Abstract
Complete and precise knowledge of the neck anatomy and its eventual anomalies is crucial while performing a safe thyroid and parathyroid surgery. Embryo-genetic malformations of the IV branchial arch can lead to an uncommon anatomical alteration known as non-recurrent inferior laryngeal nerve. Its prevalence varies between 0.7% for the dextral branch and 0.04% for the sinistral. In these cases, the inferior laryngeal nerve branches originate directly from the cervical vagus nerve, entering the larynx without hooking, on the right side around the subclavian artery or on the left around the aortic arch. The presence of a non-recurrent laryngeal nerve is challenging, due to the increased risks of iatrogenic damage to the nerve, which results in hoarseness, dysphagia, glottal obstruction, vocal cords palsy, and serious airway impairment. We present the case of a 58-year-old woman. The patient was admitted to our department for a nodule classified as Bethesda IV in the right thyroid lobe. Through the use of intraoperative neuromonitoring (IONM), surgeons detected intraoperatively a non-recurrent laryngeal nerve. A subsequent computed tomography scan confirmed an anomalous right subclavian artery branching from the left aortic arch, the Lusoria Artery. Anatomical variants represent pitfalls in this case and an accurate knowledge of the neck region is imperative while performing thyroid surgery. Devices such as IONM are useful for detecting abnormalities that may lead to iatrogenic damages.
Keywords: endocrine surgery, case report, thyroid surgery, head and neck surgery, non-recurrent laryngeal nerve
Introduction
Complete and precise knowledge of the neck anatomy and its eventual anomalies is crucial to performing safe and reliable thyroid and parathyroid surgery.
Thyroid surgery without complications relies on locating and exposing the cervical portion of the recurrent laryngeal nerve (RLN). 1 These nerves branch with different patterns from the vagus nerve. On the right side, they loop under the subclavian artery, while on the left around the aortic arch. 2 Acknowledge the relationship between the inferior thyroid arteries around the thyroid and the RLN is mandatory to avoid major complications such as paralysis of these nerves, whose nerve fibers are involved in motor, sensory, and parasympathetic functions for the larynx.3,4
Normally, the inferior laryngeal nerve is the terminal branch of the RLN. Unfortunately, RLN’s anatomy shows a high rate of variability, the two most common abnormalities reported are non-recurrency of the nerve and extra laryngeal branches. 3
The non-recurrent inferior laryngeal nerve (NRLN) originates secondarily from embryological mistakes that cause the origin of an RLN from the cervical vagal trunk which then travels into the larynx without looping under the subclavian artery. 5
The incidence of a Right-NRLN is frequently associated with vascular abnormality, with the presence of an aberrant right-subclavian artery (ARSCA) estimated at 0.7%. 6 The Left-NRLN is rarer, with an incidence of 0.04%, and is associated with a right-sided aortic arch and aberrant left subclavian artery (ALSCA).2,7,8
The RLN usually originates from the vagus on the left side at the lower border of the aortic arch and then passes downward and slightly to the side of the ligamentum arteriosum before ascending in the tracheoesophageal groove to reach the larynx. Non-recurrent laryngeal nerves branch directly from the cervical portion of the vagus nerve entering the larynx without descending toward thoracic level. 9
As an uncommon anatomical variation, when a non-recurrent laryngeal is present, there are higher risks of nerve injury.1,10 These iatrogenic damages hardly impact on quality of life affecting the vocal cord function. Injuries to the nerve can result in hoarseness, dysphagia, glottal obstruction, vocal cords palsy, and serious airway impairment. 11 Lesions rate from 13% to 14% for right NRLNs versus 2% to 4% for RLNs.
Preserving the NRLN is challenging due to its difficult preoperative diagnosis.
