, decreased inflammation and faster healing as the most common reasons (Krishna 2004). Other authors, however, do not favour routine antibiotic administration, citing a lack of evidence to support a direct causal link between infection and postoperative morbidity (Cannon 1996; O'Reilly 2003), hence the subject remains contentious. Furthermore, while traditionally secondary haemorrhage is attributed to infection and in such patients antibiotics are prescribed (Pavelic 1960), it is unclear if a causal relationship exists and whether or not antibiotics reduce the risk.
Due to the high potential for contamination by commensals, culture results of the tonsillar bed are difficult to interpret. Hence the definition and incidence of post‐tonsillectomy infection are unclear. Clinically worsening pain, continuing inability to resume diet and raised temperature are considered features of infection and these patients are typically administered antibiotics (Murthy 1998). However, it is unclear whether infection contributes to postoperative morbidity in all or a majority of patients, what the risk factors for infection are and whether they are different for adults and children. The role of routine antibiotics therefore remains unclear. For transurethral resection of the prostate, which is analogous to tonsillectomy in that tissue resection leaves denuded mucosa to heal by secondary intention in a bacterial milieu, a recent systematic review suggests that antibiotic prophylaxis improves some outcomes such as high fever and bacteraemia (Qiang 2005). A systematic review is similarly required to evaluate the effectiveness of antibiotics in reducing post‐tonsillectomy morbidity. Furthermore, while individual trials might not be sufficiently large, a meta‐analysis would potentially have sufficient power to determine whether antibiotics reduce rarer complications such as secondary haemorrhage.
Objectives
To determine whether perioperative antibiotics reduce pain, associated morbidity and complications following tonsillectomy.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (blinded and unblinded).
Types of participants
Patients undergoing tonsillectomy or adenotonsillectomy. Where explicitly stated we excluded patients undergoing the following procedures: unilateral tonsillectomy, tonsillar biopsy, tonsillectomy for known carcinoma, tonsillectomy in conjunction with palatal surgery and 'hot' tonsillectomy for peritonsillar abscess.
Types of interventions
We included trials in which an antibiotic was administered as a study medication intraoperatively and/or postoperatively in patients undergoing tonsillectomy or adenotonsillectomy. We also considered for inclusion trials in which an antibiotic was administered within the 48‐hour preoperative period. We excluded trials in which the antibiotic was administered topically, or where explicitly stated more than 48 hours before surgery, from the review.
Patients in whom an antibiotic was administered as a study medication (cases) were compared to patients not given an antibiotic (controls).
Types of outcome measures
Primary outcomes
Pain.
Consumption of analgesia.
Secondary haemorrhage using two parameters: significant haemorrhage (i.e. warranting re‐admission, blood transfusion or return to theatre for haemostasis) and total (any documented) haemorrhage.
Secondary outcomes
Fever.
Time taken to resume normal diet and activities.
Adverse events such as rash, anaphylaxis, candidiasis and diarrhoea.
Where possible we used standardised and validated scales (such as visual analogue for pain) for outcome analysis. Although typically pain is reported to resolve spontaneously beyond the first week (Murthy 1998), we investigated whether antibiotics significantly shortened this duration or that of other morbid outcomes. We classified haemorrhage occurring within 24 hours of surgery as primary, and any haemorrhage occurring beyond this time period as secondary.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 20 March 2012 following previous searches in 2010, 2007 and 2005.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2012, Issue 3); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; ISRCTN; ClinicalTrials.gov; ICTRP; Google Scholar and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for the major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned reference lists of identified studies for further trials. We searched PubMed, TRIPdatabase, The Cochrane Library, and Google to retrieve existing systematic reviews possibly relevant to this systematic review, in order to search their reference lists for additional trials.
Data collection and analysis
Study selection
From all identified studies, two authors (MD and MS) independently selected trials for possible inclusion. We initially assessed all trials examining the impact of systemic antibiotics on post‐tonsillectomy morbidity and randomised controlled trials were included in the review. The senior author (WM) resolved any disagreement in study selection.
Quality assessment
Two authors (MD and AC) independently assessed studies included in the review for quality. We assessed four components of quality.
1. Adequacy of randomisation (randomisation sequence generation, allocation concealment and implementation)
Trials were scored as follows. Grade A: all three sub‐components adequately fulfilled. Grade B: adequate allocation concealment, but one or both of the other sub‐components unsatisfactory. Grade C: unclear allocation concealment. Grade D: clearly inadequate concealment. (Grade A, B = high quality).
2. Blinding
Trials were scored as follows. Grade A: participant and care provider and outcome assessor blinded. Grade B: outcome assessor blinded. Grade C: unclear. Grade D: no blinding of outcome assessor. (Grade A = high quality).
3. Reporting of participants by allocated group (intention‐to‐treat analysis)
Trials were scored as follows. Grade A: the progress of all randomised patients in each group described. Grade B: unclear or no mention of withdrawals or drop‐outs. Grade C: the progress of all randomised patients in each group clearly not described. (Grade A = high quality).
4. Follow‐up
Trials were scored as follows. Grade A: outcomes measured in > 90%. Grade B: outcomes measured in 80% to 90%. Grade C: unclear. Grade D: outcomes measured in < 80%. (Grade A = high quality).
We then gave studies an overall quality grading:
A: Minimisation of bias (i.e. high quality) in all four components above. B: Less than high quality (but not lowest quality) in one or more of the components above. C: Lowest quality in one or more of the components above.
Where necessary, we contacted the principal author of the relevant trial for additional information regarding methodology and/or results. The senior author (WM) resolved any disagreement.
We examined any actual or potential conflicts of interest in the included trials (such as whether sponsored by a drug company). We also noted whether surgical and anaesthetic techniques were controlled and exclusion criteria explicitly applied, but this did not necessarily serve to include/exclude trials.
Data extraction
Two authors independently extracted data (MD and AC) and separately entered these into a specific, pre‐designed pro forma. One author (MD) then entered data into RevMan (RevMan 2011) for analysis.
Data analysis
For continuous outcomes, we extracted mean and standard deviation (SD) values to facilitate meta‐analysis. If mean and/or SD values were not explicitly stated, we used raw data, t values, P values and/or graphs to generate mean/SD values. For dichotomous outcomes, we converted percentage values to the nearest number for meta‐analysis.
If insufficient data were available, we considered children and adults together for outcome analysis. If an eligible trial did not evaluate or report any of the outcomes detailed above, we excluded that trial from the analysis of that particular outcome. We attempted intention‐to‐treat analysis, wherein all participants randomised into a trial, irrespective of which (or how much) treatment they actually received and regardless of other protocol irregularities such as ineligibility, were included for analysis.
Statistics
We calculated summary measures where possible for combinable data. Given the expected variability in participants, interventions, outcomes studied and trial design and quality, we used DerSimonian and Laird random‐effects models to generate summary measures. For rarer outcomes, such as secondary haemorrhage and adverse events, if sufficient numbers of patients were not available, we calculated number needed to treat or number needed to harm as appropriate. We used RevMan version 5.1 (RevMan 2011) for the analysis.
Results
Description of studies
Of the 120 abstracts retrieved from our original searches in 2007 and update searches in 2009, we excluded 98 as these were incomplete trials, did not examine post‐tonsillectomy morbidity, did not include patients undergoing tonsillectomy, studied topical antibiotics, studied 'hot' tonsillectomy for peritonsillar abscess, compared one systemic versus another systemic antibiotic, or did not have a control group. Seven further studies (Al‐Tamimi 2000; Aslam 1998; Lackmann 1992; Lee 1996; Minet 1978; Szmeja 1997; Udaipurwala 2002) were excluded after review as they were non‐randomised trials. We excluded two further trials (Akbas 2004; Inci 2009) as they compared systemic versus topical antibiotics. We excluded three further studies due to failure to complete study (Browning 1995) or unavailability of complete study copies (Novais 2003; Udaipurwala 2004).
We again updated the searches in March 2012. In total the searches retrieved 104 references; this number dropped to 85 once duplicates were removed. We screened the titles and abstracts of the 85 references and looked at five potentially relevant references in detail, however none were eligible for inclusion in the review. One had already been excluded at a previous update of the review (Inci 2009); we excluded Zagolski 2012 because tonsillotomy with incision of the tonsil was performed instead of tonsillectomy; Dawar 2011 was excluded as it was a non‐randomised trial and Miura 2009 was excluded as it assessed the efficacy of a topical antibiotic. Details of Khalil 2004 are awaited from the authors (see Characteristics of ongoing studies).
The summaries of all excluded studies are shown in the table Characteristics of excluded studies.
Ten randomised controlled trials examining the impact of systemic antibiotics on post‐tonsillectomy morbidity fulfilled the eligibility criteria and were included for analysis. The characteristics of these trials are set out in the table Characteristics of included studies. All studies compared a short course of systemic antibiotics versus placebo. Many trials included children with no explicit information about the age range, or reported children and adults together with no means to extract data separately. Hence all participants, irrespective of age, were included in the analyses.
Data extraction
No study explicitly stated standard deviation (SD) values for any continuous outcome measure. Raw data were available from bar graphs for the outcomes reported by Cannon 1996. For the rest, the following indirect data were derived to facilitate meta‐analyses, if appropriate.
Pain
Mann 1999 reported mean pain scores for each of five days as a bar graph on a scale of 0 to 100 with standard error (SE) bars. O'Reilly 2003 used a visual analogue scale of 1 to 5 over 10 days and expressed the results as a line graph. Similarly, Grandis 1992 used a scale of 1 to 10 over seven days and expressed the results as a line graph. Colreavy 1999 similarly provided mean pain scores on a scale of 1 to 10 for seven days. Ramos gave mean pain scores on a scale of 0 to 3 (duration unclear). Guerra 2008 used a pain scale of 1 to 5 for each of the first seven postoperative days. Given the variability of parameters used in these studies and the paucity of important values such as SD, meta‐analysis for pain was not possible.
Consumption of analgesics
Linden 1990 expressed subjective rating of consumption of pain medicine as a bar chart which was converted to mean scores for comparison.
Time taken to return to diet and activities
In the trial by Telian 1986, for time taken to resume normal diet and activity, pooled SDs were estimable from published mean differences, t and P values. Guerra 2008 published mean number of days (with SD) taken to resume normal diet and activities. However, Khan 1994 reported only the mean and range of the number of days taken to resume activity and oral intake, whereas Colreavy 1999 reported only the mean values of the time taken to resume normal diet. Grandis 1992 expressed the results as line graphs, from which accurate data could not be extracted. Therefore, given the variability of parameters used and paucity of important values such as SD, meta‐analysis of the time taken to resume diet and activities was not possible.
