Abstract
Invasive candidiasis (IC) is an increasingly prevalent, costly, and potentially fatal infection brought on by the opportunistic yeast, Candida. Previously, IC has predominantly been caused by C. albicans which is often drug susceptible. There has been a global trend towards decreasing rates of infection secondary to C. albicans and a rise in non-albicans species with a corresponding increase in drug resistance creating treatment challenges. With advances in management of malignancies, there has also been an increase in the population at risk from IC along with a corresponding increase in incidence of breakthrough IC infections. Additionally, the emergence of C. auris creates many challenges in management and prevention due to drug resistance and the organism’s ability to transmit rapidly in the healthcare setting. While the development of novel antifungals is encouraging for future management, understanding the changing epidemiology of IC is a vital step in future management and prevention.
Keywords: Candida, epidemiology, resistance, emerging, non-albicans Candida species
Introduction
Invasive candidiasis (IC), defined for the purposes of this review as Candida spp. in the blood (candidemia) or Candida spp. isolated from sterile tissue (hepatosplenic candidiasis and intraabdominal candidiasis, as examples) remains a costly, morbid, and often fatal infection. Candidemia has been found to increase 90-day mortality rates by >28%.1 For the purpose of this review, Nakaseomyces glabrata will be referred to as Candida glabrata along with Pichia kudriavzevii as Candida krusei due to these names predominantly still being used clinically. Since the 1990s, there have been multiple strategies aimed at reducing the incidence of invasive infection, including antifungal prophylaxis in the highest risk groups and infection prevention strategies to reduce the rate of nosocomial infections. However, while there has been an overall reduction in incidence, new challenges have emerged including a shift to non-albicans Candida spp., and novel species such as C. auris, breakthrough infections, and drug resistance.2 Here, the changing epidemiology of invasive candidiasis and impact upon treatment are reviewed.
Clinical Manifestations of Candidemia and Invasive Candidiasis
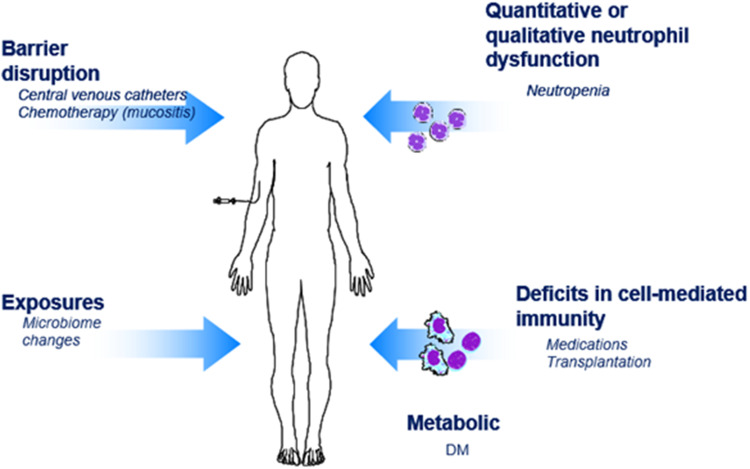
The clinical manifestations of Candida infection are broad and range from local infection of the mucus membranes to severe disseminated infection with accompanying sepsis. Candida spp. are considered a normal constituent of the human skin, gastrointestinal, and genitourinary microbiomes. Infection occurs when host defenses are impaired or via an imbalance of microbiome with ensuing Candida overgrowth (Figure 1).
Figure 1.
Common risk factors for invasive candidiasis. Risk factors: intravenous catheters, total parental nutrition, post-procedure, broad-spectrum antibiotics, colonization at non-sterile sites, chemotherapy, transplant recipients.
Host Immune Response
The mucosa is continuously exposed to Candida spp, and as such a highly coordinated immune response has evolved for host tolerance and the prevention of invasion. Epithelial cells are an essential barrier to infection and following Candida attachment via fungal adhesins, such as the agglutinin-like sequence (Als) protein family,3 epithelial cells detect pathogen-associated molecular patterns (PAMPs), such as mannan and 1,3-β-D-glucan. Immune recognition likely differs between epithelial surfaces and during systemic infection. Toll-like receptors and Dectin-1 are well recognized and critical components of host defense during invasive infection,4 however epithelial responses may require non-classical receptors such as E-cadherin, EGFR/Her2, and EphA1.5,6 The response must simultaneously kill fungi while minimizing the surrounding inflammatory reaction and maintaining immune homeostasis. These differences in host immunity may be driven by the form of Candida present with the pseudohyphal form present in invasive infections, and yeast on mucosal surfaces.7
A number of pattern recognition receptors (PRRs) have been identified including TLR2, TLR4, Dectin-1, FcγR, mannose receptor, galectin 3, MINCLE, and DC-SIGN.5 Downstream signaling involving CARD9 and SYK have also been observed as essential in the response to Candida invasion.8 A number of host polymorphisms within these genomic regions have been identified and noted to confer increased host susceptibility to infection.5
Tissue-resident macrophages play a key role in antifungal defense and produce inflammatory cytokines and chemokine to recruit and activate other immune cells including neutrophils. Neutrophil activation is essential for the clearance of Candida, with neutropenia the major risk factor for invasive disease.5 Neutrophils are additionally the only host cell capable of inhibiting Candida germination, and murine models of infection have clearly demonstrated the critical role of neutrophils in candidemia and/or invasive candidiasis.9
Following phagocytosis, killing occurs by the generation of NADPH dependent reactive oxidant species (ROS). Patients with defects within this pathway (eg, chronic granulomatous disease) exhibit invasive infection with aspergillosis and other pathogens, but have no observed increase in susceptibility to Candida spp.
Natural killer cells appear to have a limited role in host defense against candidiasis. Dendritic cells are an essential factor in the defense against fungal pathogens and are important for processing and presentation of fungal antigens for the activation of T cell responses. T cells are also essential in host defense with both CD4 and CD8 cells providing protective immunity. The production of Th-17 and IFN-γ by Th cells promotes the fungicidal activity of neutrophils and macrophages, and quantitative defects (eg HIV)10 and qualitative differences (eg, host polymorphisms)11 in this cellular response pathway have been associated with various forms of candidiasis.
Clinical Manifestations
Local mucocutaneous infections include thrush (oral candidiasis), esophageal and vaginal yeast infections, and chronic mucocutaneous candidiasis. Those with thrush most commonly have underlying diabetes with poor glycemic control, local glucocorticoid exposure via inhalation (eg, for asthma), or involve neonates with immature host responses. Esophageal infection may co-occur with oral thrush or in isolation and is typically a harbinger of more serious underlying immunologic defects primarily those with T-cell defects (eg, AIDS, solid organ, hematologic stem cell transplant recipients, neonates with immature host response). Vulvovaginal candidiasis may occur with any of the above underlying conditions or following recent antibiotic use with the ensuing loss of protective bacterial species. Chronic mucocutaneous infection occurs in those with polymorphisms in specific genomic regions particularly in those with STAT1 gain of function mutations12 or in autosomal recessive polyglandular autoimmune syndrome type I.13
In contrast, invasive disease primarily occurs in those with a clear breach in host defenses (Figure 1). Risk factors for invasive infection include significant disruptions in the mucosal barrier (mucositis following receipt of chemotherapy) in conjunction with underlying neutropenia. These patients additionally frequently receive broad-spectrum antibiotics significantly altering the gastrointestinal microbiome and have central venous catheters – both additional risk factors for infection. Total parental nutrition, hemodialysis, intravenous drug use, gastrointestinal perforation, and gastrointestinal surgery all pose additional risk factors for invasive disease.14
Diagnostics
Our understanding of IC epidemiology is significantly affected by the sensitivity and specificity of currently available diagnostics. The gold standard for diagnosis of IC is a positive culture obtained from blood or a normally sterile site. Positive results may undergo Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) for more rapid identification to the species level.15 Blood cultures, however, are positive in only 21–71% of patients later proven by autopsy to have IC.16 The poor performance of blood cultures may be related to collection methods, in particular the collected blood volume as during candidemia there is typically less than one colony forming unit of Candida per mL.17 However, blood cultures cannot be relied upon in all cases of IC given approximately one-third of patients with IC are categorized as having deep-seated infection without candidemia.16 In these patients, the diagnosis is established by positive cultures or histopathology from affected sites (eg, intraabdominal fluid collection). In concert, this data has led to the conclusion that the sensitivity for blood cultures for invasive candidiasis is only 50%.16 The relatively poor performance of blood cultures has led to the development of additional diagnostics including antigen-18 and molecular-based approaches.19
Several assays have been developed to detect the presence of Candida antigens including mannan and 1,3-β-D-glucan.18 Diagnostic testing for mannan antigen is typically performed in concert with testing for anti-mannan antibodies and this approach has shown promising results in pediatric patients and in those with central nervous system (CNS) infections,18 but performed poorly in a large prospective study of non-neutropenic intensive care unit (ICU) patients at risk of IC due to severe abdominal conditions. In this group, the sensitivity of the mannan antigen was only 43.3% with a specificity of 67.3%.20 Anti-mannan antibody performance exhibited a sensitivity of only 25.8% but a more favorable specificity of 89%.20 Others have assessed the performance of combined mannan antigen and anti-mannan antibody, but the sensitivity has been sub-optimal at only 51% with a specificity of 71%.21 Due to poor performance, this test has not been approved by the US FDA.
1,3-β-D-glucan testing has improved performance characteristics in comparison to mannan antigen testing, with a sensitivity across multiple studies averaging approximately 85% and a high negative predictive value often >95%.18,22 However, the specificity has generally been below 60% due to the potential for false-positive results associated with multiple potential sources including: hemodialysis with membranes manufactured from cellulose, the receipt of intravenous immunoglobulin or albumin, concurrent antibacterials, severe mucositis, and other fungal infections.18
Multiple molecular-based diagnostics have been approved for use and recently reviewed elsewhere in the literature.19 The T2Candida Panel (T2 Biosystems, Lexington, MA, United States) does not require positive blood cultures compared to the commonly used BioFire® FilmArray® Blood Culture Identification (BCID) Panel (BioFire Diagnostics, Salt Lake City, Utah, United States). The T2Candida panel has a sensitivity of 91% and a specificity of 94% across multiple studies.19 However, the panel is only limited to five species of Candida: C. albicans, C. tropicalis, C. krusei, C. glabrata, and C. parapsilosis. Metagenomic next-generation sequencing (mNGS) to detect microbial cell-free DNA (mcfDNA) in plasma is a promising approach, potentially allowing earlier detection and diagnosis of fungal infections when other blood biomarkers/tests are still negative.23 There is limited data thus far for the diagnosis of invasive candidiasis by this methodology, although early reports show potential.24
There are clearly limitations in current diagnostics for invasive candidiasis that subsequently affect our understanding of the epidemiology. Yet, it is encouraging that advances continue, and improved/novel techniques will hopefully allow for more rapid diagnosis and treatment while simultaneously improving epidemiologic assessment.
