Abstract
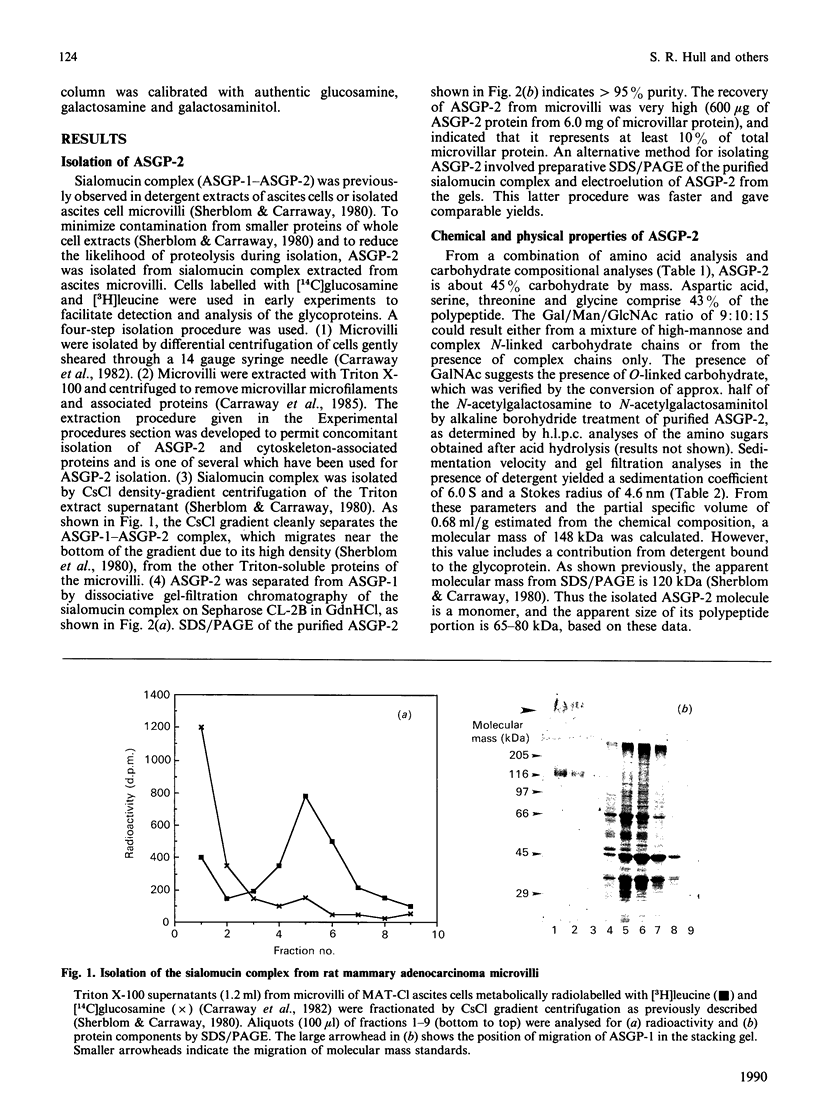
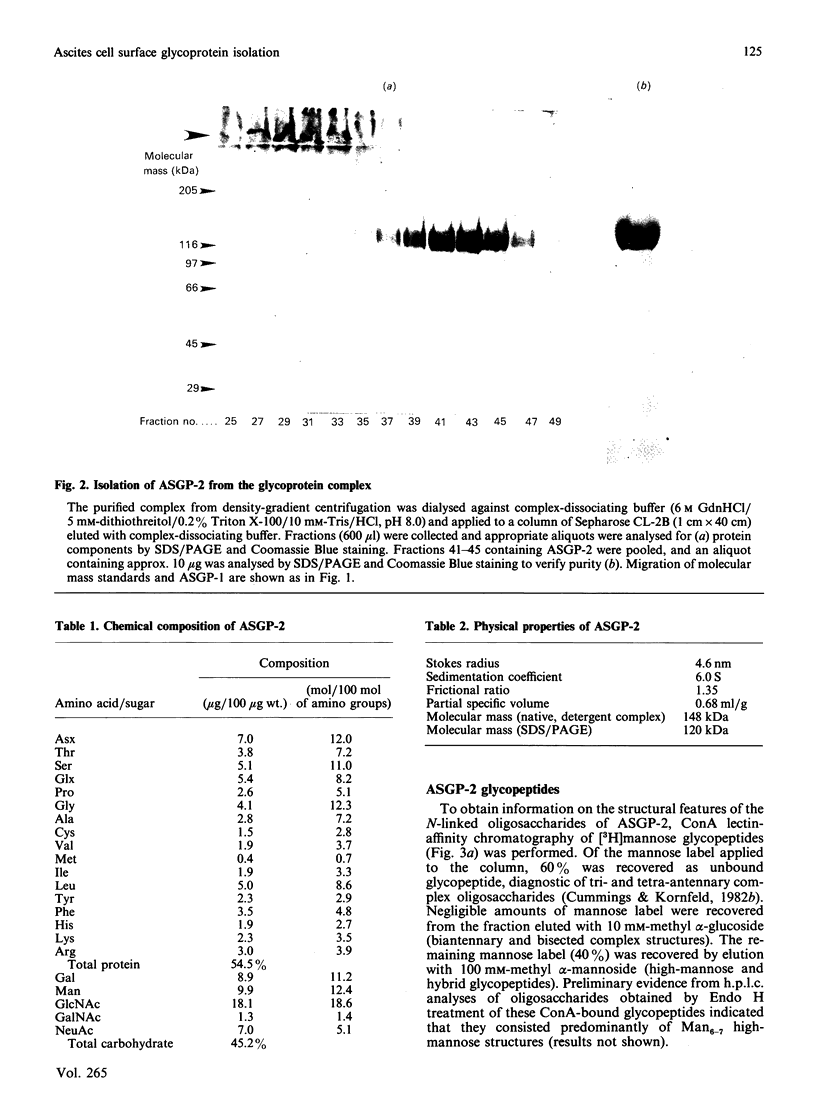
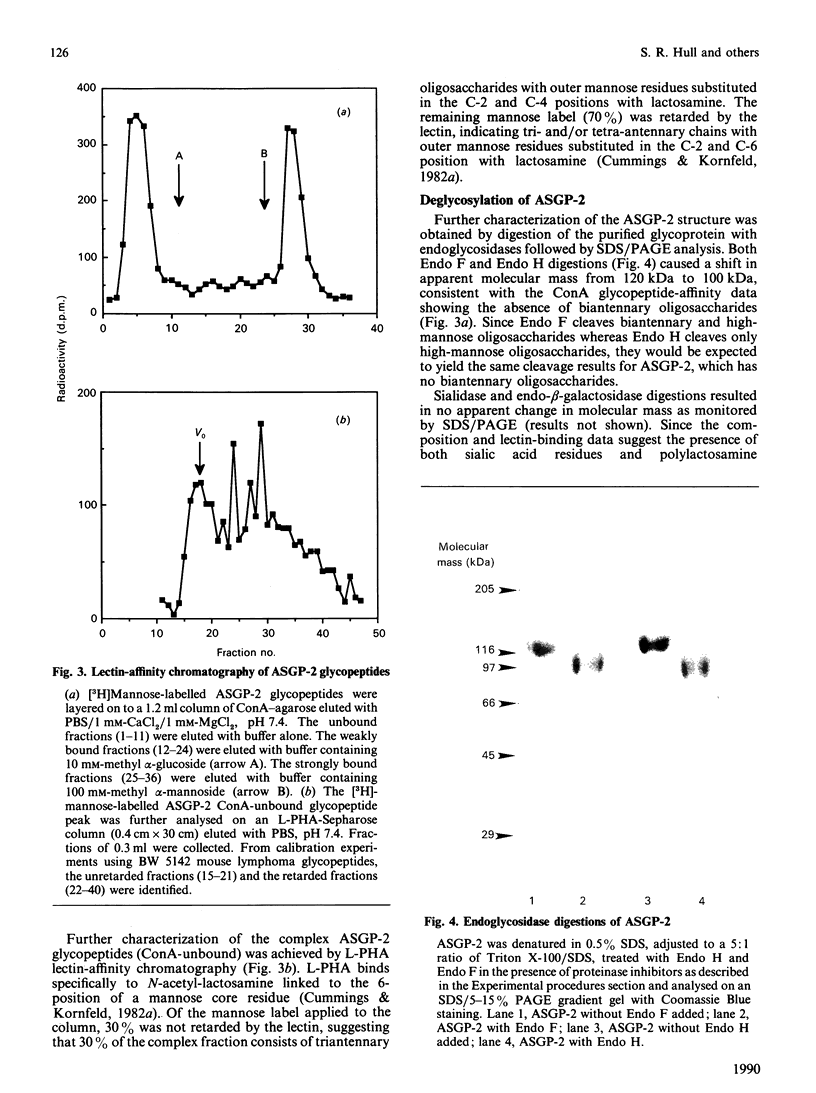
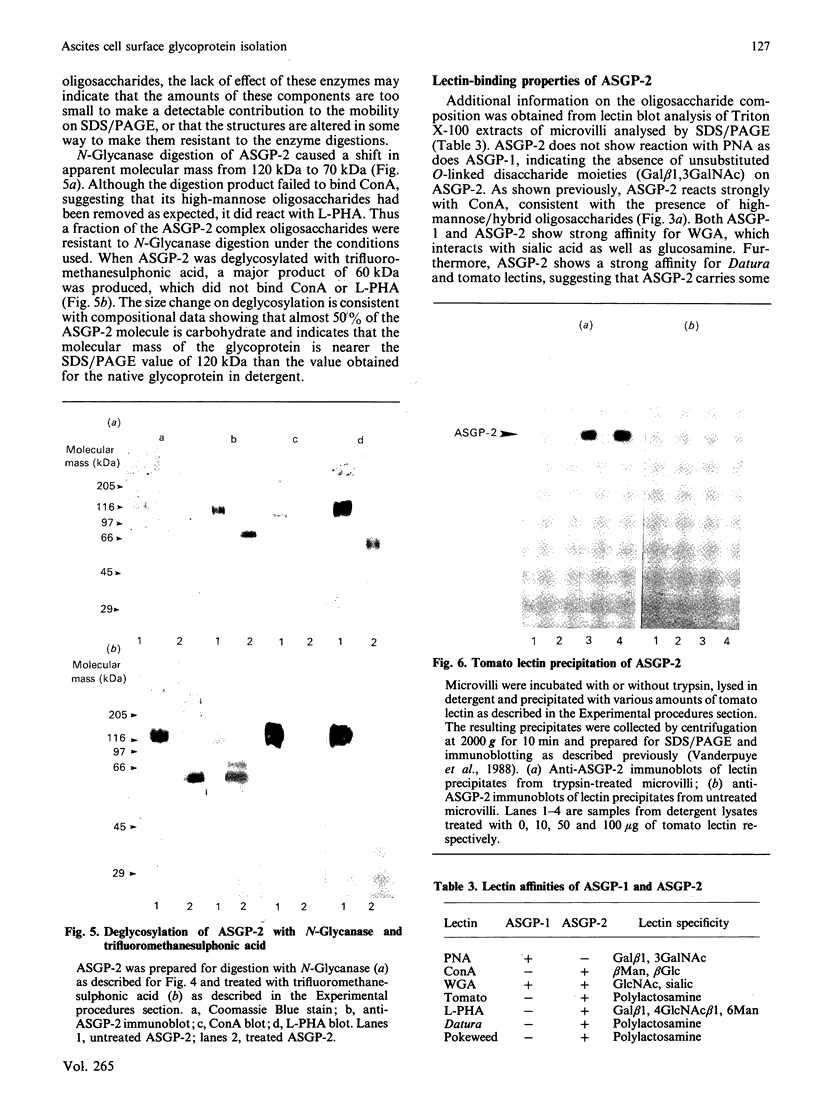
Sialomucins are the dominant components of the cell surfaces of some carcinoma ascites cells and have been postulated to inhibit recognition of tumours by the immune system. The sialomucin ASGP-1 (ascites sialoglycoprotein-1) of the 13762 rat mammary adenocarcinoma is associated with the cell surface as a complex with a concanavalin-A-binding glycoprotein called ASGP-2. This sialomucin complex has been purified from ascites cell microvilli by extraction with Triton X-100 and CsCl density-gradient centrifugation. ASGP-1 (which has been purified previously) and ASGP-2 were dissociated in 6 M-guanidine hydrochloride and separated by gel filtration. The molecular mass of the undenatured detergent complex of ASGP-2, estimated by gel filtration and velocity sedimentation in Triton X-100, was 148 kDa. Since the apparent molecular mass by SDS/polyacrylamide-gel electrophoresis was about 120 kDa, ASGP-2 must be a monomer as extracted from the membrane. Studies of its chemical composition indicate that it contains about 45% carbohydrate by weight, including both mannose and galactosamine. Alkaline borohydride treatment of ASGP-2 converted approx. half of the N-acetylgalactosamine to N-acetylgalactosaminitol, demonstrating the presence of O-linked oligosaccharides. Analyses of mannose-labelled Pronase glycopeptides from ASGP-2 by lectin-affinity chromatography on concanavalin A and leucocyte-agglutinating phytohaemagglutinin suggested that 40% of the label was present in high-mannose/hybrid oligosaccharides, 20% in triantennary oligosaccharides substituted on the C-2 and C-4 mannose positions and 40% in tri- or