Fever
Telian 1986 reported the percentage of patients manifesting fever, which was converted to the nearest number for meta‐analysis.
Secondary haemorrhage
Telian 1986 excluded from analysis patients who were non‐compliant or suffered complications. However details of secondary haemorrhage were available for the excluded patients. Hence we performed an intention‐to‐treat analysis for the meta‐analysis of secondary haemorrhage, imputing an equal number of treatment and control group patients (N = 50 each).
Risk of bias in included studies
Of the 10 included trials, none attained quality grading A. One (Telian 1986) attained quality grading B. In this trial, all components attained the highest quality except for randomisation, wherein allocation concealment was unclear. The rest attained a quality grading of C. However, the trial by O'Reilly 2003 attained the highest quality grading in all components, except for follow‐up, wherein the drop‐out rate was high at 52%.
Effects of interventions
Pain
Of the six studies that assessed pain using linear pain scores (Colreavy 1999; Grandis 1992; Guerra 2008; Mann 1999; O'Reilly 2003; Ramos 2000), only one (Colreavy 1999) found a significant reduction with antibiotics. Khan 1994 calculated the mean number of days with sore throat (10.3 in antibiotic group versus 11 in control group) and otalgia (8.1 versus 7.8), and found no significant difference. However, Guerra 2008, while assessing pain each day during the first postoperative week, found a significant reduction with antibiotics on day four, with no benefit on the other days. Telian 1986 found the mean number of days with continuous subjective pain to be improved with antibiotics (3.3 versus 4.4, P < 0.05). For the reasons previously mentioned, we could not perform meta‐analysis for pain as an outcome.
Need for analgesia
Six studies assessed for the need for analgesics (Colreavy 1999; Grandis 1992; Guerra 2008; Khan 1994; Linden 1990; O'Reilly 2003). Four did not find a significant reduction with antibiotic use, one (Colreavy 1999) found a significant reduction, while in the last (Linden 1990) the result was indeterminate. O'Reilly 2003 reported a mean of 43% and 46% patients in the antibiotic and placebo groups, respectively, needing to consume additional analgesics. A respective mean of 63% and 51% contacted primary care physicians for analgesia. Grandis 1992 found no difference between the antibiotic and placebo groups in the mean number of days wherein more than five doses of pain medicine was taken (1.8 versus 2.4 respectively), or in the mean number of doses of pain medication (19.45 versus 19.23). Guerra 2008 reported that 79% and 88% patients in the antibiotic and control groups respectively required analgesic medication with no statistical difference between the groups. Similarly Khan 1994 calculated the number of days taken until no analgesia was needed and found no significant difference (8.2 versus 8.5). However Colreavy 1999 found a significant reduction in analgesic consumption with antibiotics. The antibiotic group consumed on average 112 mg/kg of paracetamol in 24 hours as opposed to the control group which used on average 200 mg/kg in the same period (P = 0.038). Given the variability in the parameters used, we could not perform meta‐analysis for analgesic consumption.
Postoperative haemorrhage
Seven studies evaluated postoperative haemorrhage and reported incidence rates (Colreavy 1999; Grandis 1992; Guerra 2008; Khan 1994; Mann 1999; O'Reilly 2003; Telian 1986). Where not clear, correspondence with the first author of the relevant trials clarified the distinction between significant and insignificant but documented haemorrhage. Cannon 1996 reported that the rate of postoperative haemorrhage was the same in both groups, but did not give the exact incidence rates. We therefore excluded this trial from the meta‐analyses for haemorrhage. There was only one primary haemorrhage reported among all seven trials (in the control group in the trial Khan 1994). No fatality occurred and no bleeding event re‐occurred in the same patient.
Among a total of 567 participants, 14 (2.5%) significant and 70 (12.3%) total secondary haemorrhages occurred. This is comparable to rates cited in the literature (Krishna 2001; Lowe 2004).
Total secondary haemorrhage
We combined data from the seven studies in a meta‐analysis for total secondary haemorrhage. We deemed pooling appropriate because the outcome parameters used fulfilled the predefined criteria, precise incidence rates were available and there was excellent overlap of confidence intervals in the forest plot. Meta‐analysis confirmed that antibiotics did not reduce the total secondary haemorrhage rate (risk ratio (RR) 0.90, 95% confidence interval (CI) 0.56 to 1.44, P = 0.66) (Analysis 1.1). The I2 statistic revealed minimal heterogeneity.
1.1. Analysis.
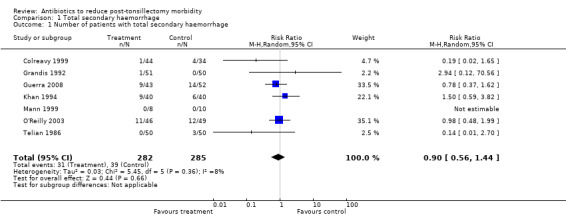
Comparison 1 Total secondary haemorrhage, Outcome 1 Number of patients with total secondary haemorrhage.
Significant secondary haemorrhage
With regard to significant secondary haemorrhage, the incidence rate was lower; in fact it was zero in several studies. Confidence intervals were therefore wider and could be derived only for a limited set of data. Nevertheless, the outcome parameter used fulfilled the predefined criteria, which were more rigid than for total haemorrhage, precise outcome data were available and there was good overlap of confidence intervals in the forest plot. Therefore, despite moderate heterogeneity demonstrated by the I2 statistic (54%), we combined data in a meta‐analysis. This confirmed that antibiotics did not reduce significant secondary haemorrhage rates (RR 0.49, 95% CI 0.08 to 3.11, P = 0.45) (Analysis 2.1). However, unlike for total secondary haemorrhage, the data for significant secondary haemorrhage may be underpowered to detect any difference.
2.1. Analysis.
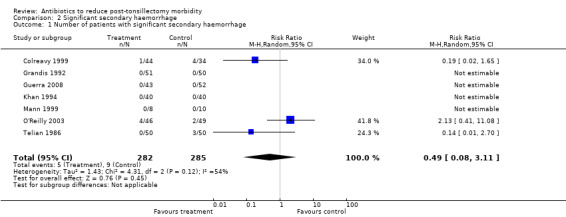
Comparison 2 Significant secondary haemorrhage, Outcome 1 Number of patients with significant secondary haemorrhage.
Fever
Two studies (Grandis 1992; Telian 1986) used the same parameter (temperature > 99.9 °F) to define fever. Both measured the outcome for the first seven days following surgery. We therefore combined these data in a meta‐analysis, which revealed antibiotics to reduce the number of patients manifesting fever (RR with antibiotics 0.63, 95% CI 0.46 to 0.85, P = 0.002) (Analysis 3.1). There was no heterogeneity (I2 statistic = 0%), excellent overlap of the confidence intervals and unidirectional outcomes. Telian 1986 also reported a significant reduction in the mean number of oral temperature recordings more than 100 °F (1.5 versus 2.9, P < 0.05) and more than 101.5 °F (0.02 versus 0.23, P < 0.05), with use of antibiotics. Similarly, Cannon 1996 reported a significant reduction in the number of patients with fever (> 99 °F, 6 versus 16, P = 0.003). Grandis 1992 reported reduction in the mean number of days with fever (> 99.9 °F) (0.35 versus 0.51, P > 0.05). On the contrary, Guerra 2008 reported no difference in the percentage of patients in the antibiotic and control groups having fever (48% versus 49%); Ramos 2000 reported mean of subjective intensity of fever on a scale of 0 to 3, and found no difference (0 versus 0.2); and Mann 1999 reported no significant difference in postoperative fever (no data available).
3.1. Analysis.
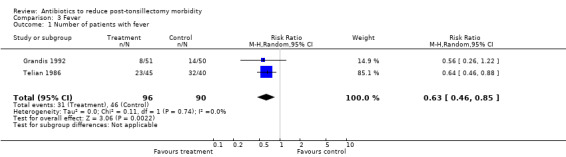
Comparison 3 Fever, Outcome 1 Number of patients with fever.
Time taken to resume diet
Seven studies analysed the time taken to resume normal or soft diet (Cannon 1996; Colreavy 1999; Grandis 1992; Guerra 2008; Khan 1994; Mann 1999; Telian 1986). Three found a significant reduction with antibiotics. Colreavy 1999 reported a mean reduction of 2.4 days (P = 0.007) and Telian 1986 reported a mean reduction of one day (P < 0.01), while Grandis 1992 did not quantify the reduction in time. However the other four studies (Cannon 1996; Guerra 2008; Khan 1994; Mann 1999) reported no significant benefit with antibiotics. Given the variability in the parameters used, we could not perform meta‐analysis for time taken to resume diet.
Time taken to resume normal activity
Six studies analysed the time taken to resume normal activity (Cannon 1996; Grandis 1992; Guerra 2008; Khan 1994; Mann 1999; Telian 1986). Two studies reported earlier return to activity with antibiotics. Telian 1986 reported a mean reduction of one day (P < 0.05), while Grandis 1992 did not quantify the reduction in time (P = 0.045). However, the other four trials (Cannon 1996; Guerra 2008; Khan 1994; Mann 1999) reported no significant benefit with antibiotics. Given the variability in the parameters used, we could not perform meta‐analysis for time taken to resume normal activity.
Adverse effects
There was no major adverse event reported in either group. With regard to minor adverse events, in the antibiotic group there were four cases manifesting a rash (in the trials by Colreavy 1999; Grandis 1992; Mann 1999 and Telian 1986), one developed oropharyngeal candidiasis (Telian 1986) and four developed diarrhoea (three in the trial by Grandis 1992 and one in the trial by Colreavy 1999). In comparison, in the control group one patient developed a rash and two others had diarrhoea (all in the trial by Grandis 1992). The RR of adverse effects with antibiotic use was 2.06 (95% CI 0.68 to 6.27, P = 0.20) (Analysis 4.1). The number needed to treat to harm for antibiotics was 26.
4.1. Analysis.
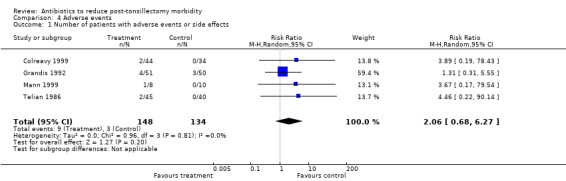
Comparison 4 Adverse events, Outcome 1 Number of patients with adverse events or side effects.