Neck ultrasound before surgery can identify ARSCA by the absence of the Y sign for the brachiocephalic artery. Preoperative ultrasonography (USG) for assessing patients for NRLN has proven effective, with an accuracy surpassing 98%. 8 Computed tomography (CT) scans may also detect the hook-like course of ARSCA (typically retro esophageal); even angiography may be useful.1,10-12
There is no clear embryological explanation for the existence of an NRLN without associated vascular anomalies. The reports of a left NRLN are anecdotical and always accompanied by other pathologies such as situs inversus. A nearly 6-fold increase in intraoperative nerve injuries in the NRLN remains undetected while using intraoperative neuromonitoring (IONM). 8
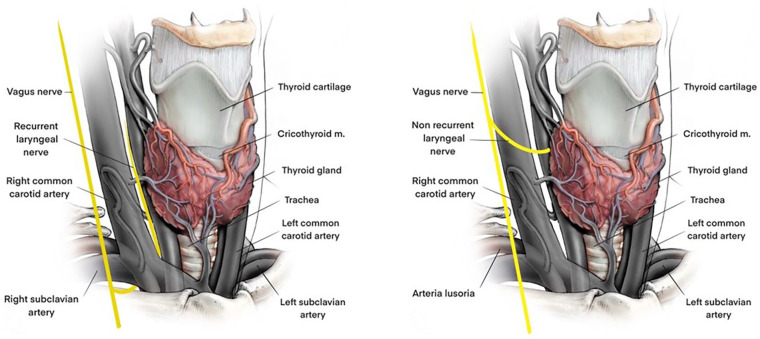
The proper application of IONM guidelines and the routine availing of this technique allows a correct NRLN identification8,13 (Figure 1).
Figure 1.
Anatomy of recurrent (left) and non-recurrent (right) laryngeal nerve.
The following case is presented according to the Context, Assessment, Intervention and Evaluation - CARE reporting checklist.
Case Description
A 58-year-old woman was admitted to our department for a nodule classified as Bethesda IV in the right thyroid lobe. According to this cytopathologic classification system, this nodule is configured as a follicular neoplasm or suspicious, posing an indication for lobectomy or thyroidectomy when malignancy is confirmed.
The patient previously suffered a pT2 N2 lung adenocarcinoma of the right lower lobe treated with lobectomy and lymphadenectomy. The thyroid nodule was identified due to a subsequent positron emission tomography (PET)-CT performed according to the oncologic follow-up.
Oncologists, endocrinologists, and surgeons discussed the case and proposed the option of total thyroidectomy with IONM.
Preoperative laryngoscopy documented bilateral cord motility. Before surgery, a tube-position verification test was performed and the tap test was positive. The procedure started with a cervicotomy with preparation of the superior and inferior flaps. The median raphe was engraved dissecting the pre-thyroid muscles approaching the thyroid lodge. The right lobe was enlarged with a central teso-elastic node.
A vagal (V) signal using IONM (V1) was not detected; the algorithm for IONM no vagal signal was applied. At first, the correct position of the tube was checked. 13 No neuromuscular block agent was administered. The vagus nerve was isolated proximally to the larynx till a nerve branch originated near the superior pedicle. Then, the vagus nerve was stimulated with a V1 signal >1000 µV. Even the stimulation upstream of the nerve branch was >1000. So, the presence of a non-recurrent inferior laryngeal nerve was identified (Figure 2). The lobectomy was performed under direct vision of the nerve preserving parathyroids. In the left lobe, a normal procedure was performed with normal V and R (recurrent) signals. No nerve injuries occurred. Postoperative laryngoscopy confirmed bilateral cord motility.
Figure 2.
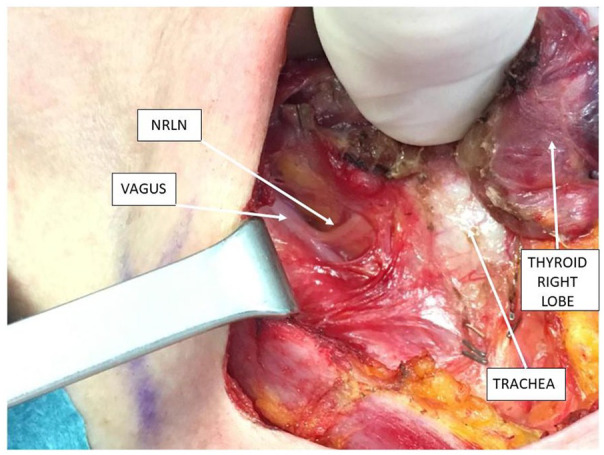
Intraoperative anatomy.