tetra-antennary oligosaccharides substituted on C-2 and C-6. The presence of polylactosamine sequences on these oligosaccharides was suggested by lectin blots and by precipitation from detergent extracts with tomato lectin. From chemical analyses and lectin-affinity studies, we estimate that ASGP-2 contains four high-mannose and 13 complex N-glycosylated oligosaccharides, plus small amounts of polylactosamine and O-linked oligosaccharides. The presence of four different classes of oligosaccharides on this glycoprotein suggests that it will be an interesting model system for biosynthetic comparisons of the different glycosylation pathways.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Kufe D. Effects of maturational agents on expression and secretion of two partially characterized high molecular weight milk-related glycoproteins in MCF-7 breast carcinoma cells. J Cell Physiol. 1986 Jan;126(1):126–132. doi: 10.1002/jcp.1041260117. [DOI] [PubMed] [Google Scholar]
- Ahrens P. B., Ankel H. The role of asparagine-linked carbohydrate in natural killer cell-mediated cytolysis. J Biol Chem. 1987 Jun 5;262(16):7575–7579. [PubMed] [Google Scholar]
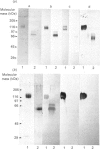
- Carraway C. A., Cerra R. F., Bell P. B., Carraway K. L. Identification of a protein associated with both membrane and cytoskeleton fractions from branched but not unbranched microvilli of 13762 rat mammary adenocarcinoma ascites tumor sublines. Biochim Biophys Acta. 1982 Oct 28;719(1):126–139. doi: 10.1016/0304-4165(82)90316-6. [DOI] [PubMed] [Google Scholar]