Discussion
The present systematic review suggests that although individual studies vary in their findings, there is no evidence to support a consistent, clinically important impact of antibiotics in reducing the main morbid outcomes following tonsillectomy (i.e. pain, need for analgesia and secondary haemorrhage rates). There is some evidence to suggest that antibiotics may reduce fever. With regard to other secondary outcomes, such as time taken to resume normal diet and activity, there is no clear evidence that antibiotics are beneficial.
These results challenge the widely held rationale for the routine prescription of antibiotics. A causal relationship between bacterial inflammation of the tonsillar fossa and postoperative morbidity, such as pain, need for analgesia and secondary haemorrhage, has not been proven. The difficulty of establishing such a correlation is complicated by the variety of commensals harboured in the oropharyngeal mucosa. This renders bacterial culture results of the postoperative tonsillar fossa as reported in a few studies (Colreavy 1999; Grandis 1992; Telian 1986) difficult to interpret. While some studies show that antibiotics reduce the bacterial count in the postoperative tonsillar fossa (Colreavy 1999; Grandis 1992), a clinical correlation in terms of reduction in morbidity is lacking. This suggests that other proposed mechanisms, such as surgical trauma to the peritonsillar tissues (Parsons 2006; Stoker 2004), ensuing inflammatory response to tissue damage (Akbas 2004), loss of pharyngeal mucosa with exposed muscle and nerve endings (D'Eredita 2004), and ensuing nerve irritation and spasm of the pharyngeal muscles (Akbas 2004) account for postoperative pain and morbidity, with minimal or nil additional morbidity conferred by bacterial inflammation.
Similarly, secondary haemorrhage is widely assumed to be caused by bacterial infection (Pai 2005; Timms 2002) and is commonly treated with antibiotics, despite scant evidence to support an infective aetiology (Pai 2005). Kumar found that of 24 patients with secondary post‐tonsillectomy haemorrhage who were not on antibiotics, only four had a positive culture on throat swab (Kumar 1984). The current review similarly fails to show that antibiotics protect against secondary haemorrhage and therefore bacterial infection as an aetiology is questionable. A more plausible explanation is that sloughing of the primary eschar, which usually occurs between day five and day 10 in the postoperative period, manifests as secondary haemorrhage (Krishna 2001). Further support for this theory comes from the large prospective audit of tonsillectomies conducted in England, in which diathermy dissection was found to increase the risk of secondary haemorrhage. The authors conclude that compared to traditional cold steel, diathermy dissection causes more tissue damage and hence a larger eschar formation, thus conferring a higher risk of secondary haemorrhage (Lowe 2004).
Reduction of fever apparent with antibiotic therapy is likely due to the amelioration of bacteraemia, which is recognised to occur during and immediately after tonsillectomy (Soldado 1998). However, given the varying parameters used, no quantification of the reduction in fever is possible and the overall clinical benefit derived is unclear.
The potential for adverse events needs to be considered while prescribing antibiotics. Antibiotic allergy is unpredictable and not dose dependent (Gruchalla 2000), therefore it is difficult to quantify the risk of allergy in an individual patient precisely (Robinson 2002). The overall frequency of allergy to beta‐lactam antibiotics such as penicillin is cited as 2% per course (Saxon 1987). However, a history of allergy to penicillin is elicited in 5% to 20% of the population (Adkinson 1998). In this population, the risk of allergy is as high as 60% on re‐exposure to beta‐lactams (Green 1977) and 13% on exposure to a different antibiotic (Moseley 1991). Further, although no major adverse event has been reported in the included trials in this review, anaphylaxis is estimated to occur in 0.01% to 0.05% of all penicillin courses (Greenberger 2002). Given the frequency and volume of tonsillectomy as a surgical procedure, this small risk of minor and major allergic events may translate into significant harm when balanced against the lack of evidence to support a consistent, clinically important impact of antibiotics. However non‐allergic adverse events such as toxicity, side effects and drug interactions may be minimised by reducing the dose or length of treatment, i.e. single perioperative administration (Gruchalla 2000).
Limitations
The main limitation of this review is the weak methodology of the included trials. First, allocation concealment, the most important criterion in the randomisation process, was adequate in only one trial (O'Reilly 2003). Studies with inadequate allocation concealment may overestimate treatment effect by 37% (Moher 1998). Second, only five out of the 10 included trials had adequate double‐blinding. Unblinded trials are well known to produce bias favouring treatment (Noseworthy 1994; Schulz 2002), particularly so when subjective outcomes such as pain are assessed (Schulz 2002). Third, intention‐to‐treat analysis was possible in only two trials (O'Reilly 2003; Telian 1986). There is some evidence to suggest that meta‐analyses of trials which do not report all participants by allocated group (i.e. inadequate intention‐to‐treat analysis) produce results favouring the treatment (Tierney 2004). It is likely that all these shortcomings have aggregated to produce significant bias favouring antibiotics. Finally, the overall drop‐out rate was high. This leaves a large potential for attrition bias, despite a comparable number being lost to follow‐up, within each trial, between the study and control groups.
There is also considerable heterogeneity between studies in terms of methodological quality, participants, interventions (type, dose, method and duration of administration of antibiotics) and outcome assessment. This is, however, unlikely to have significantly impacted on the results of this review, as results from individual trials broadly conform to one another, and to the results of meta‐analyses, where done. It is, however, not known if the dosage and antibacterial spectrum of antibiotics used in the trials have been adequate or if there exists a dose‐response effect. Further, it was not possible to analyse data stratified on the basis of indications for tonsillectomy (i.e. sleep apnoea, recurrent tonsillitis, etc.). Hence it is not clear whether a subgroup of patients exists (i.e. severe recurrent tonsillitis or peritonsillar abscess) in whom antibiotics might reduce morbidity.
Conclusion
The present review including meta‐analyses for select outcomes suggests that there is no evidence to support a consistent, clinically important impact of antibiotics in reducing the main morbid outcomes following tonsillectomy (i.e. pain, need for analgesia and secondary haemorrhage rates). Any limited benefit apparent with antibiotics may be a result of positive bias introduced by several important methodological shortcomings in the included trials. Any putative benefit of antibiotics also needs to be carefully weighed against the risk of adverse events and other negative consequences that are more difficult to evaluate and quantify, such as the possible emergence of resistant bacteria and fungal colonisation and infection.
Based on this review, therefore, we advocate against prescribing antibiotics routinely to all patients undergoing tonsillectomy. Whether a subgroup of patients who might benefit from selective administration of antibiotics exists is unknown and needs to be explored in future trials. Further well‐designed trials are recommended to confirm and expand our findings. In future studies, continuous outcome values need to be explicitly stated with mean and SD in order to facilitate meta‐analyses.
Authors' conclusions
Implications for practice.
Antibiotics should not be routinely administered to reduce postoperative morbidity in patients undergoing tonsillectomy.
Implications for research.
Whether a subgroup of patients who might benefit from selective administration of antibiotics exists is unknown and needs to be explored in future trials. Further well‐designed trials are recommended to confirm and expand our findings. In future studies, continuous outcome values need to be explicitly stated with mean and standard deviation in order to facilitate meta‐analyses.
What's new
| Date | Event | Description |
|---|---|---|
| 9 October 2012 | New citation required but conclusions have not changed | We identified no new studies which were eligible for inclusion in the review. Three further studies are excluded and one has been identified as ongoing. |
| 20 March 2012 | New search has been performed | New searches run. |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 15 March 2010 | New citation required and conclusions have changed | One new study added (Guerra 2008), resulting in a change to the review conclusions. |
| 30 October 2009 | New search has been performed | Full new searches run 30 October 2009. |
| 28 August 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors are grateful for the valuable contribution of S. Selvaraj, Biostatistics Department, Raigmore Hospital, Inverness, towards data extraction.