The patient was dismissed the day after the surgery with a calcium level of 8.7 mg/dL. No evidence of dysphonia or dysphagia was detected. Surgical stitches were removed 7 days after the intervention without signs of perilesional collections.
The patient attended a regular follow-up with oncologists and endocrinologists.
Subsequently, in order to describe eventual vascular abnormalities associated with the non-recurrent inferior right laryngeal nerve, a CT scan was performed. An aberrant right subclavian artery was identified on the left of the aortic arch, directed to the right behind the esophagus and a common origin of the carotid arteries (Figure 3).
Figure 3.
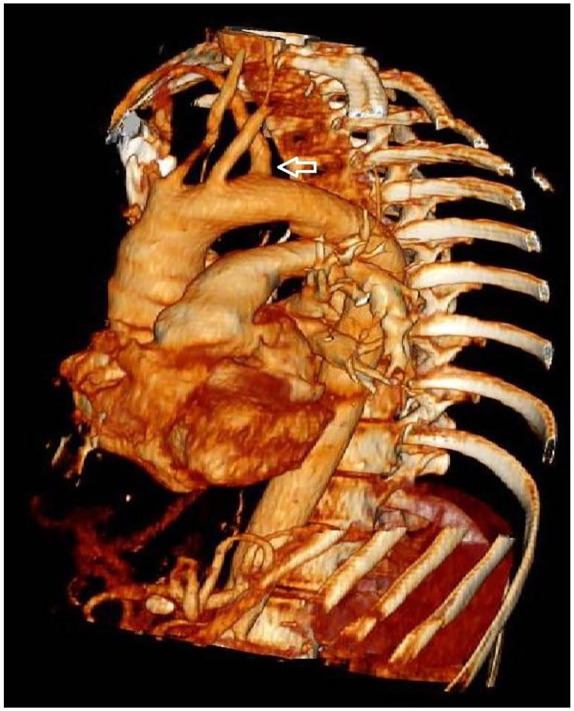
Angio CT scan reconstruction. The arrow indicates the Lusoria Artery.
Discussion
Stedman first described a NRLN in 1823. The overall reported incidence varies from 0.6% to 0.8% on the right side and 0.04% on the left.4,8
Embryologically, the RLNs derive from the VI branchial arch with a horizontal course; then with the bilateral regression of the V branchial arch and the distal portion of the VI arch, the RLN moves up to lie beneath the IV arch, remaining anchored to its developed structures: the aortic arch and the subclavian artery.
Therefore, the nerves acquire an intrathoracic position due to the descent of the heart and the elongation of the neck, assuming a recurrent course different side by side.
Arising from the right vagus nerve anterior to the first part of the subclavian artery, these nerves then wrap around the subclavian artery and move up toward the tracheoesophageal groove before reaching the larynx.
The RLN arises from the vagus nerve to the lower edge of the aortic arch on the left side and then travels beneath the aortic arch, just next to the ligamentum arteriosum, before moving upward in the tracheoesophageal groove toward the larynx. 9
In rare cases, the obliteration of the right IV arch and the proximal right dorsal aorta allows an anomalous origin of the subclavian artery that crosses the midline behind the esophagus and trachea directed to the right side. Thanks to anomalous subclavian artery (ARSCA), the inferior laryngeal nerve forms a non-recurrent pattern entering directly into the larynx. 4
Left NRLNs are rarer with an unclear underlying mechanism. The NRLNs can be categorized into 2 types: with a complete inversion of the viscera and with a right-sided aortic arch.8,14 Only 14 cases of coexisting RLN and NRLN have been described. 5
The development and regression of the branchial arches in the embryo result in the formation of the RLN. The right nerve loops beneath the right subclavian artery, while the left nerve loops under both the aortic arch and ductus arteriosus. An abnormal regression of the right fourth arch (right subclavian artery) is linked to an anomalous NRLN from an ARSCA branching off distally from the left subclavian artery on the aorta. Consequently, it does not follow its regular course but becomes non-recurrent as it moves upward. The left NRLN undergoes even more intricate regression, involving regression of both the fourth and sixth arches (adult aortic arch and ductus arteriosus).
