Abstract
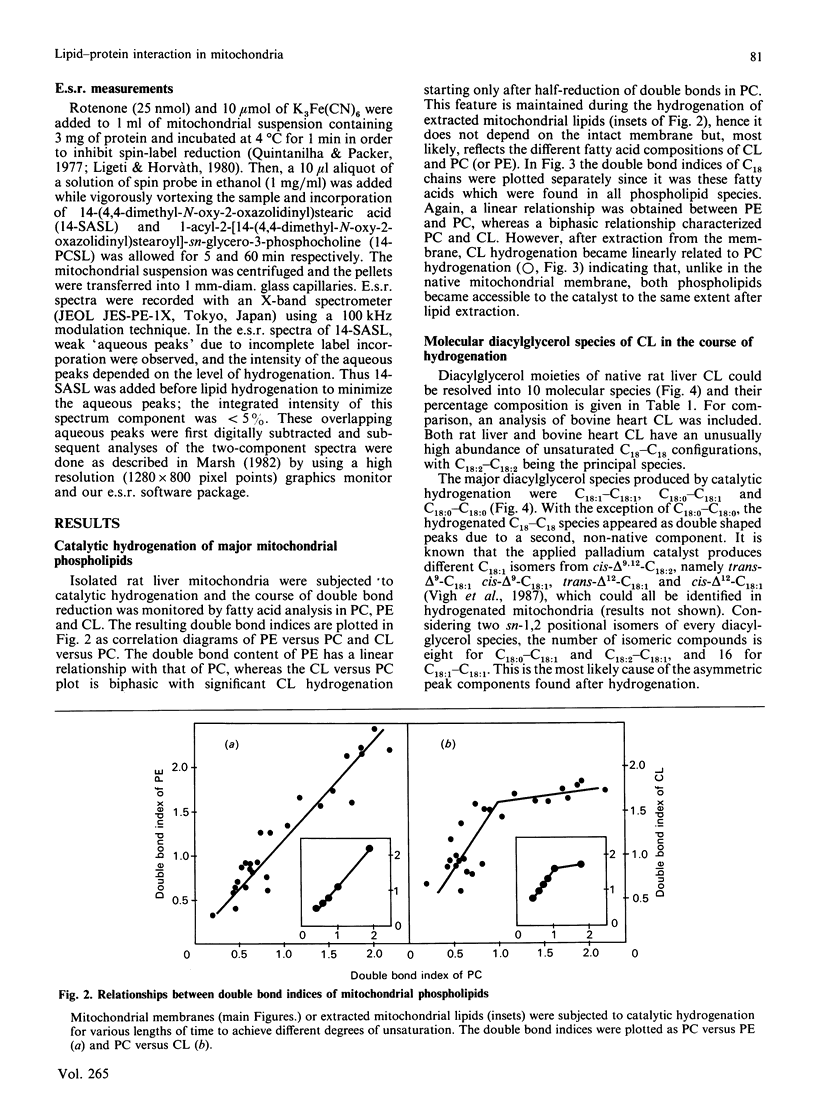
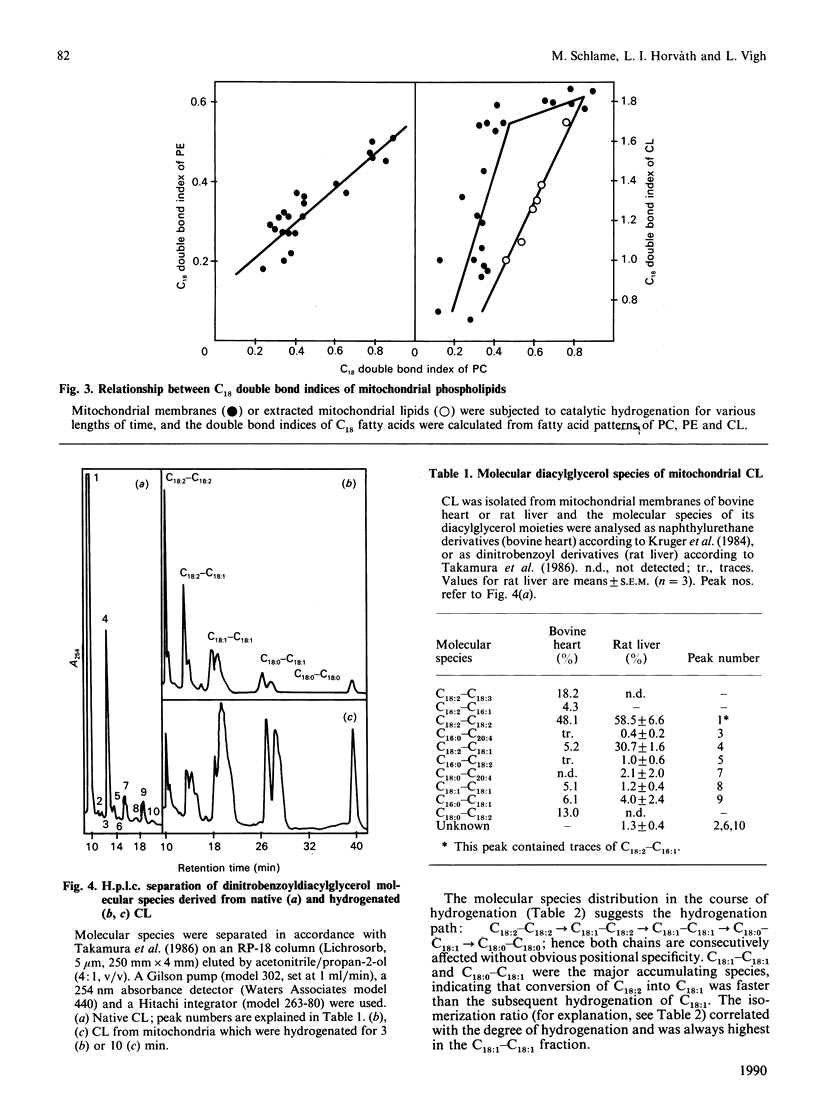
Lipid acyl double bonds in isolated mitochondrial membranes were gradually reduced by palladium-complex-catalysed hydrogenation, and the resulting saturation was monitored by fatty acid analysis of phosphatidylcholine, phosphatidylethanolamine and cardiolipin. The courses of hydrogenation of these phospholipids suggested that cardiolipin is in a membrane compartment which is less accessible to the applied catalyst. Native cardiolipin and its hydrogenation products were further characterized by analysis of their molecular diacylglycerol species. A decrease in the double bond content was accompanied by an increased amount of motionally restricted lipids at the hydrophobic interface of proteins as measured by two different spin-labelled lipids (C-14 positional isomers of spin-labelled stearic acid and phosphatidylcholine analogues). The protein-immobilized fraction of spin-labelled stearic acid increased in parallel with the hydrogenation of cardiolipin rather than of phosphatidylcholine or phosphatidylethanolamine. These data are interpreted in terms of a tight association of cardiolipin with membrane proteins, which becomes looser upon double bond reduction leading to the replacement of cardiolipin by spin-labelled stearic acid in the solvation shell. Thus the hydrophobic moiety of cardiolipin, characterized by double-unsaturated C18-C18 diacylglycerol species, seems to be an important structural requirement for the high protein affinity of this compound.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beyer K., Klingenberg M. ADP/ATP carrier protein from beef heart mitochondria has high amounts of tightly bound cardiolipin, as revealed by 31P nuclear magnetic resonance. Biochemistry. 