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE | |
| #1 TONSILLECTOMY single term (MeSH) #2 TONSIL [su] single term (MeSH) #3 tonsillectom* OR tonsilectom* #4 adenotonsillectom* OR adenotonsilectom* #5 #1 OR #2 OR #3 OR #4 #6 TONSILLITIS explode all trees (MeSH) #7 TONSIL single term (MeSH) #8 tonsil* OR peritonsil* #9 adenotonsil* #10 #6 OR #7 OR #8 OR #9 #11 SURGERY single term (MeSH) #12 surg* OR excis* OR extract* OR remov* #13 DISSECTION explode all trees (MeSH) #14 dissect* OR electrodissect* OR coblat* OR ablat* OR ultrasonic* OR harmonic* OR guillotin* OR plasma OR ultracis* #15 ion* NEAR field* OR bipolar NEAR probe* #16 DIATHERMY explode all trees (MeSH) #17 CAUTERY explode all trees (MeSH) #18 ULTRASONICS explode all trees (MeSH) #19 ELECTROSURGERY single term (MeSH) #20 electr* NEAR coagulat* OR electrocoagulat* OR electrocauter* OR electr* NEAR cauter* #21 electrosurg* OR electr* NEAR surg* OR bovie OR elmed OR somnoplast* #22 diatherm* OR thermocauter* OR thermocoagul* OR galvanocaut* #23 RADIOSURGERY single term (MeSH) #24 CRYOSURGERY single term (MeSH) #25 radiosurg* OR radiofrequenc* OR cryosurg* #26 LASER SURGERY explode all trees (MeSH) #27 LASERS single term (MeSH) #28 laser* NEAR surg* #29 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 #30 #10 AND #29 #31 #5 OR #30 #32 ANTI BACTERIAL AGENTS explode all trees (MeSH) #33 ANTIBIOTIC PROPHYLAXIS single term (MeSH) #34 LACTAMS explode all trees (MeSH) #35 QUINOLONES explode all trees (MeSH) #36 MACROLIDES explode all trees (MeSH) #37 antibiot* OR anti ADJ biot* OR antimicrobial* OR anti ADJ microbial* OR bacteriocid* OR antibacterial* OR anti ADJ bacterial* #38 penicillin* OR amoxicillin OR ampicillin OR clavulanic acid OR amoxiclav OR augmentin OR ticarcillin OR timentin OR flucloxacillin OR fluampicil OR magnapen OR piperacillin OR tazocin #39 cephalosporin* OR cefaclor OR distaclor OR cefadroxil OR baxan OR cefalexin OR ceporex OR keflex OR cefamandole OR kefadol OR cefazolin OR kefzol OR cefixime OR suprax OR cefotaxime OR claforan OR cefoxitin OR mefoxin OR cefpirome OR cefrom OR cefpodoxime OR orelox OR cefprozil OR cefzil OR cefradine OR velosel OR ceftazidime OR fortum OR kefadim OR ceftriaxone OR rocephin OR cefuroxime OR zinacef OR zinnat OR cefonicid OR aztreonam OR azactam OR imipenem OR cilastatin OR primaxin OR meropenem or meronem or tetracycline* or deteclo or demecleocyclin or ledermycin or doxycycline or vibramycin or minocycline or minocine or oxytetracycline or terramycin #40 macrolide* OR erythromycin OR erymax OR erythrocin OR erythroped OR azithromycin OR zithromax OR clarithromycin OR klaricid OR telithromycin OR ketek OR trimoxazole OR septrin OR trimethoprim OR monotrim OR trimopan OR metronidazole OR flagyl OR metrolyl #41 quinolone* OR ciprofloxacin OR ciproxin #42 #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 #43 #31 AND #42 | (("Tonsillectomy"[Mesh]) OR ("Palatine TOnsil/surgery"[Mesh]) OR (Search tonsillectom*[tiab] OR tonsilectom*[tiab] OR tonsillot*[tiab] OR adenotonsillectom*[tiab] OR adenotonsilectom*[tiab]) OR (("Tonsillitis"[Mesh] OR "Palatine TOnsil"[Mesh] OR tonsil*[tiab] OR peritonsil*[tiab] OR adenotonsil*[tiab]) AND (("Surgical Procedures, Operative"[Mesh]) OR (surg*[tiab] OR excis*[tiab] OR extract*[tiab] OR remov*[tiab]) OR ("Dissection"[Mesh] OR dissect*[tiab] OR electrodissect*[tiab] OR coblat*[tiab] OR ablat*[tiab] OR ultrasonic*[tiab] OR harmonic*[tiab] OR guillotin*[tiab] OR plasma[tiab] OR ultracis*[tiab]) OR ("Diathermy"[Mesh] OR "Cautery"[Mesh] OR "Ultrasonics"[Mesh] OR "Electrosurgery"[Mesh] OR "Radiosurgery"[Mesh] OR "Cryosurgery"[Mesh] OR "Laser Surgery"[Mesh] OR "lasers"[Mesh]) OR ((ion*[tiab] AND field*[tiab]) OR (bipolar[tiab] AND probe*[tiab]) OR (electr*[tiab] AND coagulat*[tiab]) OR eletrocoagulat*[tiab] OR (electr*[tiab] AND cauter*[tiab]) OR electrosurg*[tiab] OR (electr*[tiab] AND surg*[tiab]) OR bovie[tiab] OR elmed[tiab] OR somnoplasty[tiab]) OR (diatherm*[tiab] OR thermocauter*[tiab] OR thermocoagul*[tiab] OR galvanocaut*[tiab] OR radiosurg*[tiab] OR radiofrequenc*[tiab] OR cryosurg*[tiab])))) AND (("ANTI‐BACTERIAL AGENTS"[Mesh] OR "ANTIBIOTIC PROPHYLAXIS"[Mesh] OR "LACTAMS"[Mesh] OR "QUINOLONES"[Mesh] OR "MACROLIDES"[Mesh]) OR (antibiot*[tiab] OR (anti[tiab] AND biot*[tiab]) OR antimicrobial*[tiab] OR (anti[tiab] AND microbial*[tiab]) OR bacteriocid*[tiab] OR antibacterial*[tiab] OR (anti[tiab] AND bacterial*[tiab])) OR (penicillin*[tiab] OR amoxicillin[tiab] OR ampicillin[tiab] OR clavulanic acid[tiab] OR amoxiclav OR tiab OR augmentin[tiab] OR ticarcillin[tiab] OR timentin[tiab] OR flucloxacillin[tiab] OR fluampicil[tiab] OR magnapen[tiab] OR piperacillin[tiab] OR tazocin[tiab] OR cephalosporin*[tiab] OR cefaclor[tiab] OR distaclor[tiab] OR cefadroxil[tiab] OR baxan[tiab] OR cefalexin[tiab] OR ceporex[tiab] OR keflex[tiab] OR cefamandole[tiab] OR kefadol[tiab] OR cefazolin[tiab] OR kefzol[tiab] OR cefixime[tiab] OR suprax[tiab] OR cefotaxime[tiab] OR claforan[tiab] OR cefoxitin[tiab] OR mefoxin[tiab] OR cefpirome[tiab] OR cefrom[tiab] OR cefpodoxime[tiab] OR orelox[tiab] OR cefprozil[tiab] OR cefzil[tiab] OR cefradine[tiab] OR velosel[tiab] OR ceftazidime[tiab] OR fortum[tiab] OR kefadim[tiab] OR ceftriaxone[tiab] OR rocephin[tiab] OR cefuroxime[tiab] OR zinacef[tiab] OR zinnat[tiab] OR cefonicid[tiab] OR aztreonam[tiab] OR azactam[tiab] OR imipenem[tiab] OR cilastatin[tiab] OR primaxin[tiab] OR meropenem[tiab] OR meronem[tiab]) OR (tetracycline*[tiab] OR deteclo[tiab] OR demecleocyclin[tiab] OR ledermycin[tiab] OR doxycycline[tiab] OR vibramycin[tiab] OR minocycline[tiab] OR minocine[tiab] OR oxytetracycline[tiab] OR terramycin[tiab] OR macrolide*[tiab] OR erythromycin[tiab] OR erymax[tiab] OR erythrocin[tiab] OR erythroped[tiab] OR azithromycin[tiab] OR zithromax[tiab] OR clarithromycin[tiab] OR klaricid[tiab] OR telithromycin[tiab] OR ketek[tiab] OR trimoxazole[tiab] OR septrin[tiab] OR trimethoprim[tiab] OR monotrim[tiab] OR trimopan[tiab] OR metronidazole[tiab] OR flagyl[tiab] OR metrolyl[tiab] OR quinolone*[tiab] OR ciprofloxacin[tiab] OR ciproxin[tiab])) | 1. TONSILLECTOMY#.DE. 2. TONSIL‐DISEASE‐SU#.DE. 3. (tonsillectom$3 OR tonsilectom$3).TI,AB. 4. (adenotonsillectom$3 OR adenotonsilectom$3).TI,AB. 5. 1 OR 2 OR 3 OR 4 6. TONSILLITIS#.DE. 7. TONSIL#.DE. 8. (tonsil$3 OR peritonsil$3).TI,AB. 9. adenotonsil$3.TI,AB. 10. 6 OR 7 OR 8 OR 9 11. SURGERY#.DE. 12. (surg$6 OR excis$4 OR extract$4 OR remov$3).TI,AB. 13. SURGICAL‐TECHNIQUE#.DE. 14. (dissect$4 OR electrodissect$4 OR coblat$3 OR ablat$3 OR ultrasonic$4 OR ultracis$4 OR harmonic$4 OR guillotin$3 OR plasma).TI,AB. 15. (ion$1 NEAR field$1 OR bipolar NEAR probe$1).TI,AB. 16. SCALPEL.DE. 17. ULTRASOUND.DE. 18. (electr$6 NEAR coagula$4 OR electrocoagula$4 OR electrocauter$7 OR electro NEAR cauter$7).TI,AB. 19. (electrosurg$6 OR electr$6 NEAR surg$6 OR bovie OR elmed OR somnoplasty).TI,AB. 20. (diatherm$4 OR thermocauter$7 OR thermocoagula$4 OR galvanocauter$7).TI,AB. 21. (radiosurg$6 OR radiofrequenc$3 OR cryosurg$6).TI,AB. 22. laser$1 NEAR surg$6.TI,AB. 23. 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 24. 10 AND 23 25. 5 OR 24 26. ANTIBIOTIC‐AGENT#.DE. 27. (antibiot$3 OR anti ADJ biot$3 OR antimicrobial$2 OR anti ADJ microbial$2 OR bacteriocid$2).TI,AB. 28. (antibacterial$2 OR anti ADJ bacterial$2 OR antimycobacterial$2 OR anti ADJ mycobacterial$2).TI,AB. 29. penicillin$1 OR amoxicillin OR ampicillin OR clavulanic ADJ acid OR amoxiclav OR augmentin OR ticarcillin OR timentin OR flucloxacillin OR fluampicil OR magnapen OR piperacillin OR tazocin OR sulfisoxazole 30. cephalosporin$1 OR cefaclor OR distaclor OR cefadroxil OR baxan OR cefalexin OR cepororex OR keflex OR cefamandole OR kefadol OR cefazolin OR kefzol OR cefixime OR suprax OR cefotaxime OR claforan OR cefoxitin OR mefoxin 31. cefpirome OR cefrom OR cefpodoxime OR ORelox OR cefprozil OR cefzil OR cefradine OR velosel OR ceftazidime OR fortum OR kefadim OR ceftriaxone OR rocephin OR cefuroxime OR zinacef OR zinnat OR cefonicid OR aztreonam 32. azactam OR imipenem OR cilastatin OR primaxin OR meropenem OR meronem OR tetracycline$1 OR deteclo OR demecleocyclin OR ledermycin OR doxycycline OR vibramycin OR minocycline OR minocine OR oxytetracycline OR terramycin 33. macrolide$1 OR erythromycin OR erymax OR erythrocin OR erythroped OR azithromycin OR zithromax OR clarithromycin OR klaricid OR telithromycin OR ketek OR trimoxazole OR septrin OR trimethoprim OR monotrim OR trimopan OR metronidazole OR flagyl OR metrolyl OR quinolone$1 OR ciprofloxacin OR ciproxin 34. 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 35. 25 AND 34 | |
| BIOSIS Previews | CAB Abstracts | CINAHL | |