- Carraway C. A., Jung G., Hinkley R. E., Carraway K. L. Isolation of microvillar microfilaments and associated transmembrane complex from ascites tumor cell microvilli. Exp Cell Res. 1985 Mar;157(1):71–82. doi: 10.1016/0014-4827(85)90153-3. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., Doss R. C., Huggins J. W., Chesnut R. W., Carraway C. A. Effects of cytoskeletal perturbant drugs on ecto 5'-nucleotidase, a concanavalin A receptor. J Cell Biol. 1979 Dec;83(3):529–543. doi: 10.1083/jcb.83.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K. L., Spielman J. Structural and functional aspects of tumor cell sialomucins. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):109–120. doi: 10.1007/BF00230639. [DOI] [PubMed] [Google Scholar]
- Ceriani R. L., Blank E. W., Peterson J. A. Experimental immunotherapy of human breast carcinomas implanted in nude mice with a mixture of monoclonal antibodies against human milk fat globule components. Cancer Res. 1987 Jan 15;47(2):532–540. [PubMed] [Google Scholar]
- Ceriani R. L., Peterson J. A., Lee J. Y., Moncada R., Blank E. W. Characterization of cell surface antigens of human mammary epithelial cells with monoclonal antibodies prepared against human milk fat globule. Somatic Cell Genet. 1983 Jul;9(4):415–427. doi: 10.1007/BF01543043. [DOI] [PubMed] [Google Scholar]
- Codington J. F., Frim D. M. Cell-surface macromolecular and morphological changes related to allotransplantability in the TA3 tumor. Biomembranes. 1983;11:207–258. [PubMed] [Google Scholar]
- Codington J. F., Klein G., Cooper A. G., Lee N., Brown M. C., Jeanloz R. W. Further studies on the relationship between large glycoprotein molecules and allotransplantability in the TA3 tumor of the mouse: studies on segregating TA3-HA hybrids. J Natl Cancer Inst. 1978 Apr;60(4):811–818. doi: 10.1093/jnci/60.4.811. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J Biol Chem. 1982 Oct 10;257(19):11235–11240. [PubMed] [Google Scholar]