1985 Jul 16;24(15):3821–3826. doi: 10.1021/bi00336a001. [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Griffith O. H., Brotherus M. O., Jost P. C., Silvius J. R., Hokin L. E. Lipid--protein multiple binding equilibria in membranes. Biochemistry. 1981 Sep 1;20(18):5261–5267. doi: 10.1021/bi00521a026. [DOI] [PubMed] [Google Scholar]
- Dale M. P., Robinson N. C. Synthesis of cardiolipin derivatives with protection of the free hydroxyl: its application to the study of cardiolipin stimulation of cytochrome c oxidase. Biochemistry. 1988 Oct 18;27(21):8270–8275. doi: 10.1021/bi00421a042. [DOI] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985 Jun 12;822(1):1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Drees M., Beyer K. Interaction of phospholipids with the detergent-solubilized ADP/ATP carrier protein as studied by spin-label electron spin resonance. Biochemistry. 1988 Nov 15;27(23):8584–8591. doi: 10.1021/bi00423a012. [DOI] [PubMed] [Google Scholar]
- Ellingson J. S., Taraschi T. F., Wu A., Zimmerman R., Rubin E. Cardiolipin from ethanol-fed rats confers tolerance to ethanol in liver mitochondrial membranes. Proc Natl Acad Sci U S A. 1988 May;85(10):3353–3357. doi: 10.1073/pnas.85.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmann M., Marsh D. Spin-label studies on the origin of the specificity of lipid-protein interactions in Na+,K+-ATPase membranes from Squalus acanthias. Biochemistry. 1985 Jul 2;24(14):3572–3578. doi: 10.1021/bi00335a027. [DOI] [PubMed] [Google Scholar]
- Fry M., Green D. E. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981 Feb 25;256(4):1874–1880. [PubMed] [Google Scholar]
- Horváth L. I., Brophy P. J., Marsh D. Influence of lipid headgroup on the specificity and exchange dynamics in lipid-protein interactions. A spin-label study of myelin proteolipid apoprotein-phospholipid complexes. Biochemistry. 1988 Jul 12;27(14):5296–5304. doi: 10.1021/bi00414a052. [DOI] [PubMed] [Google Scholar]