Burden of Invasive Candidiasis
Candidemia causes an estimated 22,000 infections annually in the United States.25 Candidemia is also the second most common cause of healthcare-associated bloodstream infections (BSIs) nationally.26 A higher incidence of candidemia has been reported among older adults, males, and those of black/African-American ethnicity.27,28 Although candidemia is the most common form of IC, Candida spp. can cause infections in other sterile or deep-seated body sites, with intra-abdominal candidiasis (IAC) being the second most common type of IC in ICUs.29–31 IAC covers a wide range of disease manifestations, and a lack of standardized disease definitions has made it difficult to understand and accurately capture the burden of these infections.29
Generally, incidence rates for IC and candidemia in the US have decreased over time and plateaued in recent years, likely because of improved infection control practices and implementation of bundles for central line care.32 In the United States, IC incidence rates were higher over a decade ago, ranging from 22 to 29 infections per 100,000 population during 1996–2003.33 More recent data using US electronic medical records showed there was no significant change in the incidence between 2009–2017, with an overall incidence of inpatient IC of 90 per 100,000 hospitalizations.34 A more nuanced assessment using data from active population-based surveillance showed the incidence of candidemia decreased from 2008 to 2017 across multiple US sites,25,27,35 with a coincident increase in the incidence of IC from non-blood sources, including abdominal sterile sites, from 2009–2017.34
While an overall decrease in candidemia incidence was observed over the past decade, the incidence unfortunately increased in the setting of the COVID-19 global pandemic.36–38 In fact, some studies found higher candidemia incidence among patients with COVID-19 infections compared to those without.36–40 Candidemia in these patients with COVID-19 but no other underlying comorbidities likely resulted from healthcare-related exposures related to severe COVID-19 infection.41 Changes to healthcare systems during the COVID-19 pandemic (eg, lapses in infection control practices, increased antimicrobial prescribing) and the high acuity of care required for patients with COVID-19 infections (eg, invasive devices, long lengths of stay), likely contributed to the increased risk of IC among patients with COVID-19.41,42
IC remains a threat globally although the scope of the problem is difficult to assess. Data availability varies upon laboratory and surveillance capacity and differences in analytical methodology. The overall pooled incidence of candidemia was 3.9 per 100,000 population based on a meta-analysis of 107 European studies using data reported during 1990 and 2016.43 Similar to trends in the United States, European studies reported a decrease in candidemia incidence after 2010 and an even lower incidence of IAC compared to candidemia, with IAC incidence being approximately a third of candidemia incidence in 23 European ICUs.31,43 Currently, there are no population-based data sources for Asia, the Middle East, Africa, or Latin America.44 However, analyses using limited data from the Middle East and North Africa (MENA) region identified that Qatar had the highest candidemia incidence rate (15.4 per 100,000 population) while Iran had the lowest (0.3 per 100,000 population).45 In Asia, candidemia incidence was 1.2 episodes per 1000 patients using laboratory data from 25 hospitals in five countries.46,47 In South America, candidemia incidence ranges from 0.6–6.0 per 1000 hospital admission.48
IC is associated with prolonged hospitalizations, high healthcare costs, and increased morbidity and mortality. In a study from 2019, IC accounted for 12,770 US hospitalizations, with an average of 28 workdays lost per hospitalization.49 The estimated total US economic burden of IC is $1.8 billion.50 All-cause in-hospital mortality of candidemia has been reported as high as 36% in the US.27,51,52 In Europe, the 30-day mortality rate of IC was slightly higher at 38–42%.31,43 Mortality of IC in Asia is comparable, with studies estimating mortality rates to be 40%.46 Mortality estimates are limited in the MENA region but range from 33–60% in adult populations.53 In South America, studies found that the mortality rate varies from 30–70%.48 In the US, all-cause mortality was highest among older adults and lowest among children.25,27,52 No significant differences in mortality by race or sex were reported.52
The overall burden of IC is likely an underestimate, especially considering the challenges and gaps in surveillance globally and the performance characteristics of available diagnostic testing. Many countries have limited diagnostic laboratory capacity to test for and detect IC. Lack of standardized methodologies and denominators limit the ability to compare estimates of IC burden.44 Furthermore, most IC studies are single-center or smaller multi-center analyses. Even in countries with population-based surveillance systems, candidemia is generally not reportable to public health authorities and reporting is thus voluntary. Even with these limitations in performing a comprehensive assessment, available data confirms the high healthcare burden of IC with high associated patient mortality.
Geographic Differences of Invasive Candidiasis
Epidemiology of the Candida spp. causing IC or candidemia varies significantly by geographic region.18 Independent of these geographic variations, there has been a clear worldwide trend of decreasing proportions of C. albicans as the causative pathogen. While the proportion of C. albicans was 70–80% in the 1980–1990s,54 it has now declined to 40–60% in most geographic areas.25,55,56 While that decreasing trend is ubiquitous, with the series of multicenter European Confederation of Medical Mycology (ECMM) Candida studies showing the proportion of C. albicans causing candidemia decreased from 56.4% in 1997–1999,57 to 54% in 2006–2008,58 to 46.2% in 201856 with corresponding increases in non-albicans species (Table 1), there are important geographic differences. The actual proportion of C. albicans is well over 50–70% in Northern and Middle Europe, while mostly well below 50% in Southern Europe, Latin America, Australia, and the US.59,60
Table 1.
Causative Species for Candidemia Within the European Confederation of Medical Mycology Over Three Separate Time Points
The changes in distribution of non-albicans Candida spp. also have strong regional variations and it is imperative to understand local epidemiologic patterns when empiric treatment is prescribed rather than relying on regional assessments.18 The proportion of C. glabrata has increased particularly in the US, Australia, and Northern Europe.25,40,41,55,61 Comparatively, the proportion of C. parapsilosis has increased in Asia (including Japan and China), Latin America (including Brazil) and Southern Europe,31,62–65 and the proportion of C. tropicalis has increased particularly in Latin America, and large parts of Asia.62,66 Summarizing all of Europe together, the increase in the proportion of C. glabrata between 2006–2008 (13.8%)58 to 2018 (21.4%)56 has been striking. Of note, Candida spp. distribution may not vary only between geographical regions, but even between medical centers in close proximity, perhaps affected by factors influencing local epidemiology including prior antifungal exposure and patient age within the ICU.67 Beyond a global distribution of known pathogens, there have also been global differences in emerging species of Candida.
Emerging Species of Candida
As global temperatures rise, fungi are adapting to higher environmental temperatures,68 resulting in the emergence of new fungal species as human pathogens.69,70 The most prominent example is C. auris, which is theorized to have evolved from a plant saprophyte and possibly became a human pathogen after adaptation to higher temperatures.71 This hypothesis is strengthened by the observation that an environmental isolate grew slower at mammalian temperatures than clinical strains, a finding consistent with the notion that their ancestor recently adapted to higher temperatures.72 C. auris is now a global human threat, particularly in healthcare settings, causing large outbreaks in ICUs in India, Southern Europe, the United Kingdom, Brazil and the United States.70,71,73 As a result, C. auris has been highlighted as one of four fungal pathogens of critical importance in the recently published WHO fungal pathogens priority list in part due to the number of large outbreaks, but also due to the multi-drug resistant nature of the pathogen.74 There are still notable differences in the epidemiology of C. auris, where some countries only see isolated cases reported, potentially due to more strict hospital hygiene measures preventing nosocomial transfer and difficult to control outbreaks,75 while other countries are at a state of emergency due to larger outbreaks with this multi-drug resistant pathogen.75,76
In contrast to C. auris, which truly is an emerging pathogen, Candida spp. that were defined before as “emerging”77 (ie, C. kefyr, C. guilliermondii, C. lusitaniae, C. dubliniensis, C. famata, C. inconspicua, C. rugosa, C. norvegiensis), may have in fact already “emerged” due to better diagnostic differentiation methods, or were selected by the broad use of antifungal treatment and prophylaxis. For example, C. dubliniensis infections increased after introduction of MALDI-TOF as this method reliably differentiated between the phenotypically similar C. albicans and C. dubliniensis. Others, such as C. inconspicua, C. norvegensis, C. guilliermondii, C. digboiensis, and C. lusitaniae have intrinsic reduced susceptibility to fluconazole and/or echinocandins and may have thus increased only in patient populations exposed to antifungals.
While not an emergent species, a concerning worldwide increase of clonal outbreaks caused by fluconazole-resistant C. parapsilosis is ongoing. Fluconazole-resistant C. parapsilosis strains that carry the ERG11 Y132F mutation may not suffer an associated fitness cost (potentially a survival benefit), and may, therefore, thrive even in the absence of triazole exposure.64 These properties allow this pathogen, which is associated with high mortality rates among those infected,64 a viable threat to widescale spread. Understanding the global distribution of not only emerging species, but also emerging drug-resistance trends is vital for determining optimal preventative and treatment strategies, particularly as we attempt to prevent breakthrough infections in vulnerable populations receiving prophylactic antifungals.
Breakthrough Infections Due to Candida
Breakthrough infections due to Candida constitute a broad range of clinical entities. Breakthrough infections generally consist of organisms that tend to be antifungal resistant, and occur in patients with multiple risk-factors, have an unrecognized or unremoved source, and/or in those with unfavorable antifungal pharmacokinetic circumstances. Until recently, the term “breakthrough infection” as it relates to fungal infections in general was left to the interpretation of individual investigators and poorly defined. A recent consensus statement from the Mycoses Study Group Education and Research Consortium (MSGERC) together with the European Confederation of Medical Mycology (ECMM) has put forth a consensus definition of breakthrough fungal infections, including candidiasis, which represents a significant step forward towards standardizing these observations.78 Overall, breakthrough Candida infections are generally uncommon, occurring in fewer than 10% of at-risk patients,79 although they can constitute up to 40% of all Candida infections in selected series.80
Definition
Breakthrough (BT) Candida infection occurs in the setting of an individual receiving either prophylactic, empiric, or preemptive/targeted antifungal therapy. Unlike other fungal infections such as Aspergillus or the endemic fungi, BT Candida infections require a positive culture from a normally sterile site such as blood, or compelling culture and histologic evidence from a mucosal site such as the oropharynx or esophagus for diagnosis.81 Thus, by consensus there are only proven BT Candida infections based on European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) updated criteria.82 Serologic evidence such as Candida PCR, Candida mannan and anti-mannan, and 1.3-β-D glucan provide supporting evidence, but are not sufficient as the sole source of evidence for candidiasis.81 To meet the definition of BT, infection must occur at least 72 hours after initiation of systemic antifungal therapy and no greater than one dosing-interval (8 hours to 7 days depending on the antifungal) after the antifungal agent has been discontinued.78
Risk Factors for Breakthrough Candidiasis
There are several readily identifiable risk factors associated with BT candidiasis. Many of these events occur in three main host groups: patients with prolonged ICU stays, solid organ transplant recipients, and those with hematologic malignancies or stem cell transplant recipients.79 These host risk factors are described below.
