Abstract
Background:
Intravenous fluid therapy is a ubiquitous intervention for the management of patients with sepsis, however excessive cumulative fluid balance has been shown to result in worse outcomes. Hyperoncotic albumin is presented in low volumes, is an effective resuscitation fluid and may have effects beyond plasma volume expansion alone. This systematic review aimed to assess the efficacy, safety and effectiveness of hyperoncotic albumin solutions in the management of sepsis.
Methods:
We searched four databases and two trial registries for controlled clinical trials of hyperoncotic albumin for management of sepsis. Review outcomes were mortality, need for renal replacement therapy, cumulative-fluid balance, and need for organ support. We used methods guided by the Cochrane Handbook for reviews of clinical interventions. Studies were assessed using Cochrane’s Risk of Bias 2 tool. We performed pairwise meta-analysis where possible. Certainty of evidence was assessed using GRADE.
Results:
We included six trials; four (2772 patients) were meta-analysed. Most studies had moderate or high risk of bias. There was no significant difference in 28-day mortality for septic patients receiving hyperoncotic albumin compared to other intravenous fluids (OR 0.95, [95% CI: 0.8–1.12]); in patients with septic shock (2013 patients) there was a significant reduction (OR 0.82 [95% CI: 0.68–0.98]). There was no significant difference in safety outcomes. Hyperoncotic albumin was associated with variable reduction in early cumulative fluid balance and faster resolution of shock.
Conclusions:
There is no good-quality evidence to support the use of hyperoncotic albumin in patients with sepsis, but it may reduce short-term mortality in the sub-groups with septic shock. It appears safe in terms of need for renal replacement therapy and is associated with reduced early cumulative fluid balance and faster resolution of shock. Larger, better quality randomised controlled trials in patients with septic shock may enhance the certainty of these findings.
Review registration:
PROSPERO ref: CRD42021150674
Keywords: Sepsis, septic shock, hyperoncotic albumin, fluid resuscitation
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated immune response to infection. 1 Despite advanced treatments including organ support, this response may lead to damage and dysfunction of vital organ systems, which in turn may progress to multi-organ failure and death. Sepsis represents an increasing burden on healthcare systems, with over 19 million cases per year globally, resulting in approximately 5 million deaths. 2 International clinical practice guidelines for the management of sepsis recommend doctors urgently give intravenous fluid to patients, however this strong recommendation is based on low quality evidence. 3 There are also data linking the administration of excessive volumes of fluid with worse outcomes for patients with sepsis.4 –6 In addition, there is very little evidence to guide clinicians in the choice of fluid to use. Salt solutions, known as crystalloids, are recommended in the first instance, but human albumin solution is suggested for patients with persistent evidence of hypoperfusion after receiving a large volume of fluid. Hyperoncotic albumin preparations are presented in lower (50–100 ml) volumes compared to their isotonic counterparts, their use is associated with lower fluid resuscitation requirements for critically ill patients. 7
Unlike crystalloids, human albumin solutions may also possess beneficial effects beyond their expansion in intravascular volume. 8 Albumin has a significant influence over the maintenance of vascular endothelium, providing protection from inflammation and injury to lining of blood vessels (the glycocalyx). It also binds a range of endogenous and exogenous compounds, allowing it to assist the transport, storage and clearance of a number of potentially harmful substances. These include the reactive oxygen and nitrogen species produced during states of systemic oxidative stress seen in sepsis.9,10
Despite many published systematic reviews of fluid therapy in critically ill patients, few concentrate specifically on hyperoncotic albumin solutions or patients with sepsis. A previous Cochrane review focussed specifically on albumin solutions for fluid resuscitation, but examined trials in both adults and children, with a variety of pathology and range of different albumin preparations (4%–25%). 11 This review included data from 38 trials, concluding there was no evidence for albumin in the reduction of mortality compared to crystalloid alternatives. Very few of these trials tested hyperoncotic albumin solutions and the authors acknowledged further research with this solution in spesis was warranted. Another systematic review specific to patients with sepsis only, found lower mortality rates for patients receiving albumin compared to other resuscitation fluids. However, it also included studies of a broad range of albumin preparations (4%–20%) and some manuscripts that have since been retracted from the published literature. 12 The only systematic review specific for hyperoncotic albumin solutions included 7 (out of 20) studies of patients with sepsis. However, this review also included studies that have now been retracted and studies published since the searches were completed could alter its findings. 13
Accordingly, the objective of this systematic review was to identify the evidence for hyperoncotic albumin solutions in acutely ill hospitalised adults with a diagnosis of sepsis. Outcomes of interest included those measuring efficacy, safety and effectiveness.
Methods
Review protocol and registration
A formal protocol for this systematic review was prospectively written and approved by all authors (see online supporting information). This review is registered on the International prospective register of systematic reviews, hosted by the UK National Institute for Health Research (PROSEPERO ref: CRD42021150674). 14
Eligibility criteria
Types of studies and participants
The context of our review question was based on sepsis in human subjects. We therefore, included prospective randomised and non-randomised trials that studied hospitalised adult patients with a diagnosis of sepsis. All clinical trials included a parallel control group. Non-clinical, non-human and retrospective studies, along with case reports or narrative reviews were excluded. Systematic reviews were not included but were reference checked for studies relevant to this review.