- Kadenbach B., Mende P., Kolbe H. V., Stipani I., Palmieri F. The mitochondrial phosphate carrier has an essential requirement for cardiolipin. FEBS Lett. 1982 Mar 8;139(1):109–112. doi: 10.1016/0014-5793(82)80498-5. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Awasthi Y. C., Crane F. L. Cardiolipin from beef heart mitochondria: fatty acid positioning an molecular species distribution. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1102–1109. doi: 10.1016/0006-291x(70)90908-3. [DOI] [PubMed] [Google Scholar]
- Knowles P. F., Watts A., Marsh D. Spin-label studies of lipid immobilization in dimyristoylphosphatidylcholine-substituted cytochrome oxidase. Biochemistry. 1979 Oct 16;18(21):4480–4487. doi: 10.1021/bi00588a005. [DOI] [PubMed] [Google Scholar]
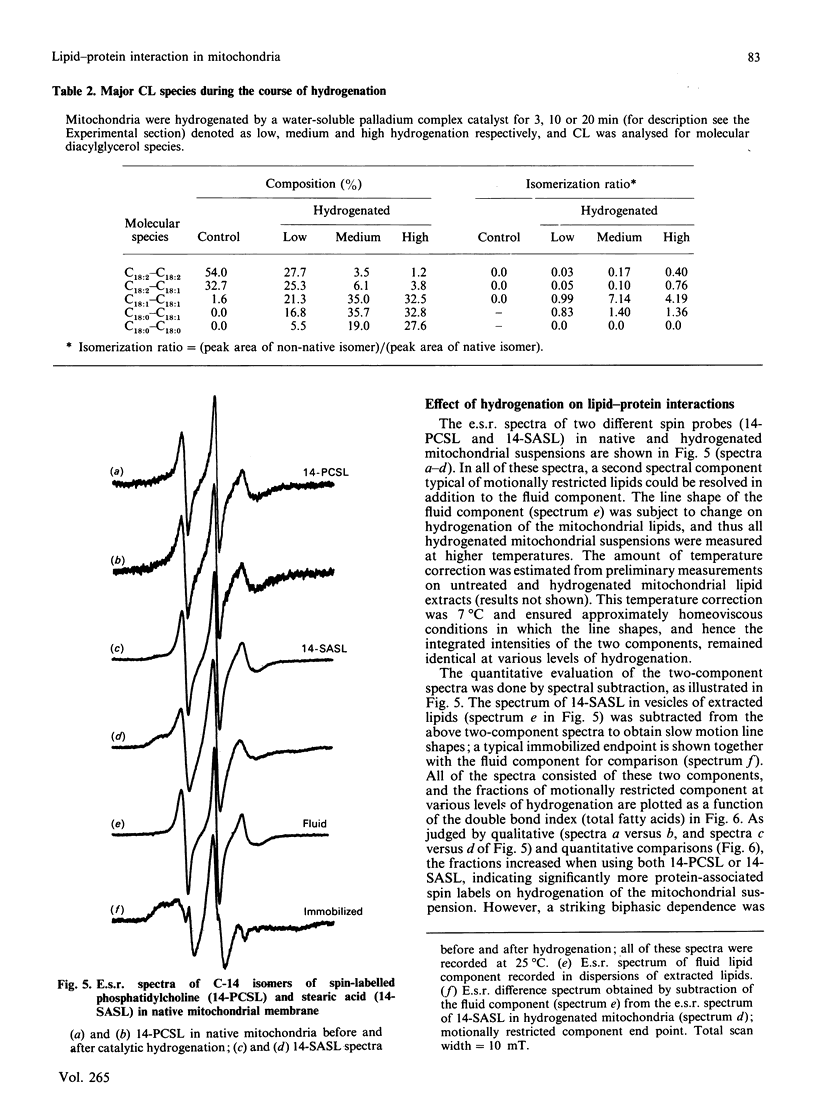
- Krüger J., Rabe H., Reichmann G., Rüstow B. Separation and determination of diacylglycerols as their naphthylurethanes by high-performance liquid chromatography. J Chromatogr. 1984 May 11;307(2):387–392. doi: 10.1016/s0378-4347(00)84110-9. [DOI] [PubMed] [Google Scholar]
- Ligeti E., Horváth L. I. Effect of Mg2+ on membrane fluidity and K+ transport in rat liver mitochondria. Biochim Biophys Acta. 1980 Jul 16;600(1):150–156. doi: 10.1016/0005-2736(80)90420-4. [DOI] [PubMed] [Google Scholar]
- Noël H., Pande S. V. An essential requirement of cardiolipin for mitochondrial carnitine acylcarnitine translocase activity. Lipid requirement of carnitine acylcarnitine translocase. Eur J Biochem. 1986 Feb 17;155(1):99–102. doi: 10.1111/j.1432-1033.1986.tb09463.x. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Ito A., Umeda M., Inoue K., Omura T. Specific binding of mitochondrial protein precursors to liposomes containing cardiolipin. J Biochem. 1988 Apr;103(4):589–595. doi: 10.1093/oxfordjournals.jbchem.a122312. [DOI] [PubMed] [Google Scholar]