The RLN normally ascends behind the carotid artery and travels medially in the dissected field of the external carotid artery (ECA). The NRLN travels in another way around the carotid artery.
According to its relationship with the inferior thyroid artery, 4 traveling patterns of the NRLN are described: descending type from the vagus nerve trunk; vertical type running vertically to the cricothyroid joint; ascending type moving upward to the cricothyroid joint; and V-shaped type crossing downward and then traveling upward the cricothyroid joint. 4
The diagnosis of ARSCA can be predictive of an NRLN. Symptoms are often silent, but patients may complain of dysphagia, dysphagia lusoria or Bayford-Autenrieth dysphagia, chronic cough, or may suffer an unexplained ischemia of the right upper limb. Aberrant right-subclavian artery may be diagnosed on scans recognizing an anomalous structure traveling to the right axilla from the distal part of the aortic. 10
The positional relationship between ARSCA and the trachea esophagus is classified into: type 1, ARSCA lies on the dorsal side of the tracheal membranous wall; type 2, ARSCA lies on the ventral side of the membranous wall of the trachea.
The most used anatomical classification of the NRLN is described by Toniato. This classification recognizes 3 different patterns of this nervous variation. A type 1, observed with an incidence of 58.3%, presents a NRLN arising over the laryngotracheal junction running with a superior thyroid artery. The NRLN type 2 (41.7%) follows a transverse path parallel to an inferior thyroid artery. It is divided into 2 subtypes: type 2A, over the trunk of the inferior thyroid artery, and type 2B under the inferior thyroid artery trunk or between its branches and ascending to the larynx 11 (Figures 4-6).
Figure 4.

Toniato type 1 non-recurrent laryngeal nerve.
Figure 5.
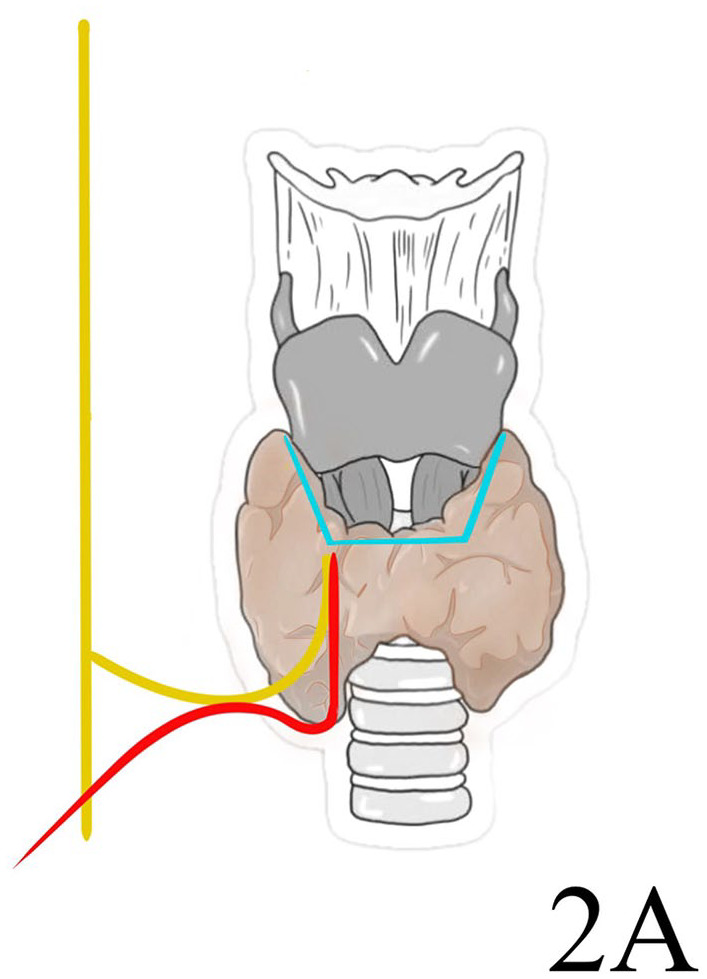
Toniato type 2A non-recurrent laryngeal nerve.