As recognised definitions for sepsis have evolved over time, we accepted any of the previous published definitions1,15 or studies where authors stated patients had sepsis. Studies of broader populations that reported data for sub-groups of patients with sepsis were also considered for inclusion if a sepsis sub-group was a well-defined and described as a proportion of the total cohort.
Types of interventions
Studies were included if participants received intravenous hyperoncotic (⩾20% concentration) albumin solution for their sepsis management. This included its use as both a resuscitation fluid (administered quickly to expand intravascular volume) or as a regular supplement. Comparator interventions could be any alternative fluid to hyperoncotic albumin.
Outcomes
We did not limit our searches or eligibility criteria to any specified outcome measures. Pre-specified outcomes we knew to be of clinical interest and identified through patient and public engagement, included measures of clinical effectiveness (mortality), measures of safety (acute kidney injury and need for renal replacement therapies) and efficacy (cumulative fluid balance and need for organ support).
Search strategy
In November 2022 the lead reviewer (JBS) performed structured computer searches of the medical literature including Medline, Embase, CINHAL and the CENTRAL Cochrane databases. Table S1 in the online supporting information outlines the structure and medical subject headings (MeSH) utilised for searches. All terms were searched for as ‘OR’ within columns and ‘AND’ across columns (see Tables S1 and S2 in the online supporting information). We also performed searches of clinical trial registries (ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform), along with conference proceedings through Web of Science (BIOSIS Previews) and the grey literature via forward citation tracking through Google Scholar.
Screening, data extraction and assessment of risk of bias
All search results were imported into EndNote for automated duplicate removal, before export into an online systematic review manager (Rayyan: https://www.rayyan.ai/). Two reviewers (JBS & ME) independently screened both study abstracts, and full text studies for inclusion. Any discrepancies or disagreements were resolved through consultation with a third reviewer (TWF).
The lead reviewers (JBS and ME) performed critical appraisal of full text articles using the Cochrane Risk of Bias 2 tool. 16 Risk of bias was assessed across the domains of: randomisation, protocol deviations, missing outcome data, outcome measurement and selective reporting. An overall risk of bias assessment was determined for each included study. All data were extracted using a structured data collection tool, based on the Cochrane Handbook for Systematic Reviews and piloted by reviewers prior to study screening. 17 Relevant data were input and analysed in RevMan (Review Manager [Computer programme]. Version 5.4.1, The Cochrane Collaboration, 2020.).
Data analysis
Binary variables such as mortality were calculated as odds ratios (ORs) with 95% confidence intervals (CIs). Continuous variables were calculated as mean differences (MDs) with CIs. Medians were converted to mean values using the method reported by Wan et al. 18 Confidence intervals were calculated using the WALD method. Data from studies reporting consistent outcomes at similar time-points were pooled for meta-analysis in RevMan. Data for comparator groups were synthesised so long as participants had not received any albumin. Statistical heterogeneity was assessed using the between-study variance (τ 2 ), I2 statistic and hypothesis test for heterogeneity, with values >50% for I2 and a significance level of p < 0.1 considered to indicate significant heterogeneity. Analyses were performed using both random (using DerSimonian and Laird methods) and fixed effect models (using generic inverse variance methods), except where between-study heterogeneity for an outcome was 0 and analysis therefore reverted to a fixed effect model. 19 To pool data across studies using inverse variance methods, the data are transformed on to an additive scale using the natural logarithm. 20 Publication bias was assessed using funnel plots and explored using trim and fill methods where applicable. 17 Due to a lack of studies included in the meta-analysis, subgroup analyses were not explored. For outcomes where it was not possible to pool data, individual effect estimates for such studies are reported where available and included in a narrative synthesis.
Quality of evidence
Following meta-analysis, an assessment of certainty of evidence was conducted for mortality as a measure of effectiveness; need for renal replacement therapy for safety; and cumulative fluid balance, dependence on mechanical ventilation and duration of shock as measures of efficacy. This was performed using the GRADE principles (Grades of Recommendation, Assessment, Development and Evaluation), deriving a rating of the evidence as either: high, moderate, low or very low. 21 We used the GRADEpro Guideline Development Tool to produce a summary of findings table, illustrating the overall certainty of evidence on the influence of hyperoncotic albumin on the outcomes analysed (GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org). Certainty of evidence was assessed across several domains including: risk of bias, inconsistency, indirectness, imprecision and publication bias.