Host Factors
Among the three major risk groups for BT infection, there are several shared risk factors including the presence of a central venous catheter, prolonged exposure (>14 days) to two or more broad-spectrum antimicrobials, and iatrogenic immunosuppression.79 Unique to the ICU group is duration of ICU stay greater than 10 days, concomitant pancreatitis, major trauma including burns, impaired renal function, total parenteral nutrition, and mechanical ventilation.83 While any of these factors can enhance the risk of BT infection in any of these three major patient groups, they are profoundly impactful and potentially synergistic in the ICU population.
Unique to the transplant population is the enhanced risk of BT Candida infections among lung, liver, and small bowel transplant recipients.80,84,85 In the largest study to date, the TRANSNET database determined that 41% of all proven Candida infections were BT candidiasis and that lung transplant recipients had the highest rates of BT infection. In this series, antifungal prophylaxis included fluconazole, other azoles, echinocandins, and amphotericin B.80 Among those with hematologic malignancy and/or stem cell transplant recipients, specific risk factors for BT candidiasis included mucositis, neutropenia, use of glucocorticoids and other immunosuppressive agents.79
A major consideration with respect to host factors pertains to source control. While this most often refers to appropriate management and removal of a central venous catheter or other intravascular device, this can also pertain to inadequate drainage of a contaminated fluid collection such as an intra-abdominal abscess, pleural empyema, or soft tissue abscess.79
Pharmacokinetic Factors
BT candidiasis can be the consequence of inadequate drug levels, poor penetration into specific tissues or spaces, or unforeseen drug-drug interactions. Sub-therapeutic drug levels are most common with the triazole antifungals despite adherence to recommended dosing regimens.86 As such, therapeutic drug monitoring, especially for itraconazole, voriconazole and posaconazole, is recommended even in the setting of antifungal prophylaxis.87 It is also crucial to recognize that some commonly use agents have limited penetration into critical spaces. For example, echinocandins do not achieve significant penetration into the central nervous system or into the urinary system. Finally, drug-drug interactions, especially between the triazoles and other commonly co-administered agents, are increasingly recognized given the complexity of modern patient management. The sheer variety of immunosuppressive agents such as tacrolimus, cyclosporine, and sirolimus as well as a number of newer anti-cancer agents require a detailed knowledge of these predictable drug interactions which can significantly influence antifungal efficacy.88
Antifungal Resistance
A review of the most recent data pertaining to BT Candida infections suggests that many, but not all, of these infections occur due to organisms that are resistant to one or more antifungals, and accordingly are dominated by non-albicans Candida species, especially C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis.89–92 Less common species such as C. lusitaniae and C. guilliermondii have also been reported.93 The majority of BT Candida infections occur in patients receiving antifungal prophylaxis with a triazole antifungal such as fluconazole, voriconazole, posaconazole or isavuconazole,94–96 but there are also ample reports of individuals who experienced breakthrough infections while receiving an echinocandins.97,98
Outcomes
If recognized early and managed appropriately, most BT Candida infections can be treated successfully with antifungal therapy including optimization of dosing, source control, and recognizing potential drug-drug interactions. In most large series, overall mortality at 30 days is similar for BT Candida infections compared to de novo Candida infections (~30%).99,100 BT infections are vital to track as they likely contribute to changes in epidemiology related to non-albicans species infections and trends in antifungal susceptibility.
Trends and Antifungal Susceptibility of Candida Species
Investigations of IC over the last three decades have led to numerous investigations of the evolving antifungal susceptibility patterns and documentation of changes over time. Regional and global surveys have documented trends in antifungal susceptibility for the major Candida species.27,101–104 For the purposes of this discussion, antifungal susceptibility is determined on the basis of CLSI and/or EUCAST methodologies, and while these methodologies and breakpoints are not entirely interchangeable, there is enough similarity to allow for general comparisons.105,106 Each of the five major species and C. auris will be reviewed.
C. albicans remains the most common cause of bloodstream infections due to Candida and other forms of invasive candidiasis in most regions of the world.18,27,62,102,103,107–109 In multiple population studies over the last two decades, C. albicans is almost uniformly susceptible to fluconazole, the echinocandins, and amphotericin B. Resistance to fluconazole is uncommon, with rates generally ranging between 0% and 3%, but averaging less than 1% overall.27,62,102,103,109
In many parts of the world, C. glabrata is the second most common cause of IC, particularly affecting older individuals and those with significant underlying immunosuppression.18 Among the more common Candida spp, C. glabrata probably poses the greatest risk with regard to the development of antifungal resistance and this may be attributed to the prevalent mutator genotype promoting multi-drug resistance phenotypes.110 Large population-based surveys conducted by the CDC have demonstrated echinocandin resistance ranges between 2% and 8%, while fluconazole resistance ranges between 7% and 11%.27 Fluconazole resistance has been reported in up to 75% of C. glabrata isolates in single center surveys, and resistance to the echinocandins ranges from 0% to 24% in larger studies.27,111–113 Rates of echinocandin resistance tend to parallel increases in fluconazole resistance as demonstrated by Alexander et al at a 10-year single center survey.111 A general consensus suggests that echinocandin resistance rates are generally higher in academic institutions compared to community-based medical centers, however this is not true across all centers. For instance, one recent survey from a large academic medical center tested >800 C. glabrata isolates over a 10-year period and detected fewer than 3% echinocandin resistant isolates in any given year, and observed no trend towards emerging resistance.114 Taken as a whole, there has been a slow but steady increase in resistance rates to both fluconazole and the echinocandins over the last two decades and continued assessment of this ongoing problem is essential.
Similar to C. glabrata, C. tropicalis prevalence varies considerably among different regions of the world. This organism is particularly common in the Asian Pacific, Caribbean and Latin America, but less so in North America and western Europe.62 Historically this organism has been pan-susceptible to triazoles, echinocandins, and amphotericin B. In recent years, however, reports of fluconazole and echinocandin resistance have become more frequent, and the current baseline level of resistance to fluconazole is 3–5% in most regions, but rates as high as 7–43% are reported in some Asian Pacific countries.115–117 Echinocandin resistance remains low at 0–2% in most surveys.27,62 Amphotericin B resistance continues to be rare, however, pan-resistant C. tropicalis may constitute 1% of isolates as reported in India.118
Among the five most common Candida species causing invasive candidiasis, C. parapsilosis is the least virulent with respect to all-cause mortality.18 Depending on geographic region, it is the first to fourth most common cause of invasive candidiasis.18,62,66 However, fluconazole resistance has emerged over the last decade and over 50% of C. parapsilosis isolates are resistant in some series.64,119 In the US, fluconazole resistance rates range between 4–10%; rates are much higher in other parts of the world including Japan, Western Europe, and Latin America. Resistance to the echinocandins has remained relatively infrequent (0–3%) despite a higher baseline susceptible MIC cutoff value compared to the other Candida spp. due to a naturally occurring polymorphism in FKS1.62,103,112,120
C. krusei is considered inherently resistant to fluconazole yet demonstrates variable susceptibility to both voriconazole and posaconazole.62,103,112 In general, voriconazole has been considered the best oral option for C. krusei infections, however, recent population surveys have demonstrated emerging resistance rates to voriconazole, approximating 10% in some regions of the world. Echinocandin and amphotericin B resistance also appears to be emerging, with rates of resistance in the 3% to 10% range among larger multicenter studies.107,112
Having only emerged globally since 2009, C. auris is relatively new in the clinical arena. While a less common cause of IC in the US, the organism is among the most common Candida bloodstream isolates in India and South Africa.118,121 It is noted for its potential multidrug antifungal resistance, but the data demonstrate resistance to fluconazole, seen in 70% to 90% of isolates, and they are non-susceptible to quaternary-ammonium disinfectants, allowing this pathogen to persist on healthcare related surfaces and cause outbreaks.122,123 Resistance to the echinocandins (up to 7%) and amphotericin B (up to 35%) vary greatly between geographic regions.122–124 Resistance to all three classes of antifungals is reported most commonly in India in South Asia (3%), whereas this is much less common in other regions of the world.122–124 Because of the potential for person-to-person transmission with C. auris, infection prevention measures are key to preventing nosocomial spread.
Although many Candida species have very predictable antifungal susceptibility patterns (eg, C. albicans) it remains essential that all clinically significant isolates be identified to species level with subsequent antifungal susceptibility testing performed.125 If testing is not routinely available, a sufficient number of local isolates should be regularly characterized, in order to be able to adequately predict susceptibility patterns at the species level in a specific hospital or geographic region.
General Management Principles of IC
The most recent Infectious Disease Society of America (IDSA)125 and European guidelines126,127 recommend echinocandins as first-line initial treatment for most invasive candidiasis in both neutropenic and non-neutropenic patients prior to identification to the species level due to their broad spectrum of activity, favorable side effect profile, mortality benefit, and the increasing incidence of azole resistance particularly among non-albicans Candida species.125,128–132 Both guidelines recommend “step-down” to fluconazole (in 5–7 days per IDSA or 10 days per European guidelines) if clinically improved and no triazole resistance is suggested by species identification or susceptibility testing. Due to intrinsic resistance to fluconazole, voriconazole is recommended as step-down for C. krusei.125,133
Non-neutropenic patients with central venous catheters should undergo catheter removal, however in neutropenic patients who may have a gastrointestinal source of candidemia, an individualized approach to CVC removal is recommended except in the cases of C. parapsilosis IC.125 Echocardiography is recommended to assess for endocarditis in cases of persistent candidemia.134 With uncomplicated candidemia, treatment duration is 2 weeks after clearance of blood cultures and symptom resolution. All infected intracardiac devices require removal or indefinite oral azole suppression following disease control.125,132
Due to potential ophthalmologic complications which significantly affect management, the IDSA recommends a dilated eye exam within one week of diagnosis for non-neutropenic patients and within a week of resolution of neutropenia for neutropenic patients, as evidence of chorioretinitis and endophthalmitis are unlikely until recovery of immune function. Per IDSA and European guidelines, intravitreal treatment is recommended in conjunction with ophthalmology, and consists of fluconazole or voriconazole, if susceptible. Otherwise, intravitreal amphotericin B with or without flucytosine is recommended for ocular involvement, with consideration of vitrectomy. Duration is 4–6 weeks or until resolution on repeat ophthalmologic exam.125,132
IDSA recommends treatment of chronic disseminated candidiasis with a liposomal amphotericin B or an echinocandin continued for several weeks with stepdown to fluconazole if susceptible, with therapy continuation until resolution of lesions on repeat imaging. Continuation of therapy throughout high-risk chemotherapy or stem cell transplantation is advised to prevent relapse.