- Paradies G., Ruggiero F. M. The effect of doxorubicin on the transport of pyruvate in rat-heart mitochondria. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1302–1307. doi: 10.1016/s0006-291x(88)80774-5. [DOI] [PubMed] [Google Scholar]
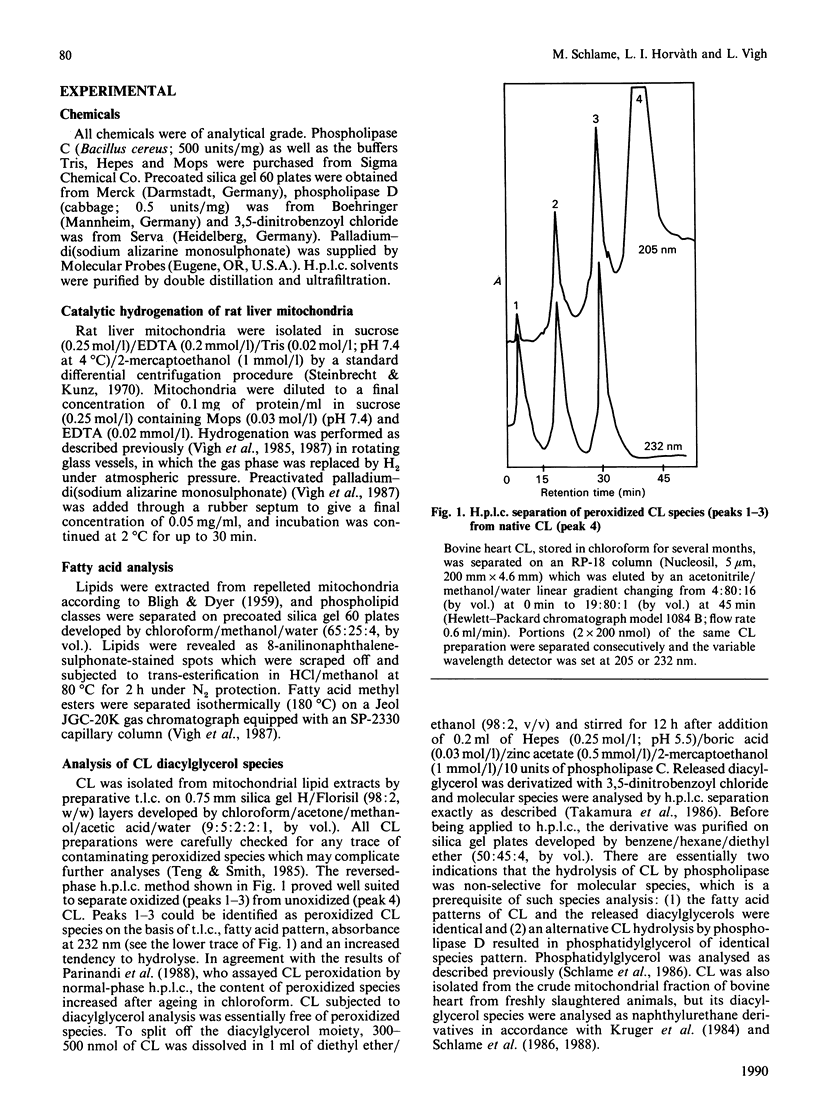
- Parinandi N. L., Weis B. K., Schmid H. H. Assay of cardiolipin peroxidation by high-performance liquid chromatography. Chem Phys Lipids. 1988 Dec;49(3):215–220. doi: 10.1016/0009-3084(88)90009-6. [DOI] [PubMed] [Google Scholar]
- Pember S. O., Powell G. L., Lambeth J. D. Cytochrome P-450scc-phospholipid interactions. Evidence for a cardiolipin binding site and thermodynamics of enzyme interactions with cardiolipin, cholesterol, and adrenodoxin. J Biol Chem. 1983 Mar 10;258(5):3198–3206. [PubMed] [Google Scholar]
- Powell G. L., Knowles P. F., Marsh D. Spin-label studies on the specificity of interaction of cardiolipin with beef heart cytochrome oxidase. Biochemistry. 1987 Dec 15;26(25):8138–8145. doi: 10.1021/bi00399a018. [DOI] [PubMed] [Google Scholar]
- Quintanilha A. T., Packer L. Surface localization of sites of reduction of nitroxide spin-labeled molecules in mitochondria. Proc Natl Acad Sci U S A. 1977 Feb;74(2):570–574. doi: 10.1073/pnas.74.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M., Rabe H., Rüstow B., Kunze D. Molecular species of mitochondrial phosphatidylcholine in rat liver and lung. Biochim Biophys Acta. 1988 Feb 19;958(3):493–496. doi: 10.1016/0005-2760(88)90236-6. [DOI] [PubMed] [Google Scholar]