Results
Literature search
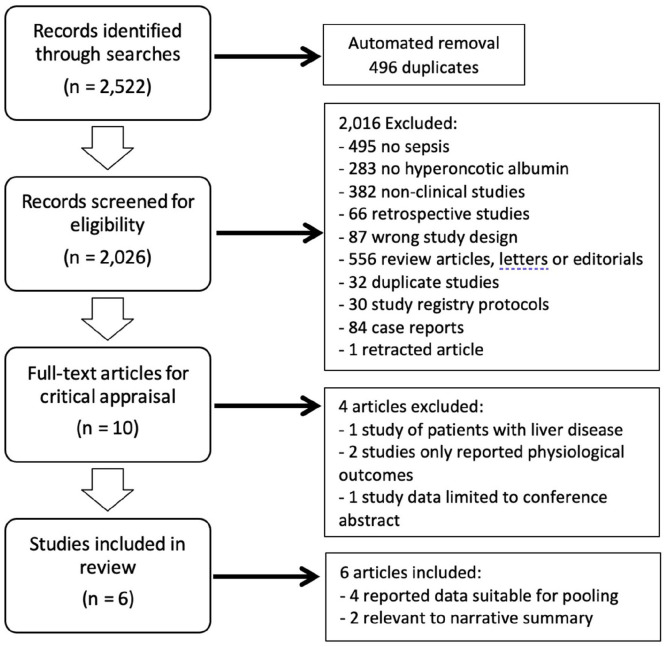
Our searches identified a total of 2522 results (Figure 1). Removal of duplicates (n = 496) through EndNote and abstract screening in Rayyan (n = 2016) resulted in 10 studies proceeding to full-text review. Four articles were excluded following full-text review for the reasons listed in Figure 1.22 –25 Of the six included studies, four reported data suitable for pooling and meta-analysis for the outcomes of 28-day mortality, need for renal replacement therapy (safety) and cumulative fluid balance.26 –29 The remaining two were deemed relevant to our review question and therefore included in Table 1 and the narrative of our results.25,30,31
Figure 1.
PRISMA flow diagram. PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Table 1.
Characteristics of included studies.
| Author & year | No of participants | Mean age (±SD) | Population | Hyperoncotic albumin intervention | Control | Duration to last follow-up (days) | Relevant outcomes |
|---|---|---|---|---|---|---|---|
| Annane 2013 | 2857 (1553 sepsis) | Albumin: 63 (±19.3)
a
Control: 62.7 (±18.6) a |
Adults requiring fluid resuscitation | Mixed colloids (including 20% albumin) for fluid therapy during ICU stay | Crystalloid solutions for all fluid therapy | 90 | Mortality @ 28 days Need for RRT Days alive & free from organ support |
| Caironi 2014 | 1818 | Albumin: 68 (±14.8)
a
Control: 68.3 (±13.7) a |
Adults with severe sepsis | 300 ml 20% albumin followed by daily dosing titrated to serum level | Crystalloid fluids | 90 | Mortality @ 28 days Need for RRT & MV Duration of shock CFB @ 7 days |
| Charpentier 2011 | 798 | 65.7 (±15.6) a b | Adults with septic shock | 100 ml 20% albumin 8-hourly for 3 days | 100 ml 0.9% saline 8-hourly for 3 days | 28 | Mortality @ 28 days Duration of shock |
| Dolecek 2009 | 56 | Albumin: 49 (±48.2)
a
Control: 44 (±34.5) a |
Adults with severe sepsis | 100 ml 20% albumin 12-hourly for 3 days | 250 ml 6% starch 6-hourly for 3 days | 28 | Mortality @ 28 days Duration of MV CFB @ 3 days |
| Maiwall 2022 | 100 | Albumin: 50.1 (±9.87) Control: 47.3 (±11.3) |
Adults with cirrhosis and sepsis-induced hypotension | 0.5–1.0 g/kg 20% albumin over 3 h | 30 ml/kg of PlasmaLyte-148 solution over 3 h | 28 | Mortality @ 28 days Need for RRT & MV Duration of shock CFB @ 24 h |
| Martensson 2018 | 321 (35 sepsis) | Albumin: 65.9 (±11.7)
a
Control: 64.3 (±12.5) a |
Adults requiring fluid resuscitation | 20% albumin for all fluid resuscitation for up to 48 h | 4%–5% albumin for all fluid resuscitation for up to 48 h | Hospital discharge | CFB @ 48 h |
RRT: renal replacement therapy; MV: mechanical ventilation; CFB: cumulative fluid balance.
Mean values mathematically derived from reported median.
Mean age reported for whole cohort only.
Study characteristics and risk of bias
The characteristics of studies included in our review are summarised in Table 1. All studies were prospective interventional trials that included a parallel control group. Allocation to treatment groups was random in all studies. The number of participants with sepsis enrolled ranged from 35 to 1818. Four studies exclusively recruited adults with sepsis or septic shock.25 –29 Annane et al. 30 and Martensson et al. 31 studied broader populations of adults requiring fluid resuscitation, of which 1553 (54%) and 35 (11%) had a diagnosis of sepsis respectively. Patients allocated to experimental groups received 20% human albumin solutions across all studies apart from Annane et al. where the interventional group received various colloid solutions. About 201 (14%) patients in the colloid arm received 20% albumin in this study, which also suffered from significant contamination through albumin use in the crystalloid control group. Those allocated to study control groups received a mixture of comparator fluids, including crystalloids (n = 5), starch solutions (n = 1), and isotonic albumin (n = 2). The longest reported follow-up period was 90 days.