Figure 6.
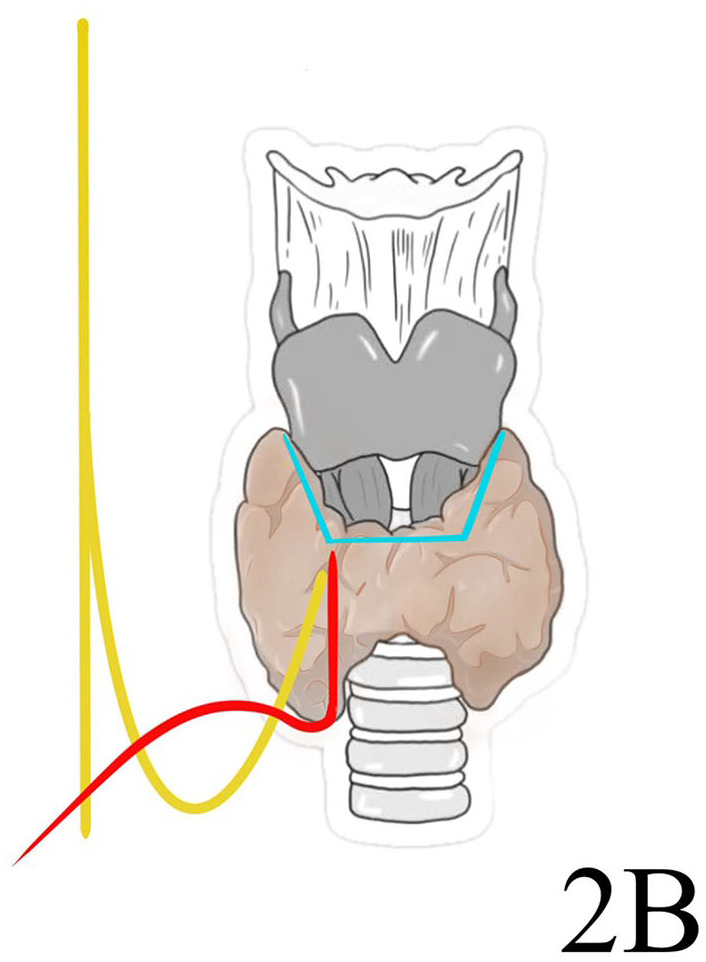
Toniato type 2B non-recurrent laryngeal nerve.
The use of USG and CT scan, which identifies preoperatively anomalous structures, such as aberrant subclavian arteries and NRLNs, with nearly 100% accuracy, provides surgeons with their best defense in those tricky cases.10,11,14
Dysphagia lusoria may be demonstrated by contrast swallow and/or esophagoscopy. In 20% of cases, a linear, oblique, mediastinal shadow may be identified in chest radiography. Computed tomography or magnetic resonance imaging with angiography can directly demonstrate an ALSCA in 100% of cases. 15 Nevertheless, the imaging mostly used to detect abnormalities such as the “Y Sign” of an abnormal subclavian artery or a Lusoria Artery branching from the aortic arch is ultrasonography. When, as expected, the right common carotid artery (RCCA) arises from the bifurcation of the brachiocephalic trunk, it may display a Y sign. In this scenario, a regular right subclavian artery and normal right RLN are assumed to be present. If the RCCA originates directly from the aortic arch, it is presumed that an aberrant right subclavian artery (Lusoria Artery) with a right NRLN exists. 3
The presence of a right-sided NRLN without vascular anomaly is reported up to 11% of cases, but its mechanism of origin is still unexplained. It might represent a false NRLN, similar in diameter and size but actually an anastomotic nerve between the sympathetic chain. This implies that the cord function is provided by the sympathetic trunk. 9
Intraoperative neuromonitoring in thyroid surgery adds a new way to identify and test nerves during dissection. The use of IONM is now a standard practice in thyroid surgery. Intraoperative neuromonitoring allows surgeons to recognize RLNs and to ensure their functional integrity. Even in challenging cases such as variation of anatomy, reopening, and malignancy, IONM could yield vital information to abridge the situation. Intraoperative neuromonitoring reduces the incidence of nerve injury by guiding the dissection and lessens the mechanical damage to its anterior branches due to excessive exposure. 16