- Davis H. M., Zurawski V. R., Jr, Bast R. C., Jr, Klug T. L. Characterization of the CA 125 antigen associated with human epithelial ovarian carcinomas. Cancer Res. 1986 Dec;46(12 Pt 1):6143–6148. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S. Recognition of asparagine-linked oligosaccharides on murine tumor cells by natural killer cells. Cancer Res. 1985 Dec;45(12 Pt 1):6034–6040. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Helm R. M., Carraway K. L. Evidence for the association of two cell surface glycoproteins of 13762 mammary ascites tumor cells. Concanavalin A-induced redistribution of peanut agglutinin-binding proteins. Exp Cell Res. 1981 Oct;135(2):418–424. doi: 10.1016/0014-4827(81)90181-6. [DOI] [PubMed] [Google Scholar]
- Howard S. C., Sherblom A. P., Chesnut R. W., Carraway C. A., Carraway K. L. Changes in expression of a major sialoglycoprotein associated with ascites forms of a mammary adenocarcinoma. Biochim Biophys Acta. 1980 Aug 1;631(1):79–89. doi: 10.1016/0304-4165(80)90055-0. [DOI] [PubMed] [Google Scholar]
- Huggins J. W., Trenbeath T. P., Sherblom A. P., Howard S. C., Carraway K. L. Glycoprotein differences in solid and ascites forms of the 13762 rat mammary adenocarcinoma. Cancer Res. 1980 Jun;40(6):1873–1878. [PubMed] [Google Scholar]
- Hull S. R., Laine R. A., Kaizu T., Rodriguez I., Carraway K. L. Structures of the O-linked oligosaccharides of the major cell surface sialoglycoprotein of MAT-B1 and MAT-C1 ascites sublines of the 13762 rat mammary adenocarcinoma. J Biol Chem. 1984 Apr 25;259(8):4866–4877. [PubMed] [Google Scholar]
- Johnson V. G., Schlom J., Paterson A. J., Bennett J., Magnani J. L., Colcher D. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 1986 Feb;46(2):850–857. [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Lan M. S., Finn O. J., Fernsten P. D., Metzgar R. S. Isolation and properties of a human pancreatic adenocarcinoma-associated antigen, DU-PAN-2. Cancer Res. 1985 Jan;45(1):305–310. [PubMed] [Google Scholar]