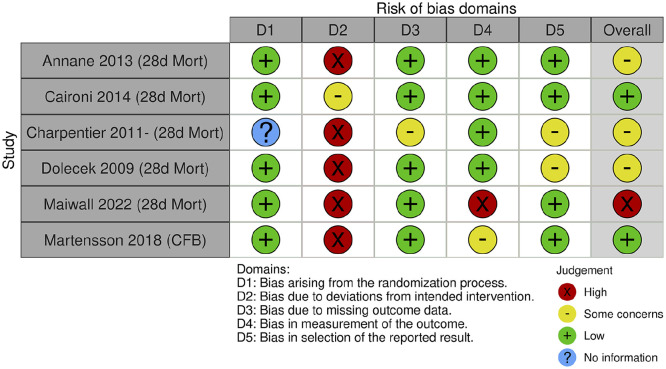
A summary of risk of bias assessments is illustrated in Figure 2. Four studies were found to have some concerns or be at a high risk of bias.27 –30 Caironi et al. 26 and Martensson et al. 31 were deemed to have an overall low risk of bias, but still presented concerns around blinding of the study interventions. Sources of bias for all studies were mainly around blinding, adherence to the intervention, or outcome measurement. Most study authors acknowledged lack of blinding as a source of bias, but cited the practical difficulties in blinding albumin infusions, as the solutions are presented in glass bottles, whereas most comparator fluids are presented in plastic containers. There were either some concerns, or a low risk of bias concerning the domains of missing data and selective outcome reporting.
Figure 2.
Risk of bias assessments for included studies. Outcomes assessed: 28d Mort: mortality 28-days after enrolment; CFB: cumulative fluid balance.
Outcomes of clinical effectiveness
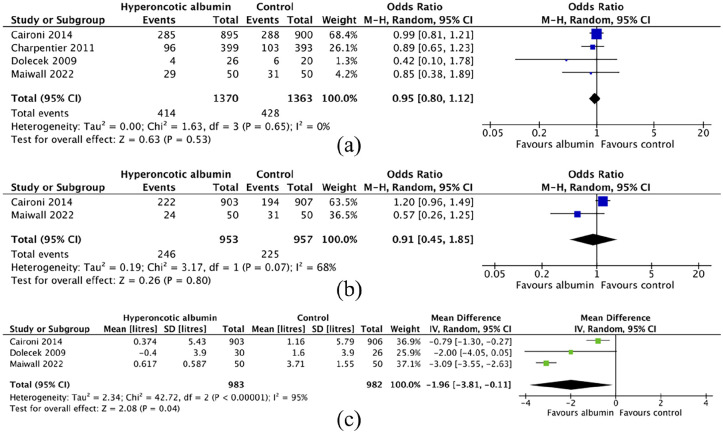
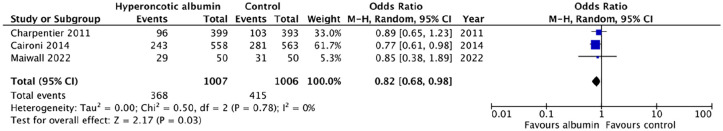
Mortality data were available for six studies, four of which reported at the same time point of 28-days after enrolment.24 –27 The pooled odds ratio (OR) for 28-day mortality in these four studies was 0.95 [95% CI: 0.8–1.12] (Figure 3(a)). Pooled data limited to studies or sub-groups of patients with septic shock resulted in an OR for 28-day mortality of 0.82 [95% CI: 0.68–0.98] (Figure 4). Statistical heterogeneity was low (I2 = 0%) across all comparisons. The certainty of evidence concerning 28-day mortality was graded as low (Table 2) for patients with sepsis. Levels of certainty were downgraded due to risk of bias caused by a lack of blinding in all trials, and point estimates of effect being largest in the smallest studies. The certainty of evidence for the sub-group analysis in patients with septic shock was graded as moderate; the point estimates were more consistent and precise when limited to these high-risk patients despite lower numbers of participants (Figure 4). A funnel plot for 28-day mortality revealed some asymmetry, suggesting some risk of publication bias (see Figures S1–S3 in the online supporting information).
Figure 3.
Forest plots for pair-wise comparisons of outcome measures of efficacy (28-day mortality) and safety (need for renal replacement therapy & cumulative fluid balance): (a) 28-day mortality, (b) need for renal replacement therapy, and (c) cumulative fluid balance.
Figure 4.
Forrest plot for pair-wise comparisons of 28-day mortality in sub-groups of patients with septic shock.
Table 2.
Summary of findings.