The lack of an early V1 response to distal stimulation of the right vagus nerve suggests that the transmission path is disrupted because the inferior laryngeal nerve branches out prematurely before reaching the point of stimulation. The separation point of NRLN can be visually and functionally identified through proximal dissection guided by IONM permitting to identify the presence of NRLN at an early stage of surgery. 1 If NRLN is close to the dissection point, IONM will show Electromyography (EMG) response, prompting careful tissue dissection to identify the nerve. 2
If a V1 signal is not identified, the algorithm for IONM should be applied. This involves evaluating function using impedance values below 5 kΩ and checking for an imbalance of less than 1 kΩ. In addition, visual confirmation of proper contact between the true vocal cord mucosa and tube surface must be repeated. Subsequently, it is essential to conduct a laryngeal twitch assessment. If present, neural function is confirmed, and any issues are managed by monitoring dysfunctions such as laryngeal electrode and/or tube displacement, and tube rotation. In the absence of laryngeal twitch, several details must be verified: delivery of stimulation current, displacement of ground sternal electrodes, malfunctioning of the probe, administration of neuromuscular blockades, and occurrence of a neural injury.
After the algorithm check, in the absence of a V1 signal and excluding any possible cause, the presence of a NRLN can be assumed and surgeons can proceed to visual and functional recognition of the nerval separation point by a proximal dissection under the enlightenment of IONM 17 (Figures 7 and 8).
Figure 7.
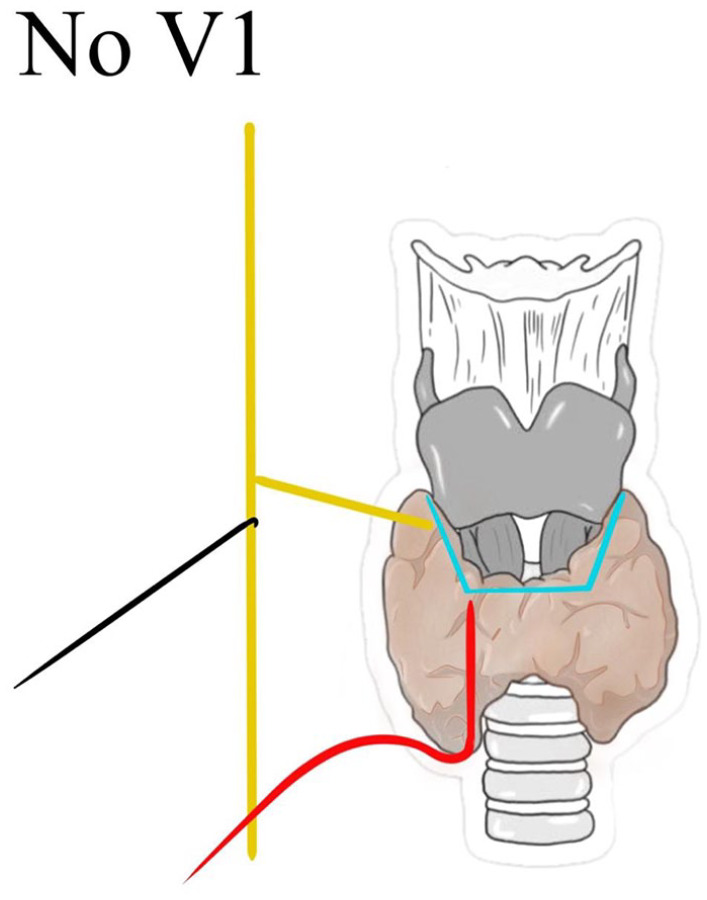
Absence of V1 signal after the arise of NRLN.
Figure 8.