For osteomyelitis, surgical debridement is indicated for large abscesses or joint instability. IDSA recommends an oral azole for 6–12 months or an echinocandin (weaker alternative liposomal amphotericin B) for 2 weeks with step-down to an oral azole for 6–12 months. European guidelines favor an oral azole for 6–12 months or liposomal amphotericin B for 2–6 weeks with step down to an oral azole to complete 6–12 months. For septic arthritis, surgical drainage and removal of any prosthetic device is recommended, with chronic oral azole suppression if a device remains. IDSA recommends an oral azole for 6 weeks or echinocandin for 2 weeks and an oral azole for ≥4 weeks or liposomal amphotericin B for 2 weeks with step down to an oral azole for ≥4 weeks. European guidelines recommend an oral azole for ≥6 weeks or liposomal amphotericin B for 2 weeks with step down to fluconazole for ≥4 weeks, with voriconazole for ≥6 weeks as an alternative.125,132
Central nervous system (CNS) candidiasis has less data on optimal treatment. The IDSA recommends treatment with liposomal amphotericin B with or without flucytosine with potential fluconazole step down if susceptible after clinical response. Duration is dependent upon resolution of symptoms, radiologic, and CSF abnormalities. Echinocandins have poor CNS penetration at normal dosing and are not recommended.125 European guidelines weakly recommend IV liposomal amphotericin B and flucytosine for 6 weeks followed by 3 weeks of fluconazole, or liposomal amphotericin B and fluconazole for 4 weeks depending on susceptibilities.132
The Need for Early Therapy
Attributable mortality from IC remains high and increases with delays in treatment. Blood cultures take an average of 2–3 days to grow Candida, or longer depending on the species. Prophylaxis and empiric treatment strategies are all thus routinely employed to decrease mortality.133,135,136 The IDSA recommends consideration of fluconazole or echinocandin prophylaxis for high-risk ICU patients in units with >5% rate of invasive candidiasis.125,137 A Cochrane review of prophylaxis in non-neutropenic ICU patients did not find an effect on mortality but did note a significant decrease in invasive fungal infection.138 European guidelines recommend fluconazole prophylaxis in ICU patients with recent abdominal surgery with recurrent perforation or leakage.132 Fluconazole, posaconazole, voriconazole, or micafungin are recommended as prophylaxis for allogeneic stem cell transplant patients during the initial neutropenic phase, with transition to fluconazole or posaconazole depending on immune recovery and immunosuppression for graft vs host disease.131
The IDSA recommends consideration of empiric antifungal therapy in deteriorating patients with risk factors and surrogate markers suggestive of infection. Therapy should be started as quickly as possible in the setting of septic shock. That said, there has been conflicting data on the benefit of empiric antifungal therapy in patients with septic shock and risk factors for IC. The EMPIRICUS trial failed to show a benefit in the use of empiric micafungin in ICU patients with septic shock and risk factors for IC.139
The Antifungal Pipeline for IC
Although morbidity and mortality associated with invasive Candida infections remains high, only four major classes of antifungal agents are currently approved for systemic therapy. The echinocandins, as first-line therapy for most Candida infections, have an overall failure rate of 25–30% in invasive candidiasis in clinical trials, and notably have little activity within the urinary tract.18,133,140–144 Multi-drug resistant Candida infections are on the rise due to a variety of mechanisms (Table 2), particularly C. auris, with widespread azole resistance and the potential for development of resistance to all classes of antifungals during therapy, highlighting the need for new agents to keep up with changes in epidemiology.133,145–153
Table 2.
Most Common Resistance Mechanisms of Major Candida Species
| Candida Species | Resistance Mechanisms |
|---|---|
| C. albicans | Fluconazole – efflux transporters (MDR1, CDR1, CDR2) with amino acid substitutions less frequently observed |
| C. glabrata | Azoles – Changes in drug efflux (Cdr1 and Cdr2) |
| Echinocandins – FKS1 polymorphisms in glucan synthase gene | |
| Mutator phenotype – Mismatch repair defect causing multiple antifungal resistance phenotypes | |
| C. krusei | Fluconazole – Intrinsically resistant due to changes within ERG11 with other azoles often spared |
| C. parapsilosis | Fluconazole – amino acid substitution (mainly Y132F) within ERG11 most commonly with resultant variable susceptibility to voriconazole |
| C. lusitaniae | Amphotericin B – Mutation or altered expression of ergosterol biosynthetic genes (ERG3, etc). |
| C. auris | Azoles – Majority are fluconazole resistant (ERG11 mutations) with variable susceptible to other azoles |
| Amphotericin B – Hypothesized to be due to alterations in ergosterol biosynthesis | |
| Echinocandins – Due to mutations in FKS1 |
Encouragingly, a new echinocandin, rezafungin, was approved by the Food and Drug Administration in 2023 for adults with invasive fungal infections after ReSTORE, a Phase 3 clinical trial comparing it to caspofungin for treatment of invasive candidiasis, demonstrated non-inferiority and a 60% cure rate.154,155 It is similar in structure to anidulafungin but with an extended half-life allowing weekly administration which will improve its suitability for outpatient therapy.149 Further studies, including ReSPECT, a Phase 3 clinical trial of prophylaxis for invasive fungal infections in patients undergoing allogeneic blood and marrow transplantation are ongoing.150,156
Ibrexafungerp is another novel agent which inhibits (1→3)-β-D-glucan synthesis at a different site than echinocandins and thus retains activity against many organisms with echinocandin resistance due to FKS mutations, including many strains of C. auris.156 Importantly, it retains solubility and activity in acidic environments, making it suitable for use in abscesses.149,157 Clinical trials for infections refractory to other agents have been promising.150 It has oral bioavailability of 35–50% and good systemic distribution except for the CNS. Ibrexafungerp is currently only FDA approved as oral therapy for vulvovaginal candidiasis, although clinical trials for invasive candidiasis step-down therapy, refractory candidiasis, and C. auris are ongoing.149,156–159
Lastly, fosmanogepix is another encouraging novel antifungal. Fosmanogepix is a first in class Gwt1 fungal protein inhibitor, which is a class of proteins necessary for mannoprotein anchoring to the cell membrane and cell wall.160 This novel mechanism provides a broad range of activity against Candida spp. including drug-resistant Candida.160 Results from a Phase 2 clinical trial for patients with candidemia from C. auris demonstrated safety and efficacy of fosmanogepix.161 Additionally, data from a Phase 2 study of patients with candidemia with C. albicans, C. glabrata, C. parapsilosis, and/or C. dubliniensis demonstrated successful outcomes in 16/20 patients without significant adverse events.162 Phase 3 trials are planned, but these preliminary results are encouraging given the increasing rates of antifungal resistance.
Prevention and Infection Control in the Healthcare Setting
Considering the morbidity and mortality associated with IC prevention is a key priority. To this end, the IDSA collaborated on a joint recommendation for multiple interventions to decrease the risk of all central line associated bloodstream infections (CLABSIs), including candidemia.163 Implementation of these CLABSI bundles has decreased the incidence of infections.18 Guidelines include education on appropriate indications for line placement and designation of specific personnel and aseptic technique to place and care for central lines. Upper extremity sites have lower infection risks than lower extremity sites.163 All insertion sites should be evaluated daily and peripheral lines removed if there are signs of phlebitis, infection, or malfunction.163 Non-tunneled subclavian lines have lower infection risks than internal jugular or femoral sites, and using the fewest lumens necessary is recommended.163 Prompt removal of unneeded central lines decreases the risk of infection, and all insertions and accessing of central lines should use with aseptic technique and sterile barrier precautions.163 Chlorhexidine skin cleansing should occur prior to insertion, and aseptic dressing change schedule based on material. Only replace midlines and central lines if malfunctioning or there is a suspicion of infection. Routine replacement does not decrease infection rate.163 Daily chlorhexidine bathing of ICU patients decreases risk of bloodstream infections in general, including candidemia.125,164
C. auris outbreaks in healthcare facilities has recently seen an increase, greatly facilitated by the COVID pandemic with the large numbers of critically ill, mechanically ventilated patients and likely breaches in infection control practices.145 This pathogen persists in the healthcare environment and patients can remain colonized for over one year. Due to the ability of C. auris to persist and spread throughout healthcare facilities, the CDC released guidance to limit outbreaks.165,166 Colonization screening is a key strategy to prevent the spread of C. auris in healthcare facilities by identifying otherwise unrecognized colonized individuals who need appropriate infection prevention and control measures. Determining who to screen depends on many factors but should be considered for individuals who are at high risk for C. auris including patients with epidemiologic linkages to other cases, encounters at high-risk facilities, and risk factors. Screening is recommended using a composite swab of the bilateral axilla and groin. Patients with C. auris should be placed under appropriate isolation precautions, which would include contact precautions for acute care hospitals, but enhanced barrier precautions can be considered in nursing home settings. Because patients often remain colonized for long periods of time and can periodically have negative results, isolation precautions should be continued indefinitely throughout the inpatient encounter and for future inpatient encounters, and it is not recommended to repeat screening to “clear” patients for removal of isolation precautions. Due to the resilient nature of C. auris in the environment, it is recommended to perform at least daily cleaning using an appropriate disinfectant167 of a colonized patient’s room along with any surfaces or shared equipment they come into contact with (eg, glucometer, hoyer lift, temperature probe, etc).
Conclusion
Invasive candidiasis continues to pose a global threat. The scope of the problem is likely underrepresented due to limited global surveillance combined with the challenges of diagnostics, even with newer methods clinically available. Nonetheless, it remains a costly and morbid condition even with advances in prevention strategies. While overall incidence does appear to be mostly declining, treatment choices and infection control practices significantly impact the epidemiology of the disease globally and locally. The shift to non-albicans Candida spp. combined with the emergence of the multi-drug resistant pathogen, C. auris, will play a large role in the evolving treatment practices and shape the future epidemiology of the disease. Recent advances in therapeutics are encouraging, however, as evident by breakthrough infections and rising drug resistance, antifungal therapy alone is unlikely to be an effective solution in the absence of a holistic approach to Candida infections and prevention. Advances in preventative strategies combined with novel agents are needed to decrease the global incidence and improve treatment outcomes.