| Hyperoncotic albumin compared to non-albumin containing fluids in the management of sepsis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: the management of sepsis Setting: hospitalised adults with a diagnosis of sepsis Intervention: hyperoncotic albumin Comparison: non-albumin containing fluids | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non-albumin containing fluids | Risk with hyperoncotic albumin | |||||
| Mortality in patients with sepsis follow-up: 28 days | 314 per 1000 | 303 per 1000 (268–339) | OR 0.95 (0.80–1.12) | 2733 (4 RCTs) | ⨁⨁◯◯ | The evidence suggests hyperoncotic albumin results in little to no difference in mortality in patients with sepsis. |
| Low a | ||||||
| Mortality in patients with septic shock follow-up: 28 days | 413 per 1000 | 365 per 1000 (323–408) | OR 0.82 (0.68–0.98) | 2013 (3 RCTs) | ⨁⨁⨁◯ | Hyperoncotic albumin probably results in a reduction in mortality in patients with septic shock. |
| Moderate b | ||||||
| Need for renal replacement therapy follow-up: 90 days | 235 per 1000 | 219 per 1000 (122–363) | OR 0.91 (0.45–1.85) | 1910 (2 RCTs) | ⨁⨁◯◯ | Hyperoncotic albumin may result in little to no difference in need for renal replacement therapy. There is no evidence linking hyperoncotic albumin to acute kidney injury. |
| Low c | ||||||
| Cumulative fluid balance assessed with: litres follow-up: range 1 days to 7 days | The mean cumulative fluid balance was 0 L | MD 1.96 L lower (3.81 to 0.11 lower) | - | 1965 (3 RCTs) | ⨁◯◯◯ | Hyperoncotic albumin may reduce cumulative fluid balance in patients with early sepsis. |
| Very low d | ||||||
| Duration of mechanical ventilation assessed with: days | Studies measured dependency on mechanical ventilation differently: one reported median [IQR] duration of mechanical ventilation in days, another reported mean (±SD) duration and another simply the number (%) who required mechnical ventilation in each group. Data were not suitable for pooling, but comparisons in individual studies were similar between groups. | 1941 (3 RCTs) | ⨁⨁◯◯ | Hyperoncotic albumin was not associated with any difference in the use or duration of mechanical ventilation. | ||
| Low e | ||||||
| Duration of shock assessed with: various | Included studies measured duration of shock differently: one measured the number of days until vasoprerssors were suspended, another used the number of patients for whom shock was reversed at 6, 12, 24 and 48 h. A third study stated that patients receiving albumin had a higher number of days free from receiving catecholamines, but there were no evaluable data reported. | 2687 (3 RCTs) | ⨁⨁◯◯ | The use of hyperoncotic albumin may result in faster resolution of shock and shorter duration of cardiovascular support | ||
| Low f | ||||||
CI: confidence interval; MD: mean difference; OR: odds ratio.
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
All studies at some or high risk of bias around blinding. Comparator fluid in Dolecek et al. 28 was 6% starch, now shown to be harmful in sepsis. Point estimates of smaller studies suggest the largest treatment effect.
All studies at risk of bias due to lack of blinding.
Smallest study suggests the largest effect size; heterogeneity in populations and duration of intervention between studies.
CFB measured at different time points across included studies (ranging from 1 to 7 days). CFB also vulnerable to measurement error and reliance on medical record keeping. High statistical heterogeneity (I2 = 95%). 95% CI either large (Dolecek et al. 28 ) or not overlapping (Caironi et al. 26 and Maiwall et al. 29 ).
Studies used different methods to measure the use and duration of mechanical ventilation and were all at risk of bias to lack of blinding.
Studies used different methods to measure reversal of shock and dependence on cardiovascular support. Studies with longest duration of intervention reported greatest effects, indicating a possible dose-response relationship.
Anane et al.’s 28 CRYSTAL trial reported mortality at both 28 and 90-days, but raw data for those patients receiving hyperoncotic albumin were not available. Martensson et al. reported survival at ICU and hospital discharge. Survival to ICU discharge was higher in patients receiving 20% albumin (RR 1.07 [95% CI: 1.01–1.13]), however this was for a mixed population of critically ill patients. Survival in both groups was >90%, illustrating the broad low-risk population enrolled into the trial. Mortality data for the sub-groups (n = 35) of patients who had sepsis were not reported. 29
Safety outcomes
Adverse renal events
There is low certainty evidence of no difference in need for RRT between the groups, based on data from three trials, of which two were suitable for pooling (OR 0.91 [95% CI: 0.45–1.85], see Figure 3(b)).24,27 As with mortality, data from Annane et al.’s 28 CRYSTAL trial did not report renal outcomes for the group receiving hyperoncotic albumin. Renal safety outcomes were vulnerable to bias due to the heterogeneity of populations and the duration of the hyperoncotic albumin intervention. Point estimates were inconsistent and imprecise when pooled, leading to downgrading certainty of evidence to low. Martensson et al. also reported similar-albeit low-risk for renal replacement therapy in patients receiving 20% versus 4%–5% albumin solutions (RR 0.79 [95% CI: 0.25–2.42]).
Other measures of safety
Maiwall et al. 29 reported significant declines in oxygenation (PaO2/FiO2 ratio) for patients receiving hyperoncotic albumin compared to plasmalyte, resulting in discontinuation of the trial drug in 11 (22%) patients. Caironi et al. 26 also reported detailed data on organ failure scores, but found no difference in measures of oxygenation between treatment groups. Dolecek et al. 28 reported extravascular lung water fell following administration of hyperoncotic albumin, but found no associated impact on oxygenation (PaO2/FiO2 ratio).