| 1 exp tonsillectomy/ 2 [exp tonsil disease/su [Surgery]] 3 (tonsillectom* or tonsilectom* or tonsillot* or adenotonsillectom* or adenotonsilectom*).tw. 4 exp tonsillitis/ or exp tonsil/ 5 (tonsil* or peritonsil* or adenotonsil*).tw. 6 4 or 5 7 exp SURGERY/ or exp surgical technique/ or scalpel/ or ultrasound/ 8 (surg* or excis* or extract* or remov*).tw. 9 (dissect* or electrodissect* or coblat* or ablat* or ultrasonic* or harmonic* or guillotin* or plasma or ultracis* or (ion* and field*) or (bipolar and probe*) or (electr* and coagulat*) or eletrocoagulat* or (electr* and cauter*) or electrosurg* or (electr* and surg*) or bovie or elmed or somnoplasty or diatherm* or thermocauter* or thermocoagul* or galvanocaut* or radiosurg* or radiofrequenc* or cryosurg*).tw. 10 8 or 7 or 9 11 6 and 10 12 11 or 1 or 3 or 2 13 exp ANTIBIOTIC AGENT/ 14 (antibiot* or (anti and biot*) or antimicrobial* or (anti and microbial*) or bacteriocid* or antibacterial* or (anti and bacterial*)).tw. 15 (penicillin* or amoxicillin or ampicillin or clavulanic acid or amoxiclav or augmentin or ticarcillin or timentin or flucloxacillin or fluampicil or magnapen or piperacillin or tazocin or cephalosporin* or cefaclor or distaclor or cefadroxil or baxan or cefalexin or ceporex or keflex or cefamandole or kefadol or cefazolin or kefzol or cefixime or suprax or cefotaxime or claforan or cefoxitin or mefoxin or cefpirome or cefrom or cefpodoxime or orelox or cefprozil or cefzil or cefradine or velosel or ceftazidime or fortum or kefadim or ceftriaxone or rocephin or cefuroxime or zinacef or zinnat or cefonicid or aztreonam or azactam or imipenem or cilastatin or primaxin or meropenem or meronem).tw. 16 (tetracycline* or deteclo or demecleocyclin or ledermycin or doxycycline or vibramycin or minocycline or minocine or oxytetracycline or terramycin or macrolide* or erythromycin or erymax or erythrocin or erythroped or azithromycin or zithromax or clarithromycin or klaricid or telithromycin or ketek or trimoxazole or septrin or trimethoprim or monotrim or trimopan or metronidazole or flagyl or metrolyl or quinolone* or ciprofloxacin or ciproxin).tw. 17 16 or 13 or 15 or 14 18 17 and 12 | 1 exp tonsillectomy/ 2 [exp tonsil disease/su [Surgery]] 3 (tonsillectom* or tonsilectom* or tonsillot* or adenotonsillectom* or adenotonsilectom*).tw. 4 exp tonsillitis/ or exp tonsil/ 5 (tonsil* or peritonsil* or adenotonsil*).tw. 6 4 or 5 7 exp SURGERY/ or exp surgical technique/ or scalpel/ or ultrasound/ 8 (surg* or excis* or extract* or remov*).tw. 9 (dissect* or electrodissect* or coblat* or ablat* or ultrasonic* or harmonic* or guillotin* or plasma or ultracis* or (ion* and field*) or (bipolar and probe*) or (electr* and coagulat*) or eletrocoagulat* or (electr* and cauter*) or electrosurg* or (electr* and surg*) or bovie or elmed or somnoplasty or diatherm* or thermocauter* or thermocoagul* or galvanocaut* or radiosurg* or radiofrequenc* or cryosurg*).tw. 10 8 or 7 or 9 11 6 and 10 12 11 or 1 or 3 or 2 13 exp ANTIBIOTIC AGENT/ 14 (antibiot* or (anti and biot*) or antimicrobial* or (anti and microbial*) or bacteriocid* or antibacterial* or (anti and bacterial*)).tw. 15 (penicillin* or amoxicillin or ampicillin or clavulanic acid or amoxiclav or augmentin or ticarcillin or timentin or flucloxacillin or fluampicil or magnapen or piperacillin or tazocin or cephalosporin* or cefaclor or distaclor or cefadroxil or baxan or cefalexin or ceporex or keflex or cefamandole or kefadol or cefazolin or kefzol or cefixime or suprax or cefotaxime or claforan or cefoxitin or mefoxin or cefpirome or cefrom or cefpodoxime or orelox or cefprozil or cefzil or cefradine or velosel or ceftazidime or fortum or kefadim or ceftriaxone or rocephin or cefuroxime or zinacef or zinnat or cefonicid or aztreonam or azactam or imipenem or cilastatin or primaxin or meropenem or meronem).tw. 16 (tetracycline* or deteclo or demecleocyclin or ledermycin or doxycycline or vibramycin or minocycline or minocine or oxytetracycline or terramycin or macrolide* or erythromycin or erymax or erythrocin or erythroped or azithromycin or zithromax or clarithromycin or klaricid or telithromycin or ketek or trimoxazole or septrin or trimethoprim or monotrim or trimopan or metronidazole or flagyl or metrolyl or quinolone* or ciprofloxacin or ciproxin).tw. 17 16 or 13 or 15 or 14 18 17 and 12 | S1 (MH "Tonsillectomy") S2 TX tonsillectom* or tonsilectom* or tonsillot* or adenotonsillectom* or adenotonsilectom* S3 (MH "Tonsil") or (MH "Tonsillitis S4 TX tonsil* or peritonsil* or adenotonsil* S5 (MH "Surgery, Operative") S6 TX surg* or excis* or extract* or remov* S7 TX dissect* or electrodissect* or coblat* or ablat* or ultrasonic* or harmonic* or guillotin* or plasma or ultracis* or (ion* and field*) or (bipolar and probe*) or (electr* and coagulat*) or eletrocoagulat* or (electr* and cauter*) or electrosurg* or (electr* and surg*) or bovie or elmed or somnoplasty or diatherm* or thermocauter* or thermocoagul* or galvanocaut* or radiosurg* or radiofrequenc* or cryosurg* S8 S3 or S4 S9 S5 or S6 or S7 S10 S8 and S9 S11 S1 or S2 or S10 S12 (MH "Antibiotics") S13 (MH "Antibiotic Prophylaxis") S14 antibiot* or (anti and biot*) or antimicrobial* or (anti and microbial*) or bacteriocid* or antibacterial* or (anti and bacterial*) S15 TX penicillin* or amoxicillin or ampicillin or clavulanic acid or amoxiclav or augmentin or ticarcillin or timentin or flucloxacillin or fluampicil or magnapen or piperacillin or tazocin or cephalosporin* or cefaclor or distaclor or cefadroxil or baxan or cefalexin or ceporex or keflex or cefamandole or kefadol or cefazolin or kefzol or cefixime or suprax or cefotaxime or claforan or cefoxitin or mefoxin or cefpirome or cefrom or cefpodoxime or orelox or cefprozil or cefzil or cefradine or velosel or ceftazidime or fortum or kefadim or ceftriaxone or rocephin or cefuroxime or zinacef or zinnat or cefonicid or aztreonam or azactam or imipenem or cilastatin or primaxin or meropenem or meronemTX penicillin* or amoxicillin or ampicillin or clavulanic acid or amoxiclav or augmentin or ticarcillin or timentin or flucloxacillin or fluampicil or magnapen or piperacillin or tazocin or cephalosporin* or cefaclor or distaclor or cefadroxil or baxan or cefalexin or ceporex or keflex or cefamandole or kefadol or cefazolin or kefzol or cefixime or suprax or cefotaxime or claforan or cefoxitin or mefoxin or cefpirome or cefrom or cefpodoxime or orelox or cefprozil or cefzil or cefradine or velos ... S16 TX tetracycline* or deteclo or demecleocyclin or ledermycin or doxycycline or vibramycin or minocycline or minocine or oxytetracycline or terramycin or macrolide* or erythromycin or erymax or erythrocin or erythroped or azithromycin or zithromax or clarithromycin or klaricid or telithromycin or ketek or trimoxazole or septrin or trimethoprim or monotrim or trimopan or metronidazole or flagyl or metrolyl or quinolone* or ciprofloxacin or ciproxin S17 S12 or S13 or S14 or S15 or S16 S18 S11 and S17 | |
| Web of Science | mRCT | ClinicalTrials.gov | |
| # 1 TS=(tonsillectom* or tonsilectom* or tonsillot* or adenotonsillectom* or adenotonsilectom*) # 2 TS=(tonsil* or peritonsil* or adenotonsil*) # 3 TS=(surg* or excis* or extract* or remov*) # 4 TS=(dissect* or electrodissect* or coblat* or ablat* or ultrasonic* or harmonic* or guillotin* or plasma or ultracis* or (ion* and field*) or (bipolar and probe*) or (electr* and coagulat*) or eletrocoagulat* or (electr* and cauter*) or electrosurg* or (electr* and surg*) or bovie or elmed or somnoplasty or diatherm* or thermocauter* or thermocoagul* or galvanocaut* or radiosurg* or radiofrequenc* or cryosurg*) # 5 #4 OR #3 # 6 #5 AND #2 # 7 #6 OR #1 # 8 TS=(antibiot* or (anti and biot*) or antimicrobial* or (anti and microbial*) or bacteriocid* or antibacterial* or (anti and bacterial*)) # 9 TS=(penicillin* or amoxicillin or ampicillin or clavulanic acid or amoxiclav or augmentin or ticarcillin or timentin or flucloxacillin or fluampicil or magnapen or piperacillin or tazocin or cephalosporin*) # 10 TS=(cefaclor or distaclor or cefadroxil or baxan or cefalexin or ceporex or keflex or cefamandole or kefadol or cefazolin or kefzol or cefixime or suprax or cefotaxime or claforan or cefoxitin or mefoxin or cefpirome or cefrom or cefpodoxime or orelox or cefprozil or cefzil or cefradine or velosel or ceftazidime or fortum or kefadim or ceftriaxone or rocephin or cefuroxime or zinacef or zinnat or cefonicid or aztreonam or azactam or imipenem or cilastatin or primaxin or meropenem or meronem) # 11 TS=(tetracycline* or deteclo or demecleocyclin or ledermycin or doxycycline or vibramycin or minocycline or minocine or oxytetracycline or terramycin or macrolide* or erythromycin or erymax or erythrocin or erythroped or azithromycin or zithromax or clarithromycin or klaricid or telithromycin or ketek or trimoxazole or septrin or trimethoprim or monotrim or trimopan or metronidazole or flagyl or metrolyl or quinolone* or ciprofloxacin or ciproxin) # 12 #11 OR #10 OR #9 OR #8 # 13 #12 AND #7 | ((tonsillectom% OR tonsilectom% OR adenotonsillectom% OR adenotonsilectom%) AND (antibiot% OR antimicrob% OR antibact% OR “anti bacterial” OR “anti microbial%” penicillin% OR amoxicillin OR ciprofloxacin)) | ((tonsillectomy OR tonsilectomy OR adenotonsillectomy) AND (antibiotic OR antimicrobial OR antibacterial OR penicillin OR amoxicillin OR ciprofloxacin)) |
Data and analyses
Comparison 1. Total secondary haemorrhage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with total secondary haemorrhage | 7 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.56, 1.44] |
Comparison 2. Significant secondary haemorrhage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with significant secondary haemorrhage | 7 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.08, 3.11] |
Comparison 3. Fever.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with fever | 2 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.46, 0.85] |
Comparison 4. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with adverse events or side effects | 4 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.68, 6.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cannon 1996.