Disclosure
The findings and conclusions of this paper are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC). D.B. was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award KL2 TR001859. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. M.H. received research funding from Gilead, Astellas, MSD, IMMY, Euroimmun, Mundipharma, Scynexis, F2G and Pfizer, outside of the submitted work. L.O.-Z. reports grants, personal fees from Pfizer, personal fees from F2G, grants, personal fees from GSK, personal fees from Melinta, personal fees from Gilead, personal fees from Viracor, grants from Scynexis, grants from Pulmocide, grants from T2 Biosystems, personal fees from Cidara, outside the submitted work; and President, Mycoses Study Group and Education Consortium. G.R.T. has received research support and consulting for: Astellas, Cidara, Mundipharma, Melinta, F2G, Scynexis. G.R.T. has served on DSMB for Pfizer. P.P. reports grants from Cidara, grants from Melinta, grants from Astellas, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Mazi PB, Olsen MA, Stwalley D, et al. Attributable mortality of candida bloodstream infections in the modern era: a propensity score analysis. Clin Infect Dis. 2022;75(6):1031–1036. PubMed PMID: 34989802; PMCID: PMC10233239. doi: 10.1093/cid/ciac004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denning DW. Global incidence and mortality of severe fungal disease. Lancet Infect Dis. 2024. PubMed PMID: 38224705. doi: 10.1016/S1473-3099(23)00692-8 [DOI] [PubMed] [Google Scholar]
- 3.Hoyer LL, Green CB, Oh SH, Zhao X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family--a sticky pursuit. Med Mycol. 2008;46(1):1–15. PubMed PMID: 17852717; PMCID: PMC2742883. doi: 10.1080/13693780701435317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10(10):2058–2066. PubMed PMID: 18549457. doi: 10.1111/j.1462-5822.2008.01188.x [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15(10):630–642. PubMed PMID: 26388329. doi: 10.1038/nri3897 [DOI] [PubMed] [Google Scholar]
- 6.Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A. 2012;109(35):14194–14199. PubMed PMID: 22891338; PMCID: PMC3435201 Inc. doi: 10.1073/pnas.1117676109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2011;10(2):112–122. PubMed PMID: 22158429; PMCID: PMC3624162. doi: 10.1038/nrmicro2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glocker EO, Hennigs A, Nabavi M, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361(18):1727–1735. PubMed PMID: 19864672; PMCID: PMC2793117. doi: 10.1056/NEJMoa0810719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kullberg BJ, van ‘t Wout JW, van Furth R. Role of granulocytes in increased host resistance to Candida albicans induced by recombinant interleukin-1. Infect Immun. 1990;58(10):3319–3324. PubMed PMID: 2144844; PMCID: PMC313656. doi: 10.1128/iai.58.10.3319-3324.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson GR, Patel PK, Kirkpatrick WR, et al. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):488–495. PubMed PMID: 20156694; PMCID: PMC2843789. doi: 10.1016/j.tripleo.2009.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zahedi N, Abedian Kenari S, Mohseni S, Aslani N, Ansari S, Badali H. Is human Dectin-1 Y238X gene polymorphism related to susceptibility to recurrent vulvovaginal candidiasis? Curr Med Mycol. 2016;2(3):15–19. PubMed PMID: 28681024; PMCID: PMC5490285. doi: 10.18869/acadpub.cmm.2.3.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada S, Asano T, Moriya K, et al. Human STAT1 gain-of-function heterozygous mutations: chronic mucocutaneous candidiasis and type I interferonopathy. J Clin Immunol. 2020;40(8):1065–1081. PubMed PMID: 32852681; PMCID: PMC8561788. doi: 10.1007/s10875-020-00847-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91(8):2843–2850. PubMed PMID: 16684821. doi: 10.1210/jc.2005-2611 [DOI] [PubMed] [Google Scholar]
- 14.McCarty TP, Pappas PG. Invasive Candidiasis. Infect Dis Clin North Am. 2016;30(1):103–124. PubMed PMID: 26739610. doi: 10.1016/j.idc.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 15.Normand AC, Gabriel F, Riat A, et al. Optimization of MALDI-ToF mass spectrometry for yeast identification: a multicenter study. Med Mycol. 2020;58(5):639–649. PubMed PMID: 31579924. doi: 10.1093/mmy/myz098 [DOI] [PubMed] [Google Scholar]
- 16.Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284–1292. PubMed PMID: 23315320. doi: 10.1093/cid/cit006 [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer CD, Samsa GP, Schell WA, Reller LB, Perfect JR, Alexander BD. Quantitation of Candida CFU in initial positive blood cultures. J Clin Microbiol. 2011;49(8):2879–2883. PubMed PMID: 21677065; PMCID: PMC3147732. doi: 10.1128/JCM.00609-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. PubMed PMID: 29749387. doi: 10.1038/nrdp.2018.26 [DOI] [PubMed] [Google Scholar]
- 19.Jenks JD, White PL, Kidd SE, et al. An update on current and novel molecular diagnostics for the diagnosis of invasive fungal infections. Expert Rev Mol Diagn. 2023:1–18. PubMed PMID: 37801397. doi: 10.1080/14737159.2023.2267977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leon C, Ruiz-Santana S, Saavedra P, et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Crit Care. 2016;20(1):149. PubMed PMID: 27181045; PMCID: PMC4867537. doi: 10.1186/s13054-016-1324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chumpitazi BF, Lebeau B, Faure-Cognet O, et al. Characteristic and clinical relevance of Candida mannan test in the diagnosis of probable invasive candidiasis. Med Mycol. 2014;52(5):462–471. PubMed PMID: 24934805. doi: 10.1093/mmy/myu018 [DOI] [PubMed] [Google Scholar]
- 22.Finkelman MA. Specificity Influences in (1-->3)-beta-d-glucan-supported diagnosis of invasive fungal disease. J Fungi. 2020;7(1). PubMed PMID: 33383818; PMCID: PMC7824349. doi: 10.3390/jof7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson MR, Sample HA, Zorn KC, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380(24):2327–2340. PubMed PMID: 31189036; PMCID: PMC6764751. doi: 10.1056/NEJMoa1803396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoenigl M, Egger M, Price J, Krause R, Prattes J, White PL. Metagenomic next-generation sequencing of plasma for diagnosis of COVID-19-associated pulmonary aspergillosis. J Clin Microbiol. 2023;61(3):e0185922. PubMed PMID: 36809121; PMCID: PMC10035327. doi: 10.1128/jcm.01859-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsay SV, Mu Y, Williams S, et al. Burden of Candidemia in the United States, 2017. Clin Infect Dis. 2020;71(9):e449–e53. PubMed PMID: 32107534. doi: 10.1093/cid/ciaa193 [DOI] [PubMed] [Google Scholar]
- 26.Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in US Hospitals. N Engl J Med. 2018;379(18):1732–1744. PubMed PMID: 30380384. doi: 10.1056/NEJMoa1801550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toda M, Williams SR, Berkow EL, et al. Population-based active surveillance for culture-confirmed candidemia - Four Sites, United States, 2012–2016. MMWR Surveill Summ. 2019;68(8):1–15. PubMed PMID: 31557145; PMCID: PMC6772189. doi: 10.15585/mmwr.ss6808a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenks JD, Aneke CI, Al-Obaidi MM, et al. Race and ethnicity: risk factors for fungal infections? PLoS Pathog. 2023;19(1):e1011025. PubMed PMID: 36602962; PMCID: PMC9815636. doi: 10.1371/journal.ppat.1011025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vergidis P, Clancy CJ, Shields RK, et al. Intra-abdominal candidiasis: the importance of early source control and antifungal treatment. PLoS One. 2016;11(4):e0153247. PubMed PMID: 27123857; PMCID: PMC4849645. doi: 10.1371/journal.pone.0153247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulrych J, Adamkova V, Matek J, et al. Intra-abdominal candidiasis in surgical intensive care unit - epidemiology characteristics and trends. Epidemiol Mikrobiol Imunol. 2020;69(2):57–63. PubMed PMID: 32819104. [PubMed] [Google Scholar]
- 31.Bassetti M, Giacobbe DR, Vena A, et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care. 2019;23(1):219. PubMed PMID: 31200780; PMCID: PMC6567430. doi: 10.1186/s13054-019-2497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, Perencevich EN, Diekema DJ, et al. Temporal trends of candidemia incidence rates and potential contributions of infection control initiatives over 18 years within the United States Veterans Health Administration System: a joinpoint time-series analysis. Clin Infect Dis. 2021;73(4):689–696. PubMed PMID: 33564858. doi: 10.1093/cid/ciab105 [DOI] [PubMed] [Google Scholar]
- 33.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–163. doi: 10.1128/cmr.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricotta EE, Lai YL, Babiker A, et al. Invasive candidiasis species distribution and trends, United States, 2009–2017. J Infect Dis. 2021;223(7):1295–1302. PubMed PMID: 32798221; PMCID: PMC8030726. doi: 10.1093/infdis/jiaa502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleveland AA, Harrison LH, Farley MM, et al. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PLoS One. 2015;10(3):e0120452. PubMed PMID: 25822249; PMCID: PMC4378850. doi: 10.1371/journal.pone.0120452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadimitriou-Olivgeris M, Kolonitsiou F, Kefala S, et al. Increased incidence of candidemia in critically ill patients during the Coronavirus Disease 2019 (COVID-19) pandemic. Braz J Infect Dis. 2022;26(2):102353. PubMed PMID: 35500645; PMCID: PMC9035354. doi: 10.1016/j.bjid.2022.102353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kayaaslan B, Eser F, Kaya Kalem A, et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021;64(9):1083–1091. PubMed PMID: 34085319; PMCID: PMC8242769. doi: 10.1111/myc.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mastrangelo A, Germinario BN, Ferrante M, et al.; Group CO-BS. Candidemia in Coronavirus Disease 2019 (COVID-19) patients: incidence and characteristics in a prospective cohort compared with historical non-COVID-19 controls. Clin Infect Dis. 2021;73(9):e2838–e9. PubMed PMID: 33124650; PMCID: PMC7665423. doi: 10.1093/cid/ciaa1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado M, Estevez A, Sanchez-Carrillo C, et al. Incidence of candidemia is higher in COVID-19 versus non-COVID-19 patients, but not driven by intrahospital transmission. J Fungi. 2022;8(3). PubMed PMID: 35330307; PMCID: PMC8950429. doi: 10.3390/jof8030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoenigl M, Seidel D, Sprute R, et al. COVID-19-associated fungal infections. Nat Microbiol. 2022;7(8):1127–1140. PubMed PMID: 35918423; PMCID: PMC9362108. doi: 10.1038/s41564-022-01172-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seagle EE, Jackson BR, Lockhart SR, et al. The landscape of candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis. 2022;74(5):802–811. PubMed PMID: 34145450. doi: 10.1093/cid/ciab562 [DOI] [PubMed] [Google Scholar]