Efficacy outcomes
Fluid volumes
Cumulative fluid balance was reported in four studies.24,26,27,29 Martensson et al. reported early CFB at 48 h to be lower in those receiving 20% albumin, but as the sample included patients without sepsis these data were not suitable for pooling. Caironi et al., Dolecek et al. and Maiwall et al. reported CFB data at 7 days, 3 days and 24 hours after enrolment respectively. Acknowledging this source of heterogeneity (I2 = 95%), Figure 3(c) illustrates the forest plot for meta-analysis of these data, revealing nearly 2 L lower CFB value for patients receiving hyperoncotic albumin compared to non-albumin control fluids (Mean difference: −1.91 L [95% CI: −3.81 to −0.11]). Evidence for effect on cumulative fluid balance was downgraded to very low due to heterogeneity of its measurement time-point and potential for confounding. The two studies utilising hyperoncotic albumin as a resuscitation fluid (as opposed to a regular supplement) both reported lower total fluid resuscitation volumes in patients receiving hyperoncotic albumin.25,30,31
Dependence on organ support
Four studies reported data concerning patients’ dependence on artificial organ support.24 –27 These outcomes included time dependent on mechanical ventilation and various measures of cardiovascular support, including duration of shock and time free from vasopressors at day 28. All studies utilised different definitions and methods of measurement for these outcomes. Caironi et al., Dolecek et al. and Maiwall et al. all reported no significant difference in the need or duration of mechanical ventilation between groups. Grading of evidence for this outcome was low, downgraded due to risk of bias arising from lack of blinding and differences in measuring dependence on respiratory support across studies. Three studies reported outcomes on cardiovascular support, the definitions of which included time to suspension of vasopressors, 26 norepenepherine dose requirements 29 and catecholamine-free days until day 28. 27 All studies found these outcomes more favourable, -that is, shorter period of cardiovascular support- for patients receiving hyperoncotic albumin. The certainty of this evidence was graded as moderate, principally due to differences in outcome measurement and lack of blinding. Reviewers noted there may-be a dose-response relationship between duration of the intervention and dependence on cardiovascular support, with studies administering the largest doses of hyperoncotic albumin to trial participants, reporting the greatest effect on duration of cardiovascular support.
Discussion
To our knowledge this is the first systematic review and meta-analysis specifically focussing on the efficacy, safety and effectiveness of hyperoncotic albumin solutions in the management of adults with sepsis. Two previous reviews have considered a range of albumin preparations (4%–20% strength solutions) in patients with sepsis, reporting similar weak evidence for reduced mortality in albumin groups.12,32 The 2011 review by Delaney et al. 12 included articles that have since been retracted from the literature, and many of these studies administered starch solutions to those in the comparator arms, which have since been shown to increase mortality in sepsis.33 –35 Xu et al. 32 and colleagues conducted a more recent review of studies testing a variety of albumin solutions ranging from 4% to 20% in patients with sepsis. Their meta-analysis revealed a trend towards improved mortality at 90 days for patients with severe sepsis, a classification of illness severity which is no longer in widespread use. In accordance with our findings however, Xu et al. found a significant reduction in mortality for patients with septic shock receiving albumin solutions. Their analysis included 2186 participants receiving a mixture of albumin products, whereas our pooled analysis included 2013 participants receiving more homogenous interventions limited to hyperoncotic albumin solutions only.
The only other review of trial data limited to hyperoncotic albumin interventions was published by Jacob et al. 13 This review considered a variety of patient populations other than those with sepsis, including adults and children undergoing cardiac surgery and patients suffering major trauma. They concluded that hyperoncotic albumin solutions were safe for fluid resuscitation, but couldn’t characterise any benefit in terms of mortality for patients with sepsis. Our review has included new trial data published since Jacob’s original review, specific to hyperoncotic albumin in patients with sepsis and septic shock.26,27,29 Finally, the most recent systematic review on this topic was published in 2023 by Geng et al. 36 Their review considered a similar question to ours on patients with sepsis, but included studies administering any concentration of albumin solution. In accordance with our results, Geng et al. also found albumin solutions resulted in a reduced risk of death for those patients with septic shock. Whilst their review question, search and analysis methods were similar to ours, Geng et al. 21 did not provide any structured assessment of the certainty of evidence, which we have provided here through Cochrane’s GRADE assessment tool.
Strengths of this review
This review was conducted according to a pre-specified protocol, published in the public domain prior to conducting any searches. The review question was intentionally and uniquely refined towards a specific intervention in well-defined populations of patients with sepsis. We utilised established high-quality methodology for searches, critical appraisal, analysis and reporting, to ensure our findings are an accurate and reliable interpretation of the available evidence in this field. Searches of the medical literature for this review were rigorous and authors of conference abstracts were contacted with requests for further data. We are therefore confident this review summarises all data and evidence available for this review question.
Limitations of review findings
The types of comparator fluids used in trials varied considerably. During data pooling and meta-analysis we limited data extraction to trials using non-albumin fluids as a comparator. One study administered starch solutions in the comparator arm, which could have had a negative impact on survival of control subjects. 28 We therefore performed a sensitivity analysis for 28-day mortality excluding this study, and found the results were robust (see Figure S7 in the online supporting information). Due to the low number of studies eligible for inclusion in this review, we were unable to utilise network meta-analysis techniques to compare outcomes between hyperoncotic albumin and different types of comparator fluids. Bansal et al. 37 published data using such methods in 2013. They compared data from studies of patients with severe sepsis, receiving a mixed range of albumin solutions versus both crystalloid and starch solutions. Their analysis of data from 13 studies, found a reduced risk of mortality for patients receiving albumin compared to crystalloids (Bayesian fixed effect model only) and starch solutions (both Bayesian fixed and random effect models).
Another significant limitation of this review is the low number of under-powered studies with data suitable for synthesis and meta-analysis. This prevented us making any power calculation, however our meta-analysis would be considered more powerful than the results of individual studies alone and whilst the optimal information size (OIS) varies between outcomes, it is very rarely achieved during meta-analysis of mortality. 38 This also limited our assessment of publication bias, which was only evident for studies reporting 28-day mortality for all patients with sepsis (see Figures S1–S5 in the online supporting information). To date, there are three large randomised controlled trials of hyperoncotic albumin in patients with septic shock currently open and recruiting.39 –41 Data from these trials are likely to be influential in fully answering this review’s question and increasing the certainty of evidence and the strength of any subsequent clinical recommendations.