- Liu Y. C., Carraway C. A., Carraway K. L. Nonmuscle tropomyosin from ascites tumor cell microvilli. J Biol Chem. 1986 Apr 5;261(10):4568–4573. [PubMed] [Google Scholar]
- Lohmander L. S. Analysis by high-performance liquid chromatography of radioactively labeled carbohydrate components of proteoglycans. Anal Biochem. 1986 Apr;154(1):75–84. doi: 10.1016/0003-2697(86)90498-7. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Steplewski Z., Koprowski H., Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983 Nov;43(11):5489–5492. [PubMed] [Google Scholar]
- Merkle R. K., Cummings R. D. Lectin affinity chromatography of glycopeptides. Methods Enzymol. 1987;138:232–259. doi: 10.1016/0076-6879(87)38020-6. [DOI] [PubMed] [Google Scholar]
- Merkle R. K., Cummings R. D. Relationship of the terminal sequences to the length of poly-N-acetyllactosamine chains in asparagine-linked oligosaccharides from the mouse lymphoma cell line BW5147. Immobilized tomato lectin interacts with high affinity with glycopeptides containing long poly-N-acetyllactosamine chains. J Biol Chem. 1987 Jun 15;262(17):8179–8189. [PubMed] [Google Scholar]
- Miotti S., Aguanno S., Canevari S., Diotti A., Orlandi R., Sonnino S., Colnaghi M. I. Biochemical analysis of human ovarian cancer-associated antigens defined by murine monoclonal antibodies. Cancer Res. 1985 Feb;45(2):826–832. [PubMed] [Google Scholar]
- Schlom J. Basic principles and applications of monoclonal antibodies in the management of carcinomas: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1986 Jul;46(7):3225–3238. [PubMed] [Google Scholar]