- 42.Sopirala MM. Predisposition of COVID-19 patients to secondary infections: set in stone or subject to change? Curr Opin Infect Dis. 2021;34(4):357–364. PubMed PMID: 34039879. doi: 10.1097/QCO.0000000000000736 [DOI] [PubMed] [Google Scholar]
- 43.Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25(10):1200–1212. PubMed PMID: 31039444. doi: 10.1016/j.cmi.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 44.Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73(suppl_1):i4–i13. PubMed PMID: 29304207. doi: 10.1093/jac/dkx444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osman M, Al Bikai A, Rafei R, Mallat H, Dabboussi F, Hamze M. Update on invasive fungal infections in the Middle Eastern and North African region. Braz J Microbiol. 2020;51(4):1771–1789. PubMed PMID: 32623654; PMCID: PMC7335363. doi: 10.1007/s42770-020-00325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y-C. Invasive Candidiasis in Asia. In: Chakrabarti A, editor. Clinical Practice of Medical Mycology in Asia. Singapore: Springer Singapore; 2020:243–255. [Google Scholar]
- 47.Tan BH, Chakrabarti A, Li RY, et al.; Asia Fungal Working Group. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clin Microbiol Infect. 2015;21(10):946–953. PubMed PMID: 26100373. doi: 10.1016/j.cmi.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 48.Riera FO, Caeiro JP, Angiolini SC, et al. Invasive candidiasis: update and current challenges in the management of this mycosis in South America. Antibiotics. 2022;11(7). PubMed PMID: 35884131; PMCID: PMC9312041. doi: 10.3390/antibiotics11070877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis. 2019;68(11):1791–1797. PubMed PMID: 30204844; PMCID: PMC6409199. doi: 10.1093/cid/ciy776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benedict K, Whitham HK, Jackson BR. Economic burden of fungal diseases in the United States. Open Forum Infect Dis. 2022;9(4):ofac097. PubMed PMID: 35350173; PMCID: PMC8946773. doi: 10.1093/ofid/ofac097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cleveland AA, Farley MM, Harrison LH, et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis. 2012;55(10):1352–1361. PubMed PMID: 22893576; PMCID: PMC4698872. doi: 10.1093/cid/cis697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyahnwi D, Siraw BB, Reingold A. Epidemiologic features, clinical characteristics, and predictors of mortality in patients with candidemia in Alameda County, California; a 2017–2020 retrospective analysis. BMC Infect Dis. 2022;22(1):843. PubMed PMID: 36371155; PMCID: PMC9652840. doi: 10.1186/s12879-022-07848-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghazi S, Rafei R, Osman M, et al. The epidemiology of Candida species in the Middle East and North Africa. J Mycol Med. 2019;29(3):245–252. PubMed PMID: 31400864. doi: 10.1016/j.mycmed.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 54.Wenzel RP. Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis. 1995;20(6):1531–1534. PubMed PMID: 7548504. doi: 10.1093/clinids/20.6.1531 [DOI] [PubMed] [Google Scholar]
- 55.Chapman B, Slavin M, Marriott D, et al. Changing epidemiology of candidaemia in Australia. J Antimicrob Chemother. 2017;72(4):1103–1108. PubMed PMID: 28364558. doi: 10.1093/jac/dkw422 [DOI] [PubMed] [Google Scholar]
- 56.Hoenigl M, Salmanton-García J, Egger M, et al. Guideline adherence and survival of patients with candidaemia in Europe: results from the ECMM Candida III multinational European observational cohort study. Lancet Infect Dis. 2023. doi: 10.1016/S1473-3099(22)00872-6 [DOI] [PubMed] [Google Scholar]
- 57.Tortorano AM, Peman J, Bernhardt H, et al.; Candidaemia EWGo. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis. 2004;23(4):317–322. PubMed PMID: 15029512. doi: 10.1007/s10096-004-1103-y [DOI] [PubMed] [Google Scholar]
- 58.Klingspor L, Tortorano AM, Peman J, et al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clin Microbiol Infect. 2015;21(1):87e1–e10. PubMed PMID: 25636940. doi: 10.1016/j.cmi.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 59.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20(Suppl 6):5–10. PubMed PMID: 24506442. doi: 10.1111/1469-0691.12539 [DOI] [PubMed] [Google Scholar]
- 60.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48(4):1366–1377. PubMed PMID: 20164282; PMCID: PMC2849609. doi: 10.1128/jcm.02117-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Risum M, Astvad K, Johansen HK, et al. Update 2016–2018 of the Nationwide Danish Fungaemia Surveillance Study: epidemiologic changes in a 15-year perspective. J Fungi. 2021;7(6). PubMed PMID: 34205349; PMCID: PMC8235436. doi: 10.3390/jof7060491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty years of the SENTRY antifungal surveillance program: results for candida species from 1997–2016. Open Forum Infect Dis. 2019;6(Suppl 1):S79–S94. PubMed PMID: 30895218; PMCID: PMC6419901. doi: 10.1093/ofid/ofy358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diaz-Garcia J, Gomez A, Machado M, et al. Blood and intra-abdominal Candida spp. from a multicentre study conducted in Madrid using EUCAST: emergence of fluconazole resistance in Candida parapsilosis, low echinocandin resistance and absence of Candida auris. J Antimicrob Chemother. 2022;77(11):3102–3109. PubMed PMID: 36031723. doi: 10.1093/jac/dkac288 [DOI] [PubMed] [Google Scholar]
- 64.Daneshnia F, de Almeida Junior JN, Ilkit M, et al. Worldwide emergence of fluconazole-resistant Candida parapsilosis: current framework and future research roadmap. Lancet Microbe. 2023;4(6):e470–e80. PubMed PMID: 37121240. doi: 10.1016/S2666-5247(23)00067-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao M, Chen SC, Kong F, et al. Distribution and antifungal susceptibility of candida species causing candidemia in China: an update from the CHIF-NET Study. J Infect Dis. 2020;221(Suppl 2):S139–S47. PubMed PMID: 32176789. doi: 10.1093/infdis/jiz573 [DOI] [PubMed] [Google Scholar]
- 66.Arastehfar A, Hilmioğlu-Polat S, Daneshnia F, et al. Recent Increase in the Prevalence of Fluconazole-Non-susceptible Candida tropicalis Blood Isolates in Turkey: clinical Implication of Azole-Non-susceptible and Fluconazole Tolerant Phenotypes and Genotyping. Front Microbiol. 2020;11:587278. PubMed PMID: 33123116. doi: 10.3389/fmicb.2020.587278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2441 patients. Antimicrob Agents Chemother. 2011;55(2):532–538. PubMed PMID: 21078946; PMCID: PMC3028765. doi: 10.1128/aac.01128-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaerger K, Schwartze VU, Dolatabadi S, et al. Adaptation to thermotolerance in Rhizopus coincides with virulence as revealed by avian and invertebrate infection models, phylogeny, physiological and metabolic flexibility. Virulence. 2015;6(4):395–403. PubMed PMID: 26065324; PMCID: PMC4604701. doi: 10.1080/21505594.2015.1029219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nnadi NE, Carter DA. Climate change and the emergence of fungal pathogens. PLoS Pathog. 2021;17(4):e1009503. PubMed PMID: 33914854; PMCID: PMC8084208. doi: 10.1371/journal.ppat.1009503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Bustos V, Cabañero-Navalon MD, Ruiz-Gaitán AC, Salavert M, Tormo-Mas M, Pemán J. Climate change, animals, and Candida auris: insights into the ecological niche of a new species from a one health approach. Clin Microbiol Infect. 2023. PubMed PMID: 36934871. doi: 10.1016/j.cmi.2023.03.016 [DOI] [PubMed] [Google Scholar]
- 71.Casadevall A, Kontoyiannis DP, Robert V. Environmental Candida auris and the global warming emergence hypothesis. mBio. 2021;12(2). PubMed PMID: 33727350; PMCID: PMC8092241. doi: 10.1128/mBio.00360-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arora P, Singh P, Wang Y, et al. Environmental isolation of Candida auris from the coastal wetlands of Andaman Islands, India. mBio. 2021;12(2). PubMed PMID: 33727354; PMCID: PMC8092279. doi: 10.1128/mBio.03181-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyman M, Forsberg K, Sexton DJ, et al. Worsening Spread of Candida auris in the United States, 2019 to 2021. Ann Intern Med. 2023;176(4):489–495. PubMed PMID: 36940442. doi: 10.7326/M22-3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher MC, Denning DW. The WHO fungal priority pathogens list as a game-changer. Nat Rev Microbiol. 2023;21(4):211–212. PubMed PMID: 36747091; PMCID: PMC9901396. doi: 10.1038/s41579-023-00861-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kohlenberg A, Monnet DL, Plachouras D. Increasing number of cases and outbreaks caused by Candida auris in the EU/EEA, 2020 to 2021. Euro Surveill. 2022;27(46). PubMed PMID: 36398575; PMCID: PMC9673237. doi: 10.2807/1560-7917.Es.2022.27.46.2200846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rhodes J, Fisher MC. Global epidemiology of emerging Candida auris. Curr Opin Microbiol. 2019;52:84–89. PubMed PMID: 31279224. doi: 10.1016/j.mib.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 77.Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog. 2013;9(9):e1003550. PubMed PMID: 24086128; PMCID: PMC3784480. doi: 10.1371/journal.ppat.1003550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cornely OA, Hoenigl M, Lass-Florl C, et al.; Mycoses Study Group E, Research C, the European Confederation of Medical M. Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses. 2019;62(9):716–729. PubMed PMID: 31254420; PMCID: PMC6692208. doi: 10.1111/myc.12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jenks JD, Cornely OA, Chen SC, Thompson GR, Hoenigl M. Breakthrough invasive fungal infections: who is at risk? Mycoses. 2020;63(10):1021–1032. PubMed PMID: 32744334. doi: 10.1111/myc.13148 [DOI] [PubMed] [Google Scholar]
- 80.Andes DR, Safdar N, Baddley JW, et al. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transplant Infect Dis. 2016;18(6):921–931. PubMed PMID: 27643395. doi: 10.1111/tid.12613 [DOI] [PubMed] [Google Scholar]
- 81.Jenks JD, Gangneux JP, Schwartz IS, et al.; European Confederation of Medical Mycology Council I. Diagnosis of breakthrough fungal infections in the clinical mycology laboratory: an ECMM consensus statement. J Fungi. 2020;6(4). PubMed PMID: 33050598; PMCID: PMC7712958. doi: 10.3390/jof6040216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. PubMed PMID: 31802125; PMCID: PMC7486838. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pasqualotto AC, Nedel WL, Machado TS, Severo LC. Risk factors and outcome for nosocomial breakthrough candidaemia. J Infect. 2006;52(3):216–222. PubMed PMID: 15936825. doi: 10.1016/j.jinf.2005.04.020 [DOI] [PubMed] [Google Scholar]