| Methods | Randomised, double‐blind, placebo‐controlled study conducted in USA; number of centres involved in study unclear Grading of quality: Randomisation = C Blinding = A Intention‐to‐treat analysis = C Follow‐up = A Overall quality grading = C |
|
| Participants | 50 children and adults (age range 13 to 40 years) undergoing tonsillectomy primarily for recurrent tonsillitis. Study and control groups were well matched in terms of age, sex and number of episodes of tonsillitis prior to surgery. Exclusion criteria: antibiotic administered within 1 week preoperatively, medical condition requiring perioperative antibiotic therapy, or allergy to antibiotic studied |
|
| Interventions | Cefonicid, IV 1 g before initiation of surgery | |
| Outcomes | Primary outcome: consumption of analgesics (number of doses of Tylenol with codeine) Secondary outcomes: fever (number of patients with fever, defined as temperature > 99 °F) and number of days required to resume soft diet and activities Period of observation: 7 days |
|
| Notes | Anaesthetic technique was not mentioned as controlled. Surgical technique was controlled, with all patients undergoing dissection and snare of the tonsils with electrocautery for haemostasis. Follow‐up: 46 (92%) patients (24 in antibiotic and 22 in control groups) completed follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Colreavy 1999.
| Methods | Single‐centre, randomised controlled trial conducted in Ireland Grading of quality: Randomisation = C Blinding = D Intention‐to‐treat analysis = C Follow‐up = D Overall quality grading = C |
|
| Participants | 78 children (2 to 12 years, mean 6.2 years) undergoing tonsillectomy with or without other lesser surgical procedures (indication not specified). Study and control groups well matched in terms of age and sex. Exclusion criteria: antibiotic administered within 1 week preoperatively, medical condition requiring perioperative antibiotic therapy, or allergy to antibiotic studied |
|
| Interventions | 1ne week of oral amoxicillin + clavulanic acid, dosage according to the British National Formulary (1996 edition) | |
| Outcomes | Primary outcomes: pain (visual analogue score (0 = little or no pain, 10 = unbearable pain)) and analgesic consumption Secondary outcomes: number of days to resume normal diet Period of observation: 7 days |
|
| Notes | Anaesthetic technique mentioned as controlled, but no details given. Similarly, surgical technique was controlled, with all patients undergoing bipolar diathermy extracapsular dissection and haemostasis with bismuth subgallate and bipolar diathermy. Follow‐up: 54 (69%) patients completed follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Grandis 1992.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled study conducted in USA Grading of quality: Randomisation = C Blinding = A Intention‐to‐treat analysis =C Follow‐up = D Overall quality grading = C |
|
| Participants | 198 adults and children aged 12 to 48 (mean 21.7) years undergoing tonsillectomy or adenotonsillectomy (indication not specified). Study and control groups were well matched with regard to age, sex and adenoidectomy (18 versus 14 respectively). Exclusion criteria: antibiotic administered within 1 week preoperatively, medical condition requiring perioperative antibiotic therapy, or allergy to antibiotic studied |
|
| Interventions | Ticarcillin + clavulanic acid, IV 3.1 g at completion of surgery, 6 and 12 hours after surgery; followed by amoxicillin + clavulanic acid 250 mg tds oral for 7 days | |
| Outcomes | Primary outcomes: pain (scale of 1 to 10, 10 being most severe) and consumption of analgesics Secondary outcomes: fever (temperature > 99.9 °F) and return to regular diet (scale of 1 to 3; 1 = regular, 2 = soft, 3 = liquid) and activities (1 = normal, 2 = moderate, 3 = bed rest) Period of observation: 7 days |
|
| Notes | Anaesthetic and surgical techniques not mentioned as being controlled Follow‐up: only 101 (51%) patients (51 in antibiotic and 50 in control groups) completed follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Guerra 2008.
| Methods | Randomised, controlled, single‐centre study conducted in Brazil Grading of quality: Randomisation = C Blinding = C Intention‐to‐treat analysis = B Follow‐up = D Overall quality grading = C |
|
| Participants | 120 children aged 14 years or less undergoing adenotonsillectomy. Study and control groups were well matched in terms of age and sex. | |
| Interventions | Postoperative amoxicillin 50 mg/kg/day for 7 days | |
| Outcomes | Primary outcomes: pain, analgesic consumption and secondary haemorrhage Secondary outcomes: fever and time taken to resume normal diet and activities Period of observation: 7 days |
|
| Notes | Exclusion criteria: allergy to amoxicillin or haematological disorder. Surgical technique was controlled: all patients underwent dissection, with no use of electrocautery, anaesthetic technique not mentioned as controlled. Follow‐up: 95 (%) patients (43 in the antibiotic group and 52 in the control group) completed follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Khan 1994.
| Methods | Single‐centre, randomised controlled trial conducted in UK Grading of quality: Randomisation = C Blinding = D Intention‐to‐treat analysis = C Follow‐up = B Overall quality grading = C |
|
| Participants | 90 children and adults, age range 6 to 36 years, undergoing tonsillectomy for recurrent tonsillitis. Study and control groups well matched with regard to age, sex, episodes of tonsillitis within previous 6 months and history of quinsy. Exclusion criteria: poor general medical condition, history of adverse drug reactions including penicillin allergy or presence of concomitant ear nose throat pathology |
|
| Interventions | One IV dose of amoxicillin (appropriate to age and body weight) at the time of induction, and 2 further oral postoperative doses at 6 and 12 hours (age 6 to 10 years: 125 mg; 10 to 16: 250 mg; over 16: 500 mg) | |
| Outcomes | Primary outcomes: pain (number of days until no sore throat and otalgia), analgesia (number of days until no analgesia) and haemorrhage Secondary outcomes: time taken to resume normal activities and intake Period of observation: 14 days | |
| Notes | Anaesthetic technique not mentioned as controlled. Surgical technique was controlled, with all patients undergoing dissection with ties for haemostasis. Follow‐up: 80 (89%) patients (40 each in antibiotic and control groups) completed follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Linden 1990.
| Methods | Randomised controlled trial conducted in USA, number of centres unclear; patients initially divided into 4 groups based on surgical technique, and then further subdivided based on whether received antibiotics or not, to attain a total of 8 subgroups; study remained open until sufficient numbers were recruited and questionnaires returned Grading of quality: Randomisation = C Blinding = D Intention‐to‐treat analysis = C Follow‐up = C Overall quality grading = C |
|
| Participants | 80 children (age range 13 months to 17 years (mean: 5 years)) undergoing tonsillectomy (indication not specified). Whether children undergoing adenotonsillectomy were included was not clear. Study and control group demographics and other characters not mentioned. Exclusion criteria: not specified |
|
| Interventions | No detail regarding the type of antibiotic or the method of administration given | |
| Outcomes | Primary outcome: analgesic consumption (mean subjective rating on a scale of 1 to 3) Period of observation: 5 days |
|
| Notes | Anaesthetic technique not mentioned as controlled. Surgical technique was quasi‐controlled: 4 different surgical and haemostatic techniques in equal numbers (electrocautery + electrocautery, dissection + electrocautery, dissection + ligature, laser + laser) were used between the study and control groups. Follow‐up: follow‐up rate of the 40 patients recruited in each of the antibiotic and control groups unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mann 1999.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled study conducted in USA Grading of quality: Randomisation = C Blinding = A (author correspondence confirmed dispensation of placebo as a capsule, but could not confirm if it was exactly identical to amoxicillin capsule; we believe this was adequate double‐blinding) Intention‐to‐treat analysis = C Follow‐up = D Overall quality grading = C |
|
| Participants | 51 adults 18 years and above undergoing tonsillectomy for tonsillitis, peritonsillar abscess or tonsillithiasis were enrolled and randomised into 4 arms: systemic antibiotic, placebo and 2 different topical antibiotics. The 2 arms that studied topical antibiotics were unsuitable for analysis and therefore excluded, leaving 18 patients in the first 2 arms (8 in antibiotic and 10 in control arms) who completed the study. Study and control groups well‐matched with regard to age and sex. Exclusion criteria: significant medical conditions (i.e. diabetes, chronic lung disease, bleeding disorders), antibiotic administered within 1 week preoperatively, medical condition requiring perioperative antibiotic therapy, or allergy to antibiotic studied |
|
| Interventions | Amoxicillin tds oral for 7 days | |
| Outcomes | Primary outcome: pain (scale 1 to 100; 0 = no pain, 100 = severe pain) Period of observation: 5 days |
|
| Notes | Anaesthetic technique not mentioned as controlled. Surgical technique was quasi‐controlled: the tonsillectomy technique is not mentioned, and instead only the haemostatic method (electrocautery) is mentioned. Follow‐up: 36 (71%) of 51 patients in the whole study completed follow‐up. Authors stated that the numbers lost to follow‐up were evenly distributed in the 4 arms, but exact follow‐up rates in the systemic antibiotic and placebo arms not available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
O'Reilly 2003.
| Methods | Randomised, double‐blind, placebo‐controlled study conducted in UK; number of centres involved in study unclear Grading of quality: Randomisation = A (author correspondence confirmed adequacy of the randomisation process) Blinding = A Intention‐to‐treat analysis = A Follow‐up = D Overall quality grading = C |
|
| Participants | 200 adults aged 16 to 53 years undergoing tonsillectomy for non‐malignant disease. Study and control groups had similar age distribution and sex ratios. Exclusion criteria: not described |
|
| Interventions | Amoxicillin IV 250 mg at induction, followed by 250 mg tds oral for 7 days | |
| Outcomes | Primary outcomes: pain (scale of 1 to 5), additional analgesic consumption and haemorrhage Period of observation: 10 days |
|
| Notes | Anaesthetic technique not mentioned as controlled. Surgical technique was quasi‐controlled, i.e. mostly electro‐dissection for tonsillectomy was used. Follow‐up: only 95 (48%) of 200 patients (46 in antibiotic and 49 in placebo groups) completed follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ramos 2000.