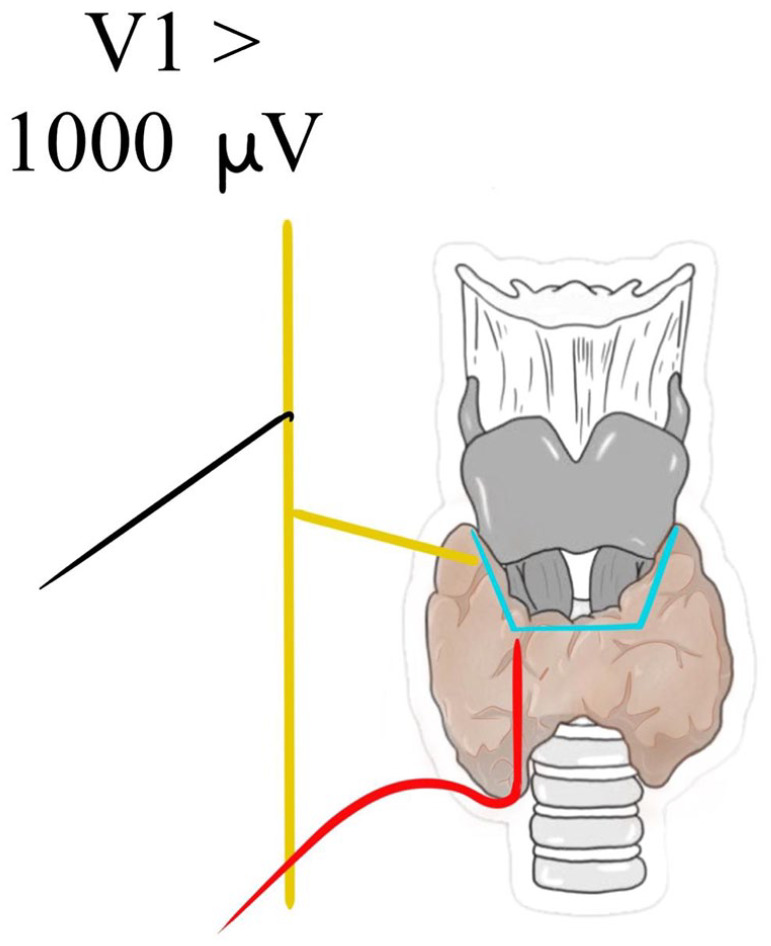
Presence of a valid V1 signal before the arise of NRLN.
In no anomalous cases, with normal RLN, the risk of injury is estimated at 1.8%, rising up to 12.9% when a NRLN is present. This incidence of nerve injury significantly increased, and the absence of clinical signs of NRLN suggests looking for Aberrant Right Subclavian Artery (ARSA) preoperatively to predict a no recurrent pattern of the inferior laryngeal nerve. 14
Recurrent laryngeal nerve palsy is acknowledged as an important complication of neck surgery. This results in hoarseness and dysphagia, glottal obstruction, and serious airway impairment.
The NRLN is a rare anatomical variation and represents a higher surgical risk of iatrogenic injury during thyroid and parathyroid surgery.
Conclusions
Complete and accurate knowledge of neck anatomy, its vascularization and innervation, and the presence of anatomical variants, such as the NRLN, is crucial to perform a safe, reliable, and successful thyroid and parathyroid surgery. Intraoperative neuromonitoring technique, especially its standard application, is a useful method to identify the presence of a NRLN before its injury. This allows avoiding dangerous and predictable iatrogenic damages that can affect vital patient functions and quality of life.
Footnotes
Author Contributions: D.I. and S.G. conceived and planned the manuscript. D.I., M.A., and A.L. performed the operation. D.C., E.F., A.P., and A.V. critically revised the literature. S.G., D.I., and G.I. performed the revision of the article. G.C. approved the final version to be published. S.G. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Informed Consent: Written informed consent for publication of this case report and accompanying images was unable to be obtained from the patient or the relatives after all possible attempts were made.
Prior Presentation of Abstract Statement: This abstract was previously presented at the 9th Biennial Congress of the European Society of Endocrine Surgeons, Athens, May 26-28, 2022.