- Schlame M., Rüstow B., Kunze D., Rabe H., Reichmann G. Phosphatidylglycerol of rat lung. Intracellular sites of formation de novo and acyl species pattern in mitochondria, microsomes and surfactant. Biochem J. 1986 Nov 15;240(1):247–252. doi: 10.1042/bj2400247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecht I., Kunz W. Anwendung der "Cycling"--Technik zur stichprobenartigen quantitativen Bestimmung der Reduktionsgrades des NAD- und NADP-Systems von Rattenlebermitochondrien bei kontinuierlich registrierenden Messungen. Acta Biol Med Ger. 1970;25(5):731–747. [PubMed] [Google Scholar]
- Takamura H., Narita H., Urade R., Kito M. Quantitative analysis of polyenoic phospholipid molecular species by high performance liquid chromatography. Lipids. 1986 May;21(5):356–361. doi: 10.1007/BF02535701. [DOI] [PubMed] [Google Scholar]
- Teng J. I., Smith L. L. High-performance liquid chromatography of cardiolipin. J Chromatogr. 1985 Apr 12;339(1):35–44. doi: 10.1016/s0378-4347(00)84625-3. [DOI] [PubMed] [Google Scholar]
- Vigh L., Horváth I., Joó F., Thompson G. A., Jr The hydrogenation of phospholipid-bound unsaturated fatty acids by a homogeneous, water-soluble, palladium catalyst. Biochim Biophys Acta. 1987 Sep 25;921(2):167–174. doi: 10.1016/0005-2760(87)90015-4. [DOI] [PubMed] [Google Scholar]
- Vigh L., Joó F., Droppa M., Horváth L. I., Horváth G. Modulation of chloroplast membrane lipids by homogeneous catalytic hydrogenation. Eur J Biochem. 1985 Mar 15;147(3):477–481. doi: 10.1111/j.0014-2956.1985.00477.x. [DOI] [PubMed] [Google Scholar]
- Vik S. B., Georgevich G., Capaldi R. A. Diphosphatidylglycerol is required for optimal activity of beef heart cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1456–1460. doi: 10.1073/pnas.78.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R., Harlow R. D. Structural analyses of rat liver phosphoglycerides. Arch Biochem Biophys. 1969 Dec;135(1):272–281. doi: 10.1016/0003-9861(69)90540-2. [DOI] [PubMed] [Google Scholar]