| Methods | Single‐centre, randomised controlled, physician‐blinded trial conducted in Brazil Grading of quality: Randomisation = C Blinding = B Intention‐to‐treat analysis = C Follow‐up = C Overall quality grading = C |
|
| Participants | 58 children (age range not given) undergoing tonsillectomy (indication not specified) randomised to 29 each in the antibiotic and control groups. Study and control groups well matched with regard to age. Exclusion criteria: not specified |
|
| Interventions | Amoxicillin + clavulanic acid during and after the operative period, with dosage calculated according to weight, for 7 days. Route of administration in the perioperative period not specified. | |
| Outcomes | Primary outcomes: pain (scale of 0 to 3; 0 = no, 1 = mild, 2 = moderate and 3 = intense) Secondary outcomes: fever (subjective intensity on a scale of 0 to 3) Period of observation: unclear |
|
| Notes | Anaesthetic technique mentioned as controlled, but no details given. Surgical technique also appears to have been controlled ‐ the authors mention Sluder's technique as being employed in all children, but no other details are given. All surgeries were performed by a single surgeon. Follow‐up: details not given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Telian 1986.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled study conducted in USA Grading of quality: Randomisation = C Blinding = A Intention‐to‐treat analysis = A Follow‐up = A Overall quality grading = B |
|
| Participants | 100 children (age range not reported) undergoing tonsillectomy or adenotonsillectomy. The number of children who underwent adenoidectomy was not specified. Most underwent surgery for obstructive sleep apnoea. Study and control groups were well matched with regard to age, sex, number of infections in the past 12 months and indications for surgery. Exclusion criteria: antibiotic administered within 1 week preoperatively, medical condition requiring perioperative antibiotic therapy, or allergy to antibiotic studied |
|
| Interventions | At completion of surgery, amoxicillin IV 1 g for children weighing >= 20 kg and 500 mg for children weighing < 20 kg was administered; this was followed by equivalent doses at 6‐hour intervals until discharge (usually 24 hours). After discharge oral amoxicillin was given tds for 7 days, at 250 mg tds in children weighing >= 20 kg and 125 mg for children weighing < 20 kg. | |
| Outcomes | Primary outcomes: pain (number of days with continuous subjective pain) Secondary outcomes: fever (temperature > 99.9 °F), time taken to resume soft or usual diet and activities Period of observation: 7 to 14 days |
|
| Notes | Anaesthetic technique not mentioned as controlled. Surgical technique was controlled, with all patients undergoing dissection and snare of the tonsils with electrocautery for haemostasis. Follow‐up: all patients completed follow‐up and were available for analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
IV = intravenous; tds = three times a day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akbas 2004 | ALLOCATION:
Randomised PARTICIPANTS: 60 children aged between 4 and 14 years undergoing tonsillectomy or adenotonsillectomy INTERVENTION: Compared systemic with topical antibiotic |
| Al‐Tamimi 2000 | ALLOCATION: Non‐randomised |
| Aslam 1998 | ALLOCATION: Study described as randomised, but alternate allocation was used |
| Browning 1995 | Study not completed (registered in National Research Register (UK) in 1995) |
| Dawar 2011 | ALLOCATION: Non‐randomised |
| Inci 2009 | ALLOCATION:
Randomised PARTICIPANTS: 78 patients undergoing tonsillectomy for recurrent tonsillitis INTERVENTION: Compared systemic with topical antibiotic regimes |
| Lackmann 1992 | ALLOCATION: Non‐randomised |
| Lee 1996 | ALLOCATION: Non‐randomised |
| Minet 1978 | ALLOCATION: Non‐randomised |
| Miura 2009 | ALLOCATION:
Randomised PARTICIPANTS: 82 children aged between four and 12 years of age undergoing adenotonsillectomy INTERVENTION: Assessed the efficacy of a topical antibiotic |
| Novais 2003 | Unable to obtain full paper |
| Szmeja 1997 | ALLOCATION: Non‐randomised; included patients undergoing otolaryngological procedures other than tonsillectomy |
| Udaipurwala 2002 | ALLOCATION: Non‐randomised |
| Udaipurwala 2004 | Unable to obtain full paper |
| Zagolski 2012 | ALLOCATION:
Randomised PARTICIPANTS: 124 children aged 5 to 7 years with obstructive symptoms INTERVENTION: Tonsillotomy with incision of the tonsil was performed instead of tonsillectomy |
Characteristics of ongoing studies [ordered by study ID]
Khalil 2004.
| Trial name or title | Peri‐operative antibiotics in tonsillectomy patients |
| Methods | Randomised, placebo‐controlled, parallel‐group trial |
| Participants | Adult patients listed for tonsillectomy |
| Interventions | Group 1: to receive 3 doses of peri‐ and postoperative cefuroxime Group 2: to receive normal saline in a similar fashion |
| Outcomes | Visual analogue scale for pain at 24 hours, 1 week and 2 weeks Analgesia requirements at the end of 2 weeks |
| Starting date | 1 March 2004 |
| Contact information | Mr H Khalil, ENT Department, North Bristol NHS Trust, Southmead Hospital, Bristol, BS10 5NB |
| Notes | ISRCTN52345875 |
Contributions of authors
MD: drafting the protocol, searching for studies, selecting studies, quality assessment, data extraction, data analysis. AC: selecting studies, quality assessment, data extraction. MS: searching for studies, selecting studies, data analysis. WM: drafting the protocol, selecting studies, quality assessment, data analysis.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cannon 1996 {published data only}
- Cannon CR. The efficacy of a single dose antibiotic regimen in adults undergoing tonsillectomy. Journal of the Mississippi State Medical Association 1996;37(11):817‐21. [PubMed] [Google Scholar]
Colreavy 1999 {published data only}
- Colreavy MP, Nanan D, Benamer M, Donnelly M, Blaney AW, O'Dwyer TP, et al. Antibiotic prophylaxis post‐tonsillectomy: is it of benefit?. International Journal of Pediatric Otorhinolaryngology 1999;50(1):15‐22. [DOI] [PubMed] [Google Scholar]
Grandis 1992 {published data only}
- Grandis JR, Johnson JT, Vickers RM, Yu VL, Wagener MM, Wagner RL, et al. The efficacy of perioperative antibiotic therapy on recovery following tonsillectomy in adults: randomized double‐blind placebo‐controlled trial. Otolaryngology ‐ Head and Neck Surgery 1992;106(2):137‐42. [DOI] [PubMed] [Google Scholar]
Guerra 2008 {published data only}
- Guerra MM, Garcia E, Pilan RR, Rapoport PB, Campanholo CB, Martinelli EO. Antibiotic use in post‐adenotonsillectomy morbidity: a randomized prospective study. Brazilian Journal of Otorhinolaryngology 2008;74(3):337‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 1994 {published data only}
- Khan MHZ, McCombie AW, Swift AC. Prophylactic antibiotic for tonsillectomy?. Pakistan Journal of Otolaryngology 1994;10:122‐6. [Google Scholar]
Linden 1990 {published data only}
- Linden BE, Gross CW, Long TE, Lazar RH. Morbidity in pediatric tonsillectomy. Laryngoscope 1990;100(2 Pt 1):120‐4. [DOI] [PubMed] [Google Scholar]
Mann 1999 {published data only}
- Mann EA, Blair EA, Levy AJ, Chang A. Effect of topical antibiotic therapy on recovery after tonsillectomy in adults. Otolaryngology ‐ Head and Neck Surgery 1999;121(3):277‐82. [DOI] [PubMed] [Google Scholar]
O'Reilly 2003 {published data only}
- O'Reilly BJ, Black S, Fernandes J, Panesar J. Is the routine use of antibiotics justified in adult tonsillectomy?. Journal of Laryngology and Otology 2003;117(5):382‐5. [DOI] [PubMed] [Google Scholar]
Ramos 2000 {published data only}
- Ramos CC, Goncalves MER, Rapoport PB. Prophylactic antibiotic therapy after tonsillectomy. Study with amoxicillin‐clavulanic acid. Revista Brasileira de Otorrinolaringologia 2000;66(6):627‐30. [Google Scholar]
Telian 1986 {published data only}
- Telian SA, Handler SD, Fleisher GR, Baranak CC, Wetmore RF, Potsic WP. The effect of antibiotic therapy on recovery after tonsillectomy in children. A controlled study. Archives of Otolaryngology ‐ Head and Neck Surgery 1986;112(6):610‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akbas 2004 {published data only}
- Akbas Y, Pata YS, Unal M, Gorur K, Micozkadioglu D. The effect of fusafungine on post‐operative pain and wound healing after pediatric tonsillectomy. International Journal of Pediatric Otorhinolaryngology 2004;68(8):1023‐6. [DOI] [PubMed] [Google Scholar]
Al‐Tamimi 2000 {published data only}
- Al‐Tamimi SF. Should antibiotics be mandatory in perioperative tonsillectomy and/or adenotonsillectomy?. Journal of the College of Physicians and Surgeons Pakistan 2000;10(6):207‐8. [Google Scholar]
Aslam 1998 {published data only}
- Aslam M, Mustafa G. Prophylactic antibiotics for tonsillectomy: experience at Rawalpindi General Hospital. Journal of the College of Physicians and Surgeons Pakistan 1998;8(1):36‐8. [Google Scholar]
Browning 1995 {published data only}
- Browning G. Randomised control trial of antibiotic cover of adult tonsillectomy to reduce post‐operative morbidity. National Research Register 1995. [N0394025087]
Dawar 2011 {published data only}
- Dawar A, Khan AR. Tonsillectomy without the use of post operative antibiotics. Journal of Medical Sciences 2011;19:54‐6. [Google Scholar]
Inci 2009 {published data only}