The studies included in our review generally administered hyperoncotic albumin as a single dose or regular supplement. Annane et al. and Martensson et al. administered albumin as a resuscitation fluid, but data from these trials were not suitable for pooling, due to the mixed nature of their patient populations.28,29 The most effective administration strategy for hyperoncotic albumin has yet to be identified, but it appears safe as both a resuscitation fluid and regular supplement. Intervention periods varied from a single dose infused over 3 hours to daily administration for up to 28 days. It was not possible to make comparisons between strategies, yet data from future clinical trials might allow such comparisons through network meta-analysis techniques.
Remaining areas of uncertainty
Our finding of reduced mortality in patients with septic shock, whilst plausible is based on low quality evidence and requires confirmation through further high-quality randomised clinical trials in this specific high-risk population. The optimal dosing strategy for hyperoncotic albumin has yet to be established. Whilst safe as both a resuscitation fluid and a regular supplement, it is unclear whether one approach is more effective or whether they might be combined. Heterogeneity of comparator fluids will limit the strengths of pairwise comparison techniques, although this maybe overcome in the future using network meta-analysis techniques once more trial data become available.
Conclusions
The use of hyperoncotic albumin appears safe in patients with sepsis. There is weak evidence suggesting a short-term mortality benefit in patients with septic shock, but no evidence to support its broader use in patients with sepsis. Its use is associated with a lower short-term cumulative fluid balance and faster resolution of shock. Further trials are needed to improve the evidence base and guide clinical care. Future trials should focus on patients with septic shock, whilst evaluating the optimal dosing strategy to achieve well defined and widely accepted clinical outcomes.
Supplemental Material
Supplemental material, sj-docx-1-inc-10.1177_17511437241259437 for The efficacy, safety and effectiveness of hyperoncotic albumin solutions in patients with sepsis: A systematic review and meta-analysis by Jonathan Bannard-Smith, Mohamed Elrakhawy, Gill Norman, Rhiannon Owen, Tim Felton and Paul Dark in Journal of the Intensive Care Society
Footnotes
Contribution of authors: JBS conceived the idea, protocol and draft manuscript of this review. JBS & ME were the principal two reviewers, TWF acted as a third reviewer where required. GN & RKO advised on appropriate methodology for this review. PMD provided advice and critical review of the protocol. All authors contributed to revisions of the final manuscript.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: None of the authors received any funding for conducting this review. JBS has previously received free product and unrestricted funding for research on this topic from CSL Behring, a commercial supplier of human albumin solutions. TWF and PMD are supported by the Manchester NIHR Biomedical Research Centre and PD is also supported by a NIHR Senior Investigator award. RKO is a member of the National Institute for Health and Care Excellence (NICE) Technology Appraisal Committee. She has served as a paid consultant to the pharmaceutical industry and international reimbursement agencies, providing unrelated methodological advice. She reports teaching fees from the Association of British Pharmaceutical Industry (ABPI) and the University of Bristol. GN is funded by the National Institute for Health and Care Research, Applied Research Collaboration-Greater Manchester (NIHR200174). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care, or its partner organisations.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Other information: This review was prospectively registered on the International prospective register of systematic reviews (PROSPERO) reference: CRD42021150674 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021150674).
ORCID iDs: Jonathan Bannard-Smith  https://orcid.org/0000-0001-7120-480X
https://orcid.org/0000-0001-7120-480X
Gill Norman  https://orcid.org/0000-0002-3972-5733
https://orcid.org/0000-0002-3972-5733
Paul Dark  https://orcid.org/0000-0003-3309-0164
https://orcid.org/0000-0003-3309-0164
Supplemental material: Supplemental material for this article is available online.