- Sheng Z., Vanderpuye O. A., Hull S. R., Carraway C. A., Carraway K. L. Topography and microfilament core association of a cell surface glycoprotein of ascites tumor cell microvilli. J Cell Biochem. 1989 Aug;40(4):453–466. doi: 10.1002/jcb.240400406. [DOI] [PubMed] [Google Scholar]
- Sherblom A. P., Buck R. L., Carraway K. L. Purification of the major sialoglycoproteins of 13762 MAT-B1 and MAT-C1 rat ascites mammary adenocarcinoma cells by density gradient centrifugation in cesium chloride and guanidine hydrochloride. J Biol Chem. 1980 Jan 25;255(2):783–790. [PubMed] [Google Scholar]
- Sherblom A. P., Carraway K. L. A complex of two cell surface glycoproteins from ascites mammary adenocarcinoma cells. J Biol Chem. 1980 Dec 25;255(24):12051–12059. [PubMed] [Google Scholar]
- Sherblom A. P., Moody C. E. Cell surface sialomucin and resistance to natural cell-mediated cytotoxicity of rat mammary tumor ascites cells. Cancer Res. 1986 Sep;46(9):4543–4546. [PubMed] [Google Scholar]
- Steck P. A., Nicolson G. L. Cell surface glycoproteins of 13762NF mammary adenocarcinoma clones of differing metastatic potentials. Exp Cell Res. 1983 Sep;147(2):255–267. doi: 10.1016/0014-4827(83)90208-2. [DOI] [PubMed] [Google Scholar]
- Vanderpuye O. A., Carraway C. A., Carraway K. L. Microfilament association of ASGP-2, the concanavalin A-binding glycoprotein of the cell-surface sialomucin complex of 13,762 rat mammary ascites tumor cells. Exp Cell Res. 1988 Oct;178(2):211–223. doi: 10.1016/0014-4827(88)90392-8. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Ohkura T., Kobata A. Enzymatic basis for the structural changes of asparagine-linked sugar chains of membrane glycoproteins of baby hamster kidney cells induced by polyoma transformation. J Biol Chem. 1985 Apr 10;260(7):3963–3969. [PubMed] [Google Scholar]