- 84.Cuervo G, Garcia-Vidal C, Nucci M, et al. Breakthrough candidaemia in the era of broad-spectrum antifungal therapies. Clin Microbiol Infect. 2016;22(2):181–188. PubMed PMID: 26460064. doi: 10.1016/j.cmi.2015.09.029 [DOI] [PubMed] [Google Scholar]
- 85.Viehman JA, Clancy CJ, Clarke L, et al. Surgical site infections after liver transplantation: emergence of multidrug-resistant bacteria and implications for prophylaxis and treatment strategies. Transplantation. 2016;100(10):2107–2114. PubMed PMID: 27479167. doi: 10.1097/TP.0000000000001356 [DOI] [PubMed] [Google Scholar]
- 86.Kably B, Launay M, Derobertmasure A, Lefeuvre S, Dannaoui E, Billaud EM. Antifungal Drugs TDM: trends and Update. Ther Drug Monit. 2022;44(1):166–197. PubMed PMID: 34923544. doi: 10.1097/FTD.0000000000000952 [DOI] [PubMed] [Google Scholar]
- 87.Stemler J, de Jonge N, Skoetz N, et al. Antifungal prophylaxis in adult patients with acute myeloid leukaemia treated with novel targeted therapies: a systematic review and expert consensus recommendation from the European Hematology Association. Lancet Haematol. 2022;9(5):e361–e73. PubMed PMID: 35483397. doi: 10.1016/S2352-3026(22)00073-4 [DOI] [PubMed] [Google Scholar]
- 88.Bruggemann RJ, Verheggen R, Boerrigter E, et al. Management of drug-drug interactions of targeted therapies for haematological malignancies and triazole antifungal drugs. Lancet Haematol. 2022;9(1):e58–e72. PubMed PMID: 34890539. doi: 10.1016/S2352-3026(21)00232-5 [DOI] [PubMed] [Google Scholar]
- 89.Sfeir MM, Jimenez-Ortigosa C, Gamaletsou MN, et al. Breakthrough bloodstream infections caused by echinocandin-resistant candida tropicalis: an emerging threat to immunocompromised patients with hematological malignancies. J Fungi. 2020;6(1). PubMed PMID: 32024039; PMCID: PMC7151208. doi: 10.3390/jof6010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shields RK, Nguyen MH, Press EG, et al. Rate of FKS mutations among consecutive Candida isolates causing bloodstream infection. Antimicrob Agents Chemother. 2015;59(12):7465–7470. PubMed PMID: 26392494; PMCID: PMC4649226. doi: 10.1128/AAC.01973-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bizerra FC, Jimenez-Ortigosa C, Souza AC, et al. Breakthrough candidemia due to multidrug-resistant Candida glabrata during prophylaxis with a low dose of micafungin. Antimicrob Agents Chemother. 2014;58(4):2438–2440. PubMed PMID: 24468776; PMCID: PMC4023795. doi: 10.1128/AAC.02189-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–1456. PubMed PMID: 26444731. doi: 10.1056/NEJMra1315399 [DOI] [PubMed] [Google Scholar]
- 93.Kabbara N, Lacroix C, Peffault de Latour R, Socie G, Ghannoum M, Ribaud P. Breakthrough C. parapsilosis and C. guilliermondii blood stream infections in allogeneic hematopoietic stem cell transplant recipients receiving long-term caspofungin therapy. Haematologica. 2008;93(4):639–640. PubMed PMID: 18379015. doi: 10.3324/haematol.11149 [DOI] [PubMed] [Google Scholar]
- 94.Gerzenshtein L, Patel SM, Scarsi KK, Postelnick MJ, Flaherty JP. Breakthrough Candida infections in patients receiving voriconazole. Ann Pharmacother. 2005;39(7–8):1342–1345. PubMed PMID: 15914520. doi: 10.1345/aph.1E627 [DOI] [PubMed] [Google Scholar]
- 95.Rausch CR, DiPippo AJ, Bose P, Kontoyiannis DP. Breakthrough fungal infections in patients with leukemia receiving isavuconazole. Clin Infect Dis. 2018;67(10):1610–1613. PubMed PMID: 29771293; PMCID: PMC6454429. doi: 10.1093/cid/ciy406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winston DJ, Bartoni K, Territo MC, Schiller GJ. Efficacy, safety, and breakthrough infections associated with standard long-term posaconazole antifungal prophylaxis in allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011;17(4):507–515. PubMed PMID: 20460163. doi: 10.1016/j.bbmt.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 97.Chen XC, Xu J, Wu DP. Clinical characteristics and outcomes of breakthrough candidemia in 71 hematologic malignancy patients and/or allogeneic hematopoietic stem cell transplant recipients: a single-center retrospective study from China, 2011–2018. Clin Infect Dis. 2020;71(Suppl 4):S394–S9. PubMed PMID: 33367573. doi: 10.1093/cid/ciaa1523 [DOI] [PubMed] [Google Scholar]
- 98.Ning Y, Xiao M, Perlin DS, et al. Decreased echinocandin susceptibility in Candida parapsilosis causing candidemia and emergence of a pan-echinocandin resistant case in China. Emerg Microbes Infect. 2023;12(1):2153086. PubMed PMID: 36440795; PMCID: PMC9793909. doi: 10.1080/22221751.2022.2153086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim SH, Choi JK, Cho SY, et al. Risk factors and clinical outcomes of breakthrough yeast bloodstream infections in patients with hematological malignancies in the era of newer antifungal agents. Med Mycol. 2018;56(2):197–206. PubMed PMID: 28525644; PMCID: PMC5896439. doi: 10.1093/mmy/myx038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Orasch C, Mertz D, Garbino J, et al.; Fungal Infection Network of S. Fluconazole non-susceptible breakthrough candidemia after prolonged low-dose prophylaxis: a prospective FUNGINOS study. J Infect. 2018;76(5):489–495. PubMed PMID: 29378240. doi: 10.1016/j.jinf.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 101.Pfaller MA, Carvalhaes CG, Smith CJ, Diekema DJ, Castanheira M. Bacterial and fungal pathogens isolated from patients with bloodstream infection: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (2012–2017). Diagnos Microbiol Infect Dis. 2020;97(2):115016. PubMed PMID: 32111415. doi: 10.1016/j.diagmicrobio.2020.115016 [DOI] [PubMed] [Google Scholar]
- 102.Desnos-Ollivier M, Lortholary O, Bretagne S, Dromer F. Azole susceptibility profiles of more than 9000 clinical yeast isolates belonging to 40 common and rare species. Antimicrob Agents Chemother. 2021;65(6). PubMed PMID: 33820766; PMCID: PMC8315974. doi: 10.1128/AAC.02615-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adam KM, Osthoff M, Lamoth F, et al.; Fungal Infection Network of S. Trends of the epidemiology of candidemia in Switzerland: a 15-year FUNGINOS Survey. Open Forum Infect Dis. 2021;8(10):ofab471. PubMed PMID: 34660836; PMCID: PMC8514178. doi: 10.1093/ofid/ofab471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan Z, Ahmad S, Al-Sweih N, et al. Changing trends in epidemiology and antifungal susceptibility patterns of six bloodstream Candida species isolates over a 12-year period in Kuwait. PLoS One. 2019;14(5):e0216250. PubMed PMID: 31042770; PMCID: PMC6494055. doi: 10.1371/journal.pone.0216250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiederhold NP. Antifungal susceptibility of yeasts and filamentous fungi by CLSI broth microdilution testing. Methods Mol Biol. 2023;2658:3–16. PubMed PMID: 37024691. doi: 10.1007/978-1-0716-3155-3_1 [DOI] [PubMed] [Google Scholar]
- 106.Arendrup MC, Friberg N, Mares M, Kahlmeter G, Meletiadis J, Guinea J; Subcommittee on Antifungal Susceptibility Testing of the EECfAST. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin Microbiol Infect. 2020;26(11):1464–1472. PubMed PMID: 32562861. doi: 10.1016/j.cmi.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 107.Kajihara T, Yahara K, Nagi M, et al. Distribution, trends, and antifungal susceptibility of Candida species causing candidemia in Japan, 2010–2019: a retrospective observational study based on national surveillance data. Med Mycol. 2022;60(9). PubMed PMID: 36095139; PMCID: PMC9521341. doi: 10.1093/mmy/myac071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bailly S, Maubon D, Fournier P, et al. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp. - Trends over 10 years. J Infect. 2016;72(1):103–111. PubMed PMID: 26518058. doi: 10.1016/j.jinf.2015.09.041 [DOI] [PubMed] [Google Scholar]
- 109.Guo LN, Yu SY, Xiao M, et al. Species distribution and antifungal susceptibility of invasive candidiasis: a 2016–2017 Multicenter Surveillance Study in Beijing, China. Infect Drug Resist. 2020;13:2443–2452. PubMed PMID: 32765018; PMCID: PMC7381087. doi: 10.2147/IDR.S255843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Healey KR, Zhao Y, Perez WB, et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat Commun. 2016;7(1):11128. PubMed PMID: 27020939; PMCID: PMC5603725. doi: 10.1038/ncomms11128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexander BD, Johnson MD, Pfeiffer CD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56(12):1724–1732. PubMed PMID: 23487382; PMCID: PMC3658363. doi: 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arastehfar A, Lass-Florl C, Garcia-Rubio R, et al. The quiet and underappreciated rise of drug-resistant invasive fungal pathogens. J Fungi. 2020;6(3). PubMed PMID: 32824785; PMCID: PMC7557958. doi: 10.3390/jof6030138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Desnos-Ollivier M, Bretagne S, Lortholary O, Dromer F; French Mycoses Study Group N. Brieu Ch Aix TCCDATCHUAJPBDCMPC. Echinocandins Susceptibility Patterns of 2787 Yeast Isolates: importance of the Thresholds for the Detection of FKS Mutations. Antimicrob Agents Chemother. 2022;66(5):e0172521. PubMed PMID: 35412354; PMCID: PMC9116480. doi: 10.1128/aac.01725-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McCarty TP, Lockhart SR, Moser SA, et al. Echinocandin resistance among Candida isolates at an academic medical centre 2005–15: analysis of trends and outcomes. J Antimicrob Chemother. 2018;73(6):1677–1680. PubMed PMID: 29506044. doi: 10.1093/jac/dky059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen PY, Chuang YC, Wu UI, et al. Clonality of fluconazole-nonsusceptible Candida tropicalis in bloodstream infections, Taiwan, 2011–2017. Emerg Infect Dis. 2019;25(9):1660–1667. PubMed PMID: 31441426; PMCID: PMC6711239. doi: 10.3201/eid2509.190520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chong Y, Shimoda S, Yakushiji H, et al. Fatal candidemia caused by azole-resistant Candida tropicalis in patients with hematological malignancies. J Infect Chemother. 2012;18(5):741–746. PubMed PMID: 22526385. doi: 10.1007/s10156-012-0412-9 [DOI] [PubMed] [Google Scholar]
- 117.Fan X, Xiao M, Liao K, et al. Notable increasing trend in azole non-susceptible candida tropicalis causing invasive candidiasis in China (August 2009 to July 2014): molecular epidemiology and clinical azole consumption. Front Microbiol. 2017;8:464. PubMed PMID: 28382028; PMCID: PMC5360734. doi: 10.3389/fmicb.2017.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chakrabarti A, Sood P, Rudramurthy SM. Critical care infections and antimicrobial resistance are complex multifactorial problems. Intensive Care Med. 2015;41(2):378. PubMed PMID: 25605471. doi: 10.1007/s00134-015-3652-1 [DOI] [PubMed] [Google Scholar]