References
- 1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudd KE, Kissoon N, Limmathurotsakul D, et al. The global burden of sepsis: barriers and potential solutions. Crit Care 2018; 22: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45: 486–552. [DOI] [PubMed] [Google Scholar]
- 4. Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39: 259–265. [DOI] [PubMed] [Google Scholar]
- 5. Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care 2015; 19: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Mourik N, Geerts BF, Binnekade JM, et al. A higher fluid balance in the days after septic shock reversal is associated with increased mortality: an observational cohort study. Crit Care Explor 2020; 2: e0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bannard-Smith J, Alexander P, Glassford N, et al. Haemodynamic and biochemical responses to fluid bolus therapy with human albumin solution, 4% versus 20%, in critically ill adults. Crit Care Resusc 2015; 17: 122–128. [PubMed] [Google Scholar]
- 8. Bihari S, Bannard-Smith J, Bellomo R. Albumin as a drug: its biological effects beyond volume expansion. Crit Care Resusc 2020; 22: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iglesias J, Abernethy VE, Wang Z, et al. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol 1999; 277: F711–F722. [DOI] [PubMed] [Google Scholar]
- 10. Kouoh F, Gressier B, Luyckx M, et al. Antioxidant properties of albumin: effect on oxidative metabolism of human neutrophil granulocytes. Farmaco 1999; 54: 695–699. [DOI] [PubMed] [Google Scholar]
- 11. Roberts I, Blackhall K, Alderson P, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev 2011; 2011: CD001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39: 386–391. [DOI] [PubMed] [Google Scholar]
- 13. Jacob M, Chappell D, Conzen P, et al. Small-volume resuscitation with hyperoncotic albumin: a systematic review of randomized clinical trials. Crit Care 2008; 12: R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bannard-Smith J, Owen R, Elrkhawy M, et al. The efficacy and safety of hyperoncotic human albumin solution in patients with sepsis: a systematic review and meta-analysis. York: The National Institute of Health Research: International Prospective Registry of Systematic Reviews, 2021. [Google Scholar]
- 15. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest physicians/society of critical care medicine. Chest 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 16. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Chandler TJ, Cumpston J, et al. (eds) Cochrane handbook for systematic reviews of interventions (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook [Google Scholar]
- 18. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1: 97–111. [DOI] [PubMed] [Google Scholar]
- 20. Deeks J, Higgins J. Statistical algorithms in Review Manager 5. 2010. [Google Scholar]
- 21. Schünemann H, Guyatt BJ, Oxman A. (eds). GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013. https://www.gradeworkinggroup.org/ [Google Scholar]
- 22. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. New Engl J Med 1999; 341: 403–409. [DOI] [PubMed] [Google Scholar]
- 23. Palumbo D, Servillo G, D’Amato L, et al. The effects of hydroxyethyl starch solution in critically ill patients. Minerva Anestesiol 2006; 72: 655–664. [PubMed] [Google Scholar]
- 24. Geoffroy H, et al. Albumin infusion had protective endothelial effects in septic shock patients. Ann Intensive Care 2018; 8. [Google Scholar]
- 25. Kongsayreepong S, Nonthiaraj S. Early 20% vs 5 % albumin in addition to crystalloid resuscitation in surgical septic shock admitting to the general SICU. Crit Care 2019; 23. [Google Scholar]
- 26. Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. New Engl J Med 2014; 370: 1412–1421. [DOI] [PubMed] [Google Scholar]
- 27. Charpentier J, Mira JP. Efficacy and tolerance of hyperoncotic albumin administration in septic shock patients: The EARSS study. Intensive Care Med 2011; 37: S115–S0438. [Google Scholar]
- 28. Dolecek M, Svoboda P, Kantorová I, et al. Therapeutic influence of 20 % albumin versus 6% hydroxyethylstarch on extravascular lung water in septic patients: a randomized controlled trial. Hepatogastroenterology 2009; 56: 1622–1628. [PubMed] [Google Scholar]
- 29. Maiwall R, Kumar A, Pasupuleti SSR, et al. A randomized-controlled trial comparing 20% albumin to plasmalyte in patients with cirrhosis and sepsis-induced hypotension [ALPS trial]. J Hepatol 2022; 77: 670–682. [DOI] [PubMed] [Google Scholar]
- 30. Annane D, Siami S, Jaber S, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 31. Mårtensson J, Bihari S, Bannard-Smith J, et al. Small volume resuscitation with 20% albumin in intensive care: physiological effects : the SWIPE randomised clinical trial. Intensive Care Med 2018; 44: 1797–1806. [DOI] [PubMed] [Google Scholar]
- 32. Xu J-Y, Chen QH, Xie JF, et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: a meta-analysis of randomized clinical trials. Crit Care 2014; 18: 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perner A. Hydroxyethyl starch 130/0.4 versus Ringer’s acetate in severe sepsis. New Engl J Med 2012; 367: 481–534. [DOI] [PubMed] [Google Scholar]
- 34. Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet 2001; 357: 911–916. [DOI] [PubMed] [Google Scholar]
- 35. Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. New Engl J Med 2008; 358: 125–139. [DOI] [PubMed] [Google Scholar]
- 36. Geng L, Tian X, Gao Z, et al. Different concentrations of albumin versus crystalloid in patients with sepsis and septic shock: a meta-analysis of randomized clinical trials. J Intensive Care Med 2023; 38: 689. [DOI] [PubMed] [Google Scholar]
- 37. Bansal M, Farrugia A, Balboni S, et al. Relative survival benefit and morbidity with fluids in severe sepsis - a network meta-analysis of alternative therapies. Curr Drug Saf 2013; 8: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia-Alamino JM, Bankhead C, Heneghan C, et al. Impact of heterogeneity and effect size on the estimation of the optimal information size: analysis of recently published meta-analyses. BMJ Open 2017; 7: e015888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakr Y, Bauer M, Nierhaus A, et al. Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): protocol for a randomized controlled trial. Trials 2020; 21: 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pesenti A. ALBumin Italian outcome septic shock -BALANCED trial (ALBIOSS-BALANCED), https://clinicaltrials.gov/study/NCT03654001 (2021, accessed 10 November 2023).
- 41. Sang-Min K. Albumin and crystalloid administration in septic shock (ALCAMIST), https://clinicaltrials.gov/study/NCT05148286 (2021, accessed 10 November 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-inc-10.1177_17511437241259437 for The efficacy, safety and effectiveness of hyperoncotic albumin solutions in patients with sepsis: A systematic review and meta-analysis by Jonathan Bannard-Smith, Mohamed Elrakhawy, Gill Norman, Rhiannon Owen, Tim Felton and Paul Dark in Journal of the Intensive Care Society