ORCID iD: Simone Gianazza  https://orcid.org/0000-0001-9270-1709
https://orcid.org/0000-0001-9270-1709
References
- 1. Gurleyik G, Torun M, Gurleyik E. Nonrecurrent laryngeal nerve: precise detection by electrophysiological nerve monitoring. Cureus. 2018;10(5):e2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akiyama T, Tanaka S, Hitotsumatsu T. Carotid endarterectomy for a patient with a right-sided aortic arch and aberrant left subclavian artery predicting a left non-recurrent inferior laryngeal nerve: a case report and literature review. NMC Case Rep J. 2021;8(1):45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Constable JD, Bathala S, Ahmed JJ, et al. Non-recurrent laryngeal nerve with a coexisting contralateral nerve demonstrating extralaryngeal branching. BMJ Case Rep. 2017;2017:bcr2016218280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong YT, Ki HH. The relationship between the non-recurrent laryngeal nerve and the inferior thyroid artery. Indian J Surg. 2017;80:109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gurleyik E, Dogan S, Cetin E. Coexistence of right nonrecurrent nerve and bifurcated recurrent laryngeal nerve pointed by Zuckerkandl’s tubercle. Cureus. 2017;9(3):e1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morais M, Capela-Costa J, Matos-Lima L, Costa-Maia J. Nonrecurrent laryngeal nerve and associated anatomical variations: the art of prediction. Eur Thyroid J. 2015;4(4):234-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furukawa T, Otsuki N, Tomotsu M, et al. Left non-recurrent inferior laryngeal nerve in a patient with right-sided aortic arch and aberrant left subclavian artery. Auris, Nasus, Larynx. 2021;48:317-321. [DOI] [PubMed] [Google Scholar]
- 8. Henry BM, Sanna S, Graves MJ, et al. The non-recurrent laryngeal nerve: a meta-analysis and clinical considerations. Peerj. 2017;5:e3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Labuschagne JJ, Hammer N. Intra-operative detection of a left-sided non-recurrent laryngeal nerve during vagus nerve stimulator implantation. Medicina. 2020;56:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Niu ZX, Zhang H, Chen LQ, et al. Preoperative computed tomography diagnosis of non-recurrent laryngeal nerve in patients with esophageal carcinoma. Thorac Cancer. 2016;8:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polednak AP. Anatomical variation in the right non-recurrent laryngeal nerve reported from studies using pre-operative arterial imaging. Surg Radiol Anat. 2019;41(8):943-949. [DOI] [PubMed] [Google Scholar]
- 12. Gong RX, Luo SH, Gong YP, et al. Prediction of nonrecurrent laryngeal nerve before thyroid surgery: experience with 1825 cases. J Surg Res. 2019;189(1):75-80. [DOI] [PubMed] [Google Scholar]
- 13. Randolph GW, Dralle H, Abdullah H, et al. ; International Intraoperative Monitoring Study Group. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;121(suppl 1):1-16. [DOI] [PubMed] [Google Scholar]
- 14. Mediouni A, Sayedi H, Chahed H, Besbes G. Non-recurrent laryngeal nerve and arteria lusoria: rare and little known association. Clin Case Rep. 2021;9(8):e04723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yetisir F, Salman AE, Ḉiftçi B, et al. Efficacy of ultrasonography in identification of non-recurrent laryngeal nerve. Int J Surg. 2012;10:506-509. [DOI] [PubMed] [Google Scholar]
- 16. Zhu Y, Gao DS, Lin J, et al. Intraoperative neuromonitoring in thyroid and parathyroid surgery. J Laparoendosc Adv Surg Tech A. 2021;31(1):18-23. [DOI] [PubMed] [Google Scholar]
- 17. Dionigi G, Chiang FY, Rausei S, et al. Surgical anatomy and neurophysiology of the vagus nerve (VN) for standardized intraoperative neuromonitoring (IONM) of the inferior laryngeal nerve (ILN) during thyroidectomy. Langenbecks Arch Surg. 2010;395:893-899. [DOI] [PubMed] [Google Scholar]