- Inci N, Basut O, Kasapoglu F, Coskun H. Management of pain after tonsillectomy: a prospective, randomized clinical study. Kulak Burun Bogaz Ihtis Derg 2009;19(1):1‐8. [PubMed] [Google Scholar]
Lackmann 1992 {published data only}
- Lackmann GM, Buttner AM, Tollner U, Draf W. Indication, preoperative diagnostic process and antibiotic prophylaxis in (adeno)‐tonsillectomies in children. Padiatrische Praxis 1992;44:107‐14. [Google Scholar]
Lee 1996 {published data only}
- Lee WC, Duignan MC, Walsh RM, McRae‐Moore JR. An audit of prophylactic antibiotic treatment following tonsillectomy in children. Journal of Laryngology and Otology 1996;110(4):357‐9. [DOI] [PubMed] [Google Scholar]
Minet 1978 {published data only}
- Minet JC, Delire M, Clotuche J, Monnoye JP. Bacteremia and tonsillectomy. Acta Otorhinolaryngologica Belgica 1978;32(3):211‐7. [PubMed] [Google Scholar]
Miura 2009 {published data only}
- Miura MS, Saleh C, Andrade M, Assmann M, Lima LH, Lubianca Neto JF. Topical clindamycin in post‐adenotonsillectomy analgesia in children: a double‐blind, randomized clinical trial. Otolaryngology ‐ Head and Neck Surgery 2009;141:509‐15. [DOI] [PubMed] [Google Scholar]
Novais 2003 {published data only}
- Novais LA, Sallum AC, Garcia RID, Cecatto SB, Costa KS, Rapoport PB. Efficacy of antibiotic therapy after tonsillectomy [Eficácia da antibioticoterapia após tonsilectomia]. Arquivos Médicos do ABC 2003;28(1):37‐40. [Google Scholar]
Szmeja 1997 {published data only}
- Szmeja Z, Golusinski W, Kruk‐Zagajewska A, Szyfter W, Wierzbicka M, Leszczynska M, et al. The evaluation of ceftibuten (Cedax) effectiveness in the prevention of perioperative infections. Otolaryngologia Polska 1997;51(4):390‐5. [PubMed] [Google Scholar]
Udaipurwala 2002 {published data only}
- Udaipurwala IH. Role of prophylactic antibiotic in reducing post‐tonsillectomy morbidity. Pakistan Journal of Otolaryngology 2002;18(1):6‐8. [Google Scholar]
Udaipurwala 2004 {published data only}
- Udaipurwala IH, Soomro MS. Use of antibiotic in tonsillectomy: is it really beneficial?. Medical Channel 2004;10(3):17‐20. [Google Scholar]
Zagolski 2012 {published data only}
- Zagolski O, Kulisiewicz J. Perioperative antibiotic in adenoidectomy with partial tonsillectomy: a randomized trial. ORL; Journal of Oto‐Rhino‐Laryngology and Its Related Specialties 2012;74(2):86‐92. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Khalil 2004 {published data only}
- Peri‐operative antibiotics in tonsillectomy patients. http://www.controlled‐trials.com/ISRCTN52345875. [ISRCTN52345875]
Additional references
Adkinson 1998
- Adkinson NF. Drug allergy. In: Middleton E, Reed CE, Ellis EF, Adkinson FA, Yunginger JW, Busse WW editor(s). Allergy: Principles and Practice. 5th Edition. St. Louis: CV Mosby, 1998:1212‐24. [Google Scholar]
D'Eredita 2004
- D'Eredita R, Marsh RR. Contact diode laser tonsillectomy in children. Otolaryngology ‐ Head and Neck Surgery 2004;131(5):732‐5. [DOI] [PubMed] [Google Scholar]
Dhiwakar 2005
- Dhiwakar M, Brown PM. Are adjuvant therapies for tonsillectomy evidence based?. Journal of Laryngology and Otology 2005;119(8):614‐9. [DOI] [PubMed] [Google Scholar]
Evans 2003
- Evans AS, Khan AM, Young D, Adamson R. Assessment of secondary haemorrhage rates following adult tonsillectomy ‐ a telephone survey and literature review. Clinical Otolaryngology and Allied Sciences 2003;28(6):489‐91. [DOI] [PubMed] [Google Scholar]
Green 1977
- Green GR, Rosenblum AH, Sweet LC. Evaluation of penicillin hypersensitivity: value of clinical history and skin testing with penicilloyl‐polylysine and penicillin G. A cooperative prospective study of the penicillin study group of the American Academy of Allergy. Journal of Allergy and Clinical Immunology 1977;60:339‐45. [DOI] [PubMed] [Google Scholar]
Greenberger 2002
- Greenberger PA. Drug allergy, part B: allergic reactions to individual drugs: low molecular weight. In: Grammer LC, Greenberger PA editor(s). Patterson's Allergic Diseases. 6th Edition. Philadelphia: Lippincott, Williams & Wilkins, 2002:335‐59. [Google Scholar]
Gruchalla 2000
- Gruchalla R. Understanding drug allergies. Journal of Allergy and Clinical Immunology 2000;105(6 (Suppl 1)):637‐44. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
- Hollis LJ, Burton MJ, Millar JM. Perioperative local anaesthesia for reducing pain following tonsillectomy. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001874] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 2003
- Kay DJ, Mehta V, Goldsmith AJ. Perioperative adenotonsillectomy management in children: current practices. Laryngoscope 2003;113(4):592‐7. [DOI] [PubMed] [Google Scholar]
Krishna 2001
- Krishna P, Lee D. Post‐tonsillectomy bleeding a meta‐analysis. Laryngoscope 2001;111:1358‐61. [DOI] [PubMed] [Google Scholar]
Krishna 2004
- Krishna P, LaPage MJ, Hughes LF, Lin SY. Current practice patterns in tonsillectomy and perioperative care. International Journal of Pediatric Otorhinolaryngology 2004;68(6):779‐84. [DOI] [PubMed] [Google Scholar]
Kumar 1984
- Kumar R. Secondary hemorrhage following tonsillectomy/adenoidectomy. Journal of Laryngology and Otology 1984;98:997‐8. [DOI] [PubMed] [Google Scholar]
Lowe 2004
- Lowe D, Meulen J. National Prospective Tonsillectomy Audit. Tonsillectomy technique as a risk factor for postoperative haemorrhage. Lancet 2004;364(9435):697‐702. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moseley 1991
- Moseley EK, Sullivan TJ. Allergic reactions to antimicrobial drugs in patients with a history of prior drug allergy (abstract). Journal of Allergy and Clinical Immunology 1991;87:226. [Google Scholar]
Murthy 1998
- Murthy P, Laing MR. Dissection tonsillectomy: pattern of post‐operative pain, medication and resumption of normal activity. Journal of Laryngology and Otology 1998;112(1):41‐4. [DOI] [PubMed] [Google Scholar]
Noseworthy 1994
- Noseworthy JH, Ebers GC, Vandervoort MK, Farquhar RE, Yetisir E, Roberts R. The impact of blinding on the results of a randomized, placebo‐controlled multiple sclerosis clinical trial. Neurology 1994;44:16‐20. [DOI] [PubMed] [Google Scholar]
Pai 2005
- Pai I, Lo S, Brown S, Toma AG. Does hydrogen peroxide mouthwash improve the outcome of secondary post‐tonsillectomy bleed? A 10‐year review. Otolaryngology ‐ Head and Neck Surgery 2005;133(2):202‐5. [DOI] [PubMed] [Google Scholar]
Parsons 2006
- Parsons SP, Cordes SR, Comer B. Comparison of posttonsillectomy pain using the ultrasonic scalpel, coblator, and electrocautery. Otolaryngology ‐ Head and Neck Surgery 2006;134(1):106‐13. [DOI] [PubMed] [Google Scholar]
Pavelic 1960
- Pavelic RA. Use of an analgesic‐antibiotic chewing troche (Orabitic) in post‐tonsillectomy and adenoidectomy complications. Ear, Nose and Throat Journal 1960;39:644–5. [PubMed] [Google Scholar]
Qiang 2005
- Qiang W, Jianchen W, MacDonald R, Monga M, Wilt TJ. Antibiotic prophylaxis for transurethral prostatic resection in men with preoperative urine containing less than 100,000 bacteria per ml: a systematic review. Journal of Urology 2005;173(4):1175‐81. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robinson 2002
- Robinson JL, Hameed T, Carr S. Practical aspects of choosing an antibiotic for patients with a reported allergy to an antibiotic. Clinical Infectious Diseases 2002;35(1):26‐31. [DOI] [PubMed] [Google Scholar]
Salonen 2002
- Salonen A, Kokki H, Nuutinen J. Recovery after tonsillectomy in adults: a three‐week follow‐up study. Laryngoscope 2002;112(1):94‐8. [DOI] [PubMed] [Google Scholar]
Saxon 1987
- Saxon A, Beall GN, Rohr AS, Adelman DC. Immediate hypersensitivity reactions to beta‐lactam antibiotics. Annals of Internal Medicine 1987;107:204‐15. [DOI] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Chalmers I, Altman DG. The landscape and lexicon of blinding in randomized trials. Annals of Internal Medicine 2002;136:254‐9. [DOI] [PubMed] [Google Scholar]
Soldado 1998
- Soldado L, Esteban F, Delgado‐Rodriguez M, Solanellas J, Florez C, Martin E. Bacteraemia during tonsillectomy: a study of the factors involved and clinical implications. Clinical Otolaryngology and Allied Sciences 1998;23(1):63‐6. [DOI] [PubMed] [Google Scholar]
Stoker 2004
- Stoker KE, Don DM, Kang DR, Haupert MS, Magit A, Madgy DN. Pediatric total tonsillectomy using coblation compared to conventional electrosurgery: a prospective, controlled single blind study. Otolaryngology ‐ Head and Neck Surgery 2004;130:666‐75. [DOI] [PubMed] [Google Scholar]
Tierney 2004
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. International Journal of Epidemiology 2005;34(1):79‐87. [DOI] [PubMed] [Google Scholar]
Timms 2002
- Timms MS, Temple RH. Coblation tonsillectomy: a double blind randomized controlled study. Journal of Laryngology and Otology 2002;116(6):450‐2. [DOI] [PubMed] [Google Scholar]
Wei 2000
- Wei JL, Beatty CW, Gustafson RO. Evaluation of posttonsillectomy hemorrhage and risk factors. Otolaryngology ‐ Head and Neck Surgery 2000;123(3):229‐35. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dhiwakar 2008
- Dhiwakar M, Clement WA, Supriya M, McKerrow W. Antibiotics to reduce post‐tonsillectomy morbidity. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005607.pub2] [DOI] [PubMed] [Google Scholar]
Dhiwakar 2010
- Dhiwakar M, Clement WA, Supriya M, McKerrow W. Antibiotics to reduce post‐tonsillectomy morbidity. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD005607.pub3] [DOI] [PubMed] [Google Scholar]