- 119.Trevijano-Contador N, Torres-Cano A, Carballo-Gonzalez C, et al. Global emergence of resistance to fluconazole and voriconazole in Candida parapsilosis in Tertiary Hospitals in Spain During the COVID-19 pandemic. Open Forum Infect Dis. 2022;9(11):ofac605. PubMed PMID: 36467290; PMCID: PMC9709632. doi: 10.1093/ofid/ofac605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pfaller M, Boyken L, Hollis R, et al. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Aspergillus species to the triazoles. J Clin Microbiol. 2011;49(2):586–590. PubMed PMID: 21123534; PMCID: PMC3043512. doi: 10.1128/JCM.02136-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Schalkwyk E, Mpembe RS, Thomas J, et al. Epidemiologic shift in candidemia driven by Candida auris, South Africa, 2016–2017(1). Emerg Infect Dis. 2019;25(9):1698–1707. PubMed PMID: 31441749; PMCID: PMC6711229. doi: 10.3201/eid2509.190040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64(2):134–140. PubMed PMID: 27988485; PMCID: PMC5215215. doi: 10.1093/cid/ciw691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chowdhary A, Sharma C, Meis JF. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017;13(5):e1006290. PubMed PMID: 28542486; PMCID: PMC5436850. doi: 10.1371/journal.ppat.1006290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chowdhary A, Prakash A, Sharma C, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73(4):891–899. PubMed PMID: 29325167. doi: 10.1093/jac/dkx480 [DOI] [PubMed] [Google Scholar]
- 125.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–50. PubMed PMID: 26679628; PMCID: PMC4725385. doi: 10.1093/cid/civ933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ullmann AJ, Cornely OA, Donnelly JP, et al.; Group EFIS. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. Clin Microbiol Infect. 2012;18(Suppl 7):1–8. PubMed PMID: 23137133. doi: 10.1111/1469-0691.12037 [DOI] [PubMed] [Google Scholar]
- 127.Martin-Loeches I, Antonelli M, Cuenca-Estrella M, et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45(6):789–805. PubMed PMID: 30911804. doi: 10.1007/s00134-019-05599-w [DOI] [PubMed] [Google Scholar]
- 128.Ostrosky-Zeichner L, Al-Obaidi M. Invasive fungal infections in the intensive care unit. Infect Dis Clin North Am. 2017;31(3):475–487. PubMed PMID: 28687215. doi: 10.1016/j.idc.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 129.Ben-Ami R. Treatment of invasive candidiasis: a narrative review. J Fungi. 2018;4(3):97. doi: 10.3390/jof4030097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110–1122. PubMed PMID: 22412055. doi: 10.1093/cid/cis021 [DOI] [PubMed] [Google Scholar]
- 131.Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18:53–67. doi: 10.1111/1469-0691.12041 [DOI] [PubMed] [Google Scholar]
- 132.Cornely OA, Bassetti M, Calandra T, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18:19–37. doi: 10.1111/1469-0691.12039 [DOI] [PubMed] [Google Scholar]
- 133.McCarty TP, White CM, Pappas PG. Candidemia and Invasive Candidiasis. Infect Dis Clin North Am. 2021;35(2):389–413. PubMed PMID: 34016283. doi: 10.1016/j.idc.2021.03.007 [DOI] [PubMed] [Google Scholar]
- 134.Thompson GR, Jenks JD, Baddley JW, et al. Fungal endocarditis: pathophysiology, epidemiology, clinical presentation, diagnosis, and management. Clin Microbiol Rev. 2023;36(3):e0001923. PubMed PMID: 37439685; PMCID: PMC10512793. doi: 10.1128/cmr.00019-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31. PubMed PMID: 16758414. doi: 10.1086/504810 [DOI] [PubMed] [Google Scholar]
- 136.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–3645. doi: 10.1128/aac.49.9.3640-3645.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ostrosky-Zeichner L, Shoham S, Vazquez J, et al. MSG-01: a randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis. 2014;58(9):1219–1226. PubMed PMID: 24550378. doi: 10.1093/cid/ciu074 [DOI] [PubMed] [Google Scholar]
- 138.Cortegiani A, Russotto V, Maggiore A, et al. Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients. Cochrane Database Syst Rev. 2016. doi: 10.1002/14651858.cd004920.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Timsit JF, Azoulay E, Schwebel C, et al. Empirical micafungin treatment and survival without invasive fungal infection in adults with ICU-acquired sepsis, Candida colonization, and multiple organ failure: the EMPIRICUS randomized clinical trial. JAMA. 2016;316(15):1555–1564. PubMed PMID: 27706483. doi: 10.1001/jama.2016.14655 [DOI] [PubMed] [Google Scholar]
- 140.Kuse ER, Chetchotisakd P, Da Cunha CA, et al.; Micafungin Invasive Candidiasis Working G. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a Phase III randomised double-blind trial. Lancet. 2007;369(9572):1519–1527. PubMed PMID: 17482982. doi: 10.1016/S0140-6736(07)60605-9 [DOI] [PubMed] [Google Scholar]
- 141.Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus Fluconazole for Invasive Candidiasis. N Engl J Med. 2007;356(24):2472–2482. doi: 10.1056/nejmoa066906 [DOI] [PubMed] [Google Scholar]
- 142.Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45(7):883–893. PubMed PMID: 17806055. doi: 10.1086/520980 [DOI] [PubMed] [Google Scholar]
- 143.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347(25):2020–2029. doi: 10.1056/nejmoa021585 [DOI] [PubMed] [Google Scholar]
- 144.Betts RF, Nucci M, Talwar D, et al. A Multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis. 2009;48(12):1676–1684. PubMed PMID: 19419331. doi: 10.1086/598933 [DOI] [PubMed] [Google Scholar]
- 145.ASSESSMENT RR. Candida auris outbreak in healthcare facilities in northern Italy, 2019-20212022; 2022.
- 146.Alfouzan WA, Dhar R, Alabbad J, Rabaan AA. Infection control measures against candida auris in healthcare facilities. Processes. 2022;10(8):1625. [Google Scholar]
- 147.Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg Infect Dis. 2020; 26(7). PubMed PMID: 32275497. doi: 10.3201/eid2607.200885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hanson BM, Dinh AQ, Tran TT, et al. Candida auris invasive infections during a COVID-19 case surge. Antimicrob Agents Chemother. 2021;65(10):e01146–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seiler GT, Ostrosky-Zeichner L. Investigational agents for the treatment of resistant yeasts and molds. Curr Fungal Infect Rep. 2021;15(3):104–115. PubMed PMID: 34075318; PMCID: PMC8162489. doi: 10.1007/s12281-021-00419-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johnson MD. Antifungals in clinical use and the pipeline. Infect Dis Clin North Am. 2021;35(2):341–371. PubMed PMID: 34016281. doi: 10.1016/j.idc.2021.03.005 [DOI] [PubMed] [Google Scholar]
- 151.Sabino R, Veríssimo C, Pereira ÁA, Antunes F. Candida auris, an agent of hospital-associated outbreaks: which challenging issues do we need to have in mind? Microorganisms. 2020;8(2):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pandya N, Cag Y, Pandak N, et al. International multicentre study of candida auris infections. J Fungi. 2021;7(10):878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Watkins RR, Gowen R, Lionakis M, Ghannoum M. Update on the pathogenesis, virulence, and treatment of Candida auris. Pathog Immun. 2022;7(2):46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.fda.gov. Novel drug approvals for 2023; 2023. [March 30, 2023]. Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023. Accessed July 31, 2024.
- 155.Thompson GR, Soriano A, Cornely OA, et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet. 2023;401(10370):49–59. PubMed PMID: 36442484. doi: 10.1016/S0140-6736(22)02324-8 [DOI] [PubMed] [Google Scholar]
- 156.Hoenigl M, Sprute R, Egger M, et al. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs. 2021;81(15):1703–1729. doi: 10.1007/s40265-021-01611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mccarthy MW. Pharmacokinetics and pharmacodynamics of ibrexafungerp. Drugs R D. 2022;22(1):9–13. doi: 10.1007/s40268-021-00376-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spec A, Pullman J, Thompson GR, et al. MSG-10: a Phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J Antimicrob Chemother. 2019;74(10):3056–3062. PubMed PMID: 31304536. doi: 10.1093/jac/dkz277 [DOI] [PubMed] [Google Scholar]
- 159.Jallow S, Govender NP. Ibrexafungerp: a first-in-class oral triterpenoid glucan synthase inhibitor. J Fungi. 2021;7(3):163. doi: 10.3390/jof7030163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shaw KJ, Ibrahim AS. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi. 2020;6(4). PubMed PMID: 33105672; PMCID: PMC7711534. doi: 10.3390/jof6040239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vazquez JA, Pappas PG, Boffard K, et al. Clinical efficacy and safety of a novel antifungal, fosmanogepix, in patients with candidemia caused by Candida auris: results from a Phase 2 trial. Antimicrob Agents Chemother. 2023;67(5):e0141922. PubMed PMID: 37022196; PMCID: PMC10190264. doi: 10.1128/aac.01419-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pappas PG, Vazquez JA, Oren I, et al. Clinical safety and efficacy of novel antifungal, fosmanogepix, for the treatment of candidaemia: results from a Phase 2 trial. J Antimicrob Chemother. 2023;78(10):2471–2480. PubMed PMID: 37596890; PMCID: PMC10545531. doi: 10.1093/jac/dkad256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.O’Grady NP, Alexander M, Burns LA, et al.; Healthcare Infection Control Practices Advisory C. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 Suppl 1):S1–34. PubMed PMID: 21511081. doi: 10.1016/j.ajic.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 164.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–542. doi: 10.1056/nejmoa1113849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Prevention CfDCa. Infection Prevention and Control for Candida auris: CDC; 2023. [December 12, 2023]. Available from: https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html. Accessed July 31, 2024.
- 166.Prevention CfDCa. Strategies for Prevention and Response to Novel & Targeted Multidrug-Resistant Organisms (MDROs). CDC; 2023. [cited December 12, 2023]. Available from: https://www.cdc.gov/hai/mdro-guides/index.html. Accessed July 31, 2024. [Google Scholar]
- 167.Agency USEP. List P: antimicrobial products registered with EPA for claims against candida auris: EPA; 2023. [December 12, 2023]. Available from: https://www.epa.gov/pesticide-registration/list-p-antimicrobial-products-registered-epa-claims-against-candida-auris. Accessed July 31